Photoredox-Catalyzed Synthesis of 3-Sulfonylated Pyrrolin-2-ones via a Regioselective Tandem Sulfonylation Cyclization of 1,5-Dienes
Abstract
:1. Introduction
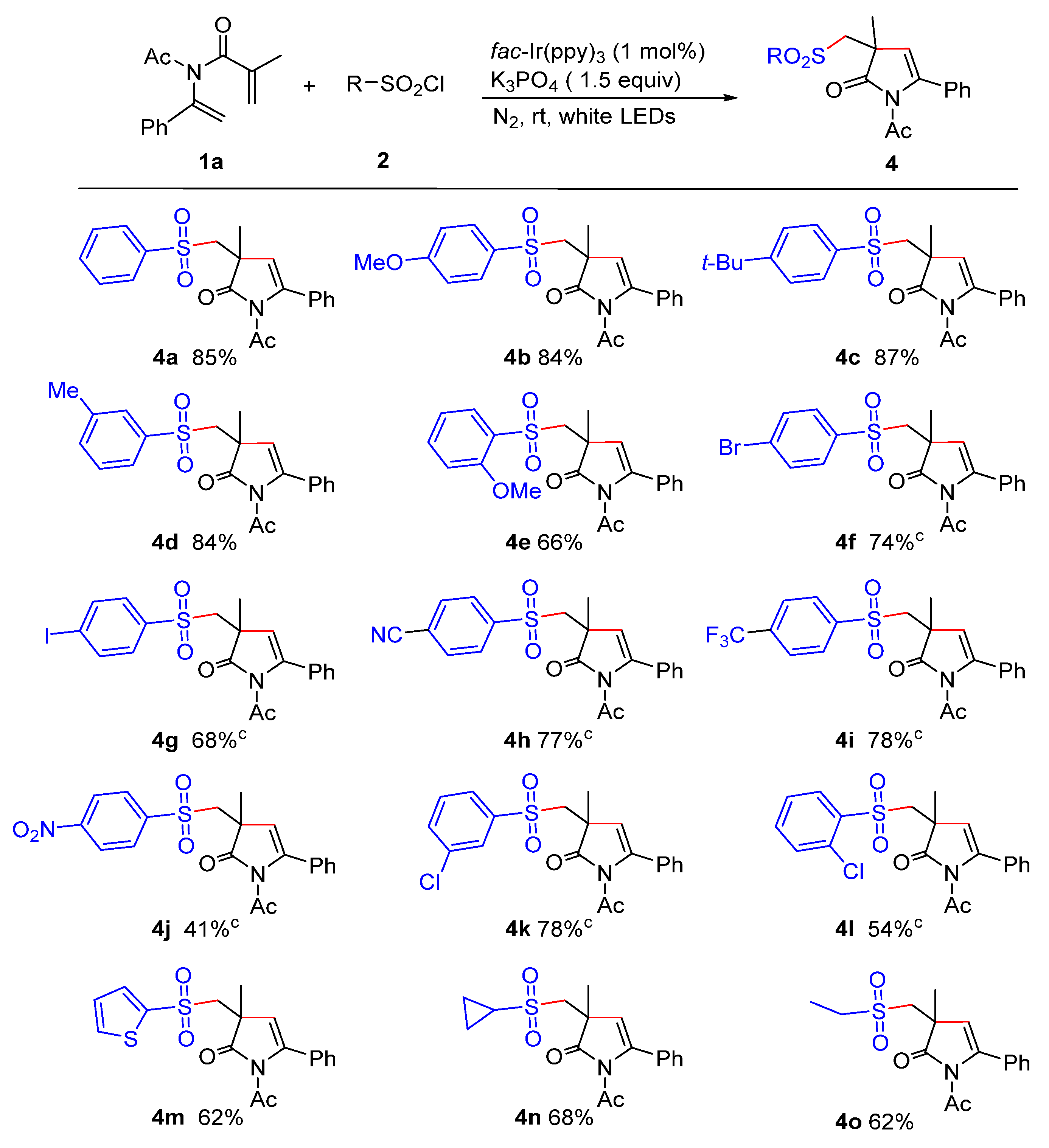
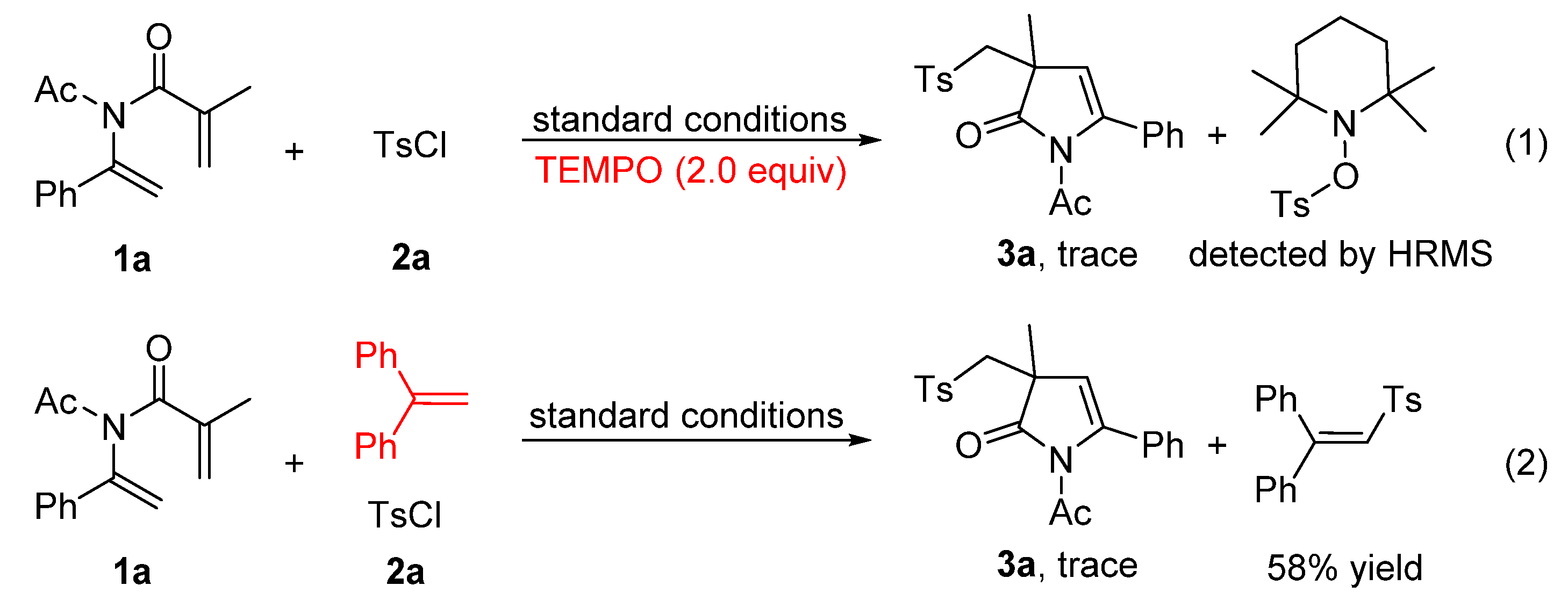
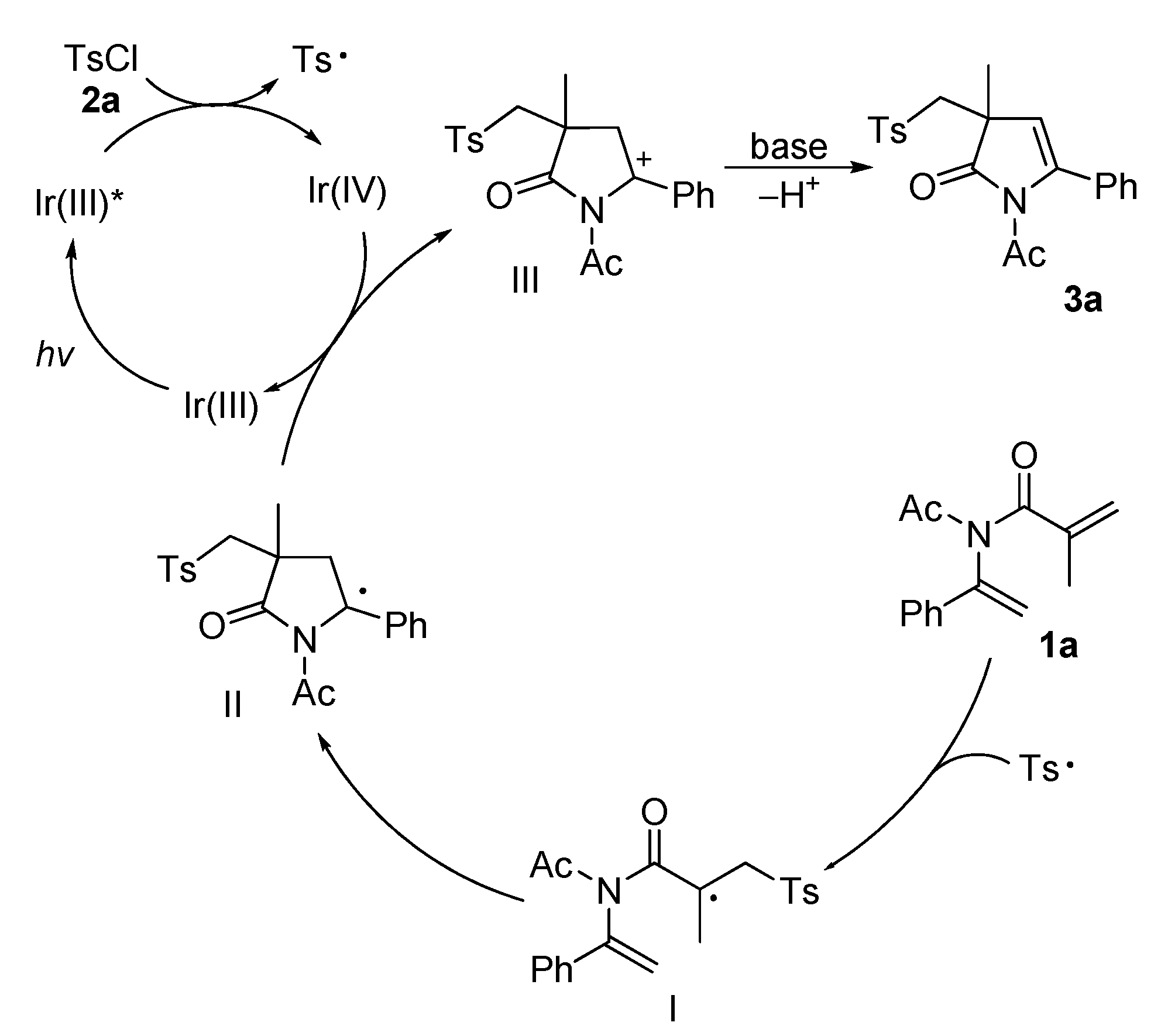
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Typical Procedure for the Preparation of 3a
3.3. Procedure for the Synthesis of the Coupling Product 4aa
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Drews, J. Drug Discovery: A Historical Perspective. Science 2000, 287, 1960–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metzner, P.; Thuillier, A.; Katritzky, A.; MethCohn, O.; Rees, C.W. Sulfur Reagents in Organic Synthesis; Academic Press: London, UK, 1994. [Google Scholar]
- Lin, S.; Yeh, T.; Kuo, C.; Song, J.; Cheng, M.; Liao, F.; Chao, M.; Huang, H.; Chen, Y.; Yang, C.; et al. Phenyl benzenesulfonylhydrazides exhibit selective indoleamine 2,3-dioxygenase inhibition with potent in vivo pharmacodynamic activity and antitumor efficacy. J. Med. Chem. 2016, 59, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Dunny, E.; Doherty, W.; Evans, P.; Malthouse, J.; Nolan, D.; Knox, A. Vinyl sulfone-based peptidomimetics as anti-trypanosomal agents: Design, synthesis, biological and computational evaluation. J. Med. Chem. 2013, 56, 6638–6647. [Google Scholar] [CrossRef]
- Zhao, Z.; Pissarnitski, D.; Josien, H.; Wu, W.; Xu, R.; Li, H.; Clader, J.W.; Burnett, D.A.; Terracina, G.; Hyde, L.; et al. Discovery of a novel, potent spirocyclic series of γ-secretase inhibitors. J. Med. Chem. 2015, 58, 8806–8813. [Google Scholar] [CrossRef]
- Procopiou, P.A.; Barrett, V.; Biggadike, K.; Butchers, P.R.; Craven, A.; Ford, A.J.; Guntrip, S.; Holmes, D.; Hughes, S.; Jones, A.; et al. Discovery of a rapidly metabolized, long-acting β2 adrenergic receptor agonist with a short onset time incorporating a sulfone group suitable for once-daily dosing. J. Med. Chem. 2014, 57, 159–170. [Google Scholar] [CrossRef]
- Yang, L.; Wang, D.-X.; Huang, Z.-T.; Wang, M.-X. Cr(III)(salen)Cl catalyzed enantioselective intramolecular addition of tertiary enamides to ketones: A general access to enantioenriched 1H-pyrrol-2(3H)-one derivatives bearing a hydroxylated quaternary carbon atom. J. Am. Chem. Soc. 2009, 131, 10390–10394. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Tao, Y.; Zhang, X.; Su, W. 1,2-Aryl migration induced by amide C–N bond-formation: Reaction of alkyl aryl ketones with primary amines towards α, α-diaryl β, γ-unsaturated γ-lactams. Angew. Chem. Int. Ed. 2021, 60, 8425–8428. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Song, H.; Zhang, C.; Liu, Y.-J.; Shi, B.-F. Copper-catalyzed modular access to N-fused polycyclic indoles and 5-aaroyl-pyrrol-2-ones via intramolecular N–H/C–H annulation with alkynes: Scope and mechanism probes. Chin. J. Chem. 2020, 38, 1545–1552. [Google Scholar] [CrossRef]
- Zhao, Z.; Kong, X.; Wang, W.; Hao, J.; Wang, Y. Direct use of unprotected aliphatic amines to generate N-heterocycles via β-C–H malonylation with iodonium ylide. Org. Lett. 2020, 22, 230–234. [Google Scholar] [CrossRef]
- Kumarasamy, E.; Raghunathan, R.; Kandappa, S.K.; Sreenithya, A.; Jockusch, S.; Sunoj, R.B.; Sivaguru, J. Transposed paternò–büchi reaction. J. Am. Chem. Soc. 2017, 139, 655–659. [Google Scholar] [CrossRef]
- Koronatov, A.N.; Rostovskii, N.V.; Khlebnikov, A.F.; Novikov, M.S. Synthesis of 3-alkoxy-4-pyrrolin-2-ones via Rhodium(II)-catalyzed denitrogenative transannulation of 1H-1,2,3-Triazoles with diazo esters. Org. Lett. 2020, 22, 7958–7963. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Arseniyadis, S.; Cossy, J. Asymmetric synthesis of α-quaternary γ-lactams through palladium-catalyzed asymmetric allylic alkylation. Org. Lett. 2019, 21, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Mao, M.-H.; Jia, W.-Z.; Fu, J.-M.; Liu, L.; Mao, Y.-Y.; Guo, Y.; Wang, P.-L. Synthesis of sulfonylated pyrrolines and pyrrolinones via Ag mediated radical cyclization of olefinic enamides with sodium sulfinates. Asian J. Org. Chem. 2021, 10, 366–370. [Google Scholar] [CrossRef]
- He, J.-Q.; Yang, Z.-X.; Zhou, X.-L.; Li, Y.; Gao, S.; Shi, L.; Liang, D. Exploring the regioselectivity of the cyanoalkylation of 3-aza-1,5-dienes: Photoinduced synthesis of 3-cyanoalkyl-4-pyrrolin-2-ones. Org. Chem. Front. 2022, 9, 4575–4579. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.; Wu, X.; Du, F.; Zeng, L.; Wu, J.; Chen, J. Regioselective radical-relay sulfonylation/cyclization protocol to sulfonylated pyrrolidones under transition-metal-free conditions. J. Org. Chem. 2022, 87, 6137–6145. [Google Scholar] [CrossRef]
- Wang, X.; You, F.; Xiong, B.; Chen, L.; Zhang, X.; Lian, Z. Metal- and base-free tandem sulfonylation/cyclization of 1,5-dienes with aryldiazonium salts via the insertion of sulfur dioxide. RSC Adv. 2022, 12, 16745–16748. [Google Scholar] [CrossRef]
- Wang, P.; Leng, Y.; Wu, Y. Copper(I)-catalyzed regioselective tandem cyanoalkylative cyclization of 1,5-dienes with Cyclobutanone Oxime Esters. Eur. J. Org. Chem. 2022, 2022, e202201091. [Google Scholar] [CrossRef]
- Chen, B.; Wu, L.-Z.; Tung, C.-H. Photocatalytic activation of less reactive bonds and their functionalization via hydrogen-evolution cross-couplings. Acc. Chem. Res. 2018, 51, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lu, L.-Q.; Yu, D.-G.; Zhu, C.-J.; Xiao, W.-J. Visible light-driven organic photochemical synthesis in China. Sci. China Chem. 2019, 62, 2462–2468. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.-Z. Recent Advances in Visible-light-driven organic reactions. Natl. Sci. Rev. 2017, 4, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Xuan, J.; Xiao, W.-J. Visible-light photoredox catalysis. Angew. Chem. Int. Ed. 2012, 51, 6828–6832. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-Y.; Jiang, J.; Wu, C.; Hou, J.-C.; Lu, Y.-H.; Wang, K.-L.; Yang, T.-B.; He, W.-M. Semi-heterogeneous g-C3N4/NaI dual catalytic C–C bond formation under visible light. Green Chem. 2023, 25, 3292–3296. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Liua, R.; Ji, Z.; Li, Y.; Zhao, X.; Wei, W. Visible-light-initiated 4CzIPN catalyzed multi-component tandem reactions to assemble sulfonated quinoxalin-2(1H)-ones. Chin. Chem. Lett. 2022, 33, 1479–1482. [Google Scholar] [CrossRef]
- Hu, X.; Tao, M.; Ma, Z.; Zhang, Y.; Li, Y.; Liang, D. Regioselective photocatalytic dialkylation/cyclization sequence of 3-aza-1,5-dienes: Access to 3,4-dialkylated 4-pyrrolin-2-ones. Adv. Synth. Catal. 2022, 364, 2163–2168. [Google Scholar] [CrossRef]
- Cassani, C.; Bernardi, L.; Fini, F.; Ricci, A. Catalytic asymmetric mannich reactions of sulfonylacetates. Angew. Chem. Int. Ed. 2009, 48, 5694–5697. [Google Scholar] [CrossRef]
- González, P.B.; Lopez, R.; Palomo, C. Catalytic enantioselective mannich-type reaction with β-Phenyl sulfonyl acetonitrile. J. Org. Chem. 2010, 75, 3920–3922. [Google Scholar] [CrossRef]
- Carreno, M.C. Applications of sulfoxides to asymmetric synthesis of biologically active compounds. Chem. Rev. 1995, 95, 1717–1760. [Google Scholar] [CrossRef]
- Liu, K.-G.; Robichaud, A.J.; Bernotas, R.C.; Yan, Y.; Lo, J.R.; Zhang, M.-Y.; Hughes, Z.A.; Huselton, C.; Zhang, G.-M.; Zhang, J.-Y.; et al. 5-Piperazinyl-3-sulfonylindazoles as potent and selective 5-hydroxytryptamine-6 antagonists. J. Med. Chem. 2010, 53, 7639–7646. [Google Scholar] [CrossRef]
- Ivachtchenko, A.V.; Golovina, E.S.; Kadieva, M.G.; Kysil, V.M.; Mitkin, O.D.; Tkachenko, S.E.; Okun, I.M. Synthesis and structure-activity relationship (SAR) of (5,7-disubstituted 3-phenylsulfonyl-pyrazolo[1,5-a]pyrimidin-2-yl) methylamines as potent serotonin 5-HT6 receptor (5-HT6R) antagonists. J. Med. Chem. 2011, 54, 8161–8173. [Google Scholar] [CrossRef]
- Huang, Y.; Huo, L.; Zhang, S.; Guo, X.; Han, C.C.; Li, Y.; Hou, J. Sulfonyl: A new application of electron-withdrawing substituent in highly efficient photovoltaic polymer. Chem. Commun. 2011, 47, 8904–8906. [Google Scholar] [CrossRef]
- Barbuceanu, S.-F.; Almajan, G.L.; Saramet, I.; Draghici, C.; Tarcomnicu, A.I.; Bancescu, G. Synthesis, characterization and evaluation of antibacterial activity of some thiazolo[3,2-b][1,2,4]triazole incorporating diphenylsulfone moieties. Eur. J. Med. Chem. 2009, 44, 4752–4755. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-Q.; Zou, J.-P.; Yi, W.-B.; Zhang, W. Recent advances in sulfur- and phosphorous-centered radical reactions for the formation of S-C and P-C bonds. Tetrahedron 2015, 71, 7481–7844. [Google Scholar] [CrossRef]
- Fang, Y.; Luo, Z.; Xu, X. Recent advances in the synthesis of vinyl sulfones. RSC Adv. 2016, 6, 59661–59676. [Google Scholar] [CrossRef]
- Shaaban, S.; Liang, S.; Liu, N.-W.; Manolikakes, G. Manolikakes, Synthesis of sulfones via selective C–H-functionalization. Org. Biomol. Chem. 2017, 15, 1947–1955. [Google Scholar] [CrossRef]
- Chaudhary, R.; Natarajan, P. Visible light photoredox activation of sulfonyl chlorides: Applications in organic synthesis. ChemistrySelect 2017, 2, 6458–6472. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, W.-C.; Wang, X.-D.; Wu, L. Photoredox catalysis in c-s bond construction: Recent progress in photo-catalyzed formation of sulfones and sulfoxides. Adv. Synth. Catal. 2018, 360, 386–399. [Google Scholar] [CrossRef]
- Lv, Y.; Cui, H.; Meng, N.; Yue, H.; Wei, W. Recent advances in the application of sulfinic acids for the construction of sulfur-containing compounds. Chin. Chem. Lett. 2022, 33, 97–109. [Google Scholar] [CrossRef]
- Rao, W.-H.; Jiang, L.-L.; Liu, X.-M.; Chen, M.-J.; Chen, F.-Y.; Jiang, X.; Zhao, J.-X.; Zou, G.-D.; Zhou, Y.-Q.; Tang, L. Copper(II)-catalyzed alkene aminosulfonylation with sodium sulfinates for the synthesis of sulfonylated pyrrolidones. Org. Lett. 2019, 21, 2890–2893. [Google Scholar] [CrossRef]
- Wang, L.-J.; Chen, J.-M.; Dong, W.; Hou, C.-Y.; Pang, M.; Jin, W.-B.; Dong, F.-G.; Xu, Z.-D.; Li, W. Synthesis of sulfonylated lactams by copper-mediated aminosulfonylation of 2-vinylbenzamides with sodium sulfinates. J. Org. Chem. 2019, 84, 2330–2338. [Google Scholar] [CrossRef]
- Dong, W.; Qi, L.; Song, J.-Y.; Chen, J.-M.; Guo, J.-X.; Shen, S.; Li, L.-J.; Li, W.; Wang, L.-J. Direct synthesis of sulfonylated spiro[indole-3,3′-pyrrolidines] by silver-mediated sulfonylation of acrylamides coupled with indole dearomatization. Org. Lett. 2020, 22, 1830–1835. [Google Scholar] [CrossRef]
- Wei, W.; Liu, C.; Yang, D.; Wen, J.; You, J.; Suo, Y.; Wang, H. Copper-catalyzed direct oxysulfonylation of alkenes with dioxygen and sulfonylhydrazides leading to β-ketosulfones. Chem. Commun. 2013, 49, 10239–10241. [Google Scholar] [CrossRef]
- Zhu, R.; Buchwald, S.J. Versatile enantioselective synthesis of functionalized lactones via copper-catalyzed radical oxyfunctionalization of alkenes. J. Am. Chem. Soc. 2015, 137, 8069–9077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.-J.; Chen, M.; Qi, L.; Xu, Z.; Li, W. Copper-mediated oxysulfonylation of alkenyl oximes with sodium sulfinates: A facile synthesis of isoxazolines featuring a sulfone substituent. Chem. Commun. 2017, 53, 2056–2059. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-T.; Lv, L.; Wang, T.; Gu, Q.-S.; Xu, G.-X.; Li, Z.-L.; Ye, L.; Zhang, X.; Cheng, G.-J.; Liu, X.-Y. Diastereo- and enantioselective catalytic radical oxysulfonylation of alkenes in β,γ unsaturated ketoximes. Chem 2020, 6, 1692–1706. [Google Scholar] [CrossRef]
- Gao, Y.; Tang, X.; Peng, J.; Hu, M.; Wu, W.; Jiang, H. Copper-catalyzed oxysulfenylation of enolates with sodium sulfinates: A strategy To construct sulfenylated cyclic ethers. Org. Lett. 2016, 18, 1158–1161. [Google Scholar] [CrossRef]
- He, F.-S.; Wu, Y.; Zhang, J.; Xia, H.; Wu, J. Thiosulfonylation of alkenes with the insertion of sulfur dioxide under non-metallic conditions. Org. Chem. Front. 2018, 5, 2940–2944. [Google Scholar] [CrossRef]
- Niu, T.-F.; Xue, L.-S.; Jiang, D.-Y.; Ni, B.-Q. Visible-Light-Induced chemoselective synthesis of α-chloro and vinyl sulfones by sulfonylation of alkenes. Synlett 2018, 29, 364–367. [Google Scholar] [CrossRef]
- Wang, C.; Sun, G.; Huang, H.-L.; Liu, J.; Tang, H.; Li, Y.; Hu, H.; He, S.; Gao, F. Visible-light-driven sulfonylation/cyclization to Access Sulfonylated Benzo[4,5]imidazo[2,1-a]isoquinolin-6(5H)-ones. Chem. Asian. J. 2021, 16, 2618–2622. [Google Scholar] [CrossRef]
- Sun, B.; Tian, H.-X.; Ni, Z.-G.; Huang, P.-Y.; Ding, H.; Li, B.-Q.; Jin, C.; Wu, C.-L.; Shen, R.-P. Photocatalyst-, metal- and additive-free regioselective radical cascade sulfonylation/cyclization of benzimidazole derivatives with sulfonyl chlorides induced by visible light. Org. Chem. Front. 2022, 9, 3669–3676. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, X.; Lu, H.; Deng, G.; Liang, Y.; Yang, Y.; Li, J.-H. Copper-catalyzed [3 + 2]/[3 + 2] carboannulation of dienynes and arylsulfonyl chlorides enabled by smiles rearrangement: Access to cyclopenta[a]indene-fused quinolinones. Org. Chem. Front. 2021, 8, 5092–5097. [Google Scholar] [CrossRef]
- Liu, X.; Cong, T.; Liu, P.; Sun, P. Visible light-promoted synthesis of 4-(sulfonylmethyl)isoquinoline-1,3(2H,4H)-diones via a tandem radical cyclization and sulfonylation reaction. Org. Biomol. Chem. 2016, 14, 9416–9422. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-F.; Zhu, S.-L.; Wang, D.; Liang, Y.-M. Sulfide and sulfonyl chloride as sulfonylating precursors for the synthesis of sulfone-containing isoquinolinonediones. Adv. Synth. Catal. 2017, 359, 859–862. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.-L.; Chen, Z.; Zhou, Q.; Li, H.; Zhou, C.-S.; Xiong, B.-Q.; Zhang, P.-L.; Tang, K.-W. Visible-light-catalyzed C−C bond difunctionalization of methylenecyclopropanes with sulfonyl chlorides for the synthesis of 3-sulfonyl-1,2-dihydronaphthalenes. J. Org. Chem. 2019, 84, 2829–2839. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.-L.; Zheng, D.-G.; Zhu, X.-H.; Zhou, A.-X.; Yang, S.-D. Visible-light-induced sulfonylation/cyclization of vinyl azides: One-pot construction of 6-(sulfonylmethyl)phenanthridines. Org. Chem. Front. 2018, 5, 232–236. [Google Scholar] [CrossRef]
- Riggi, I.D.; Gastaldi, S.; Surzur, J.-M.; Bertrand, M.P. Chemoselective ring construction from unsymmetrical 1,6-dienes via radical addition of sulfonyl halides. J. Org. Chem. 1992, 57, 6118–6125. [Google Scholar] [CrossRef]
- Wang, C.; Russell, G.A. Chemoselective lactam formation in the addition of benzenesulfonyl bromide to N-allyl acrylamides and N-allyl 3,3-dimethylacrylamides. J. Org. Chem. 1999, 64, 2346–2352. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, Y.; Wan, J.-P. Transition metal-free synthesis of 3-trifluoromethyl chromones via tandem C-H trifluoromethylation and chromone annulation of enaminones. Org. Chem. Front. 2020, 7, 2770–2775. [Google Scholar] [CrossRef]
- Du, K.; Zhang, Z.; Sheng, W. Copper-Catalyzed the Synthesis of 3-Trifluoromethylchromone via Trifluoromethyl Radical Addition Tandem Cyclization Reaction of 2-Hydroxyphenyl Enaminones. Chin. J. Org. Chem. 2021, 41, 3242–3248. [Google Scholar] [CrossRef]
- Gilmore, K.; Mohamed, R.K.; Alabugin, I.V. The Baldwin rules: Revised and extended. Comput. Mol. Sci. 2016, 6, 487–514. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Timokhin, V.I.; Abrams, J.N.; Mariappan, M.; Abrams, R.; Ghiviriga, I. In Search of Efficient 5-Endo-dig Cyclization of a Carbon-Centered Radical: 40 Years from a Prediction to Another Success for the Baldwin Rules. J. Am. Chem. Soc. 2008, 130, 10984–10995. [Google Scholar] [CrossRef]
- Studer, A.; Curran, D.P. The electron is a catalyst. Nat. Chem. 2014, 6, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Dixon, J.A.; Rodriguez, R.A.; Baxter, R.D.; Dixon, D.D.; Collins, M.R.; Blackmond, D.G.; Baran, P.S. A New Reagent for Direct Difluoromethylation. J. Am. Chem. Soc. 2012, 134, 1494–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Ruffoni, A.; Gianatassio, R.; Fujiwara, Y.; Sella, E.; Shabat, D.; Baran, P.S. Direct Synthesis of Fluorinated Heteroarylether Bioisosteres. Angew. Chem. Int. Ed. 2013, 52, 3949–3952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hara, F.; Blackmond, D.G.; Baran, P.S. Radical-Based Regioselective C–H Functionalization of Electron-Deficient Heteroarenes: Scope, Tunability, and Predictability. J. Am. Chem. Soc. 2013, 135, 12122–12134. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.U.; Straková, K.; Slanina, T.; König, B. Eosin Y (EY) Photoredox-Catalyzed Sulfonylation of Alkenes: Scope and Mechanism. Chem. Eur. J. 2016, 22, 8694–8699. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Mulina, O.M.; Pirgach, D.A.; Demchuk, D.V.; Syroeshkin, M.A.; Nikishin, G.I. Copper(I)-mediated synthesis of β-hydroxysulfones from styrenes and sulfonylhydrazides: An electrochemical mechanistic study. RSC Adv. 2016, 6, 93476–93485. [Google Scholar] [CrossRef] [Green Version]
- Gomes, G.; Wimmer, A.; Smith, J.; König, B.; Alabugin, I.V. CO2 or SO2: Should It Stay, or Should It Go? J. Org. Chem. 2019, 84, 6232–6243. [Google Scholar] [CrossRef]




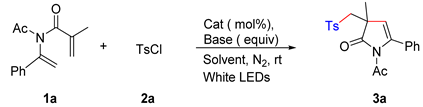 | ||||
|---|---|---|---|---|
| Entry | Catalyst (1 mol%) | Base (Equiv.) | Solvent | Yield b |
| 1 | fac-Ir(ppy)3 | Na2CO3 (1.0) | CH2Cl2 | 57% |
| 2 | Ru(bpy)3Cl2 | Na2CO3 (1.0) | CH2Cl2 | trace |
| 3 | Eosin Y | Na2CO3 (1.0) | CH2Cl2 | trace |
| 4 | fac-Ir(ppy)3 | K2CO3 (1.0) | CH2Cl2 | 66% |
| 5 | fac-Ir(ppy)3 | Li2CO3 (1.0) | CH2Cl2 | 59% |
| 6 | fac-Ir(ppy)3 | NaHCO3 (1.0) | CH2Cl2 | 64% |
| 7 | fac-Ir(ppy)3 | K3PO4 (1.0) | CH2Cl2 | 72% |
| 8 | fac-Ir(ppy)3 | Na3PO4 (1.0) | CH2Cl2 | 63% |
| 9 | fac-Ir(ppy)3 | K3PO4 (1.0) | DCE | 55% |
| 10 | fac-Ir(ppy)3 | K3PO4 (1.0) | CHCl3 | 62% |
| 11 | fac-Ir(ppy)3 | K3PO4 (1.0) | Acetone | 67% |
| 12 | fac-Ir(ppy)3 | K3PO4 (1.0) | Toluene | 44% |
| 13 | fac-Ir(ppy)3 | K3PO4 (1.0) | THF | 59% |
| 14 | fac-Ir(ppy)3 | K3PO4 (1.0) | EtOAc | 32% |
| 15 | fac-Ir(ppy)3 | K3PO4 (1.5) | CH2Cl2 | 79% |
| 16 | fac-Ir(ppy)3 | K3PO4 (2.0) | CH2Cl2 | 79% |
| 17 c | fac-Ir(ppy)3 | K3PO4 (1.5) | CH2Cl2 | 79% |
| 18 c | ------ | K3PO4 (1.5) | CH2Cl2 | 0% |
| 19 c | fac-Ir(ppy)3 | ------ | CH2Cl2 | 16% |
| 20 d | fac-Ir(ppy)3 | K3PO4 (1.5) | CH2Cl2 | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, R.; Li, L.; Yu, Y.-T.; Zhang, B.; Wang, P.-L. Photoredox-Catalyzed Synthesis of 3-Sulfonylated Pyrrolin-2-ones via a Regioselective Tandem Sulfonylation Cyclization of 1,5-Dienes. Molecules 2023, 28, 5473. https://doi.org/10.3390/molecules28145473
Ding R, Li L, Yu Y-T, Zhang B, Wang P-L. Photoredox-Catalyzed Synthesis of 3-Sulfonylated Pyrrolin-2-ones via a Regioselective Tandem Sulfonylation Cyclization of 1,5-Dienes. Molecules. 2023; 28(14):5473. https://doi.org/10.3390/molecules28145473
Chicago/Turabian StyleDing, Ran, Liang Li, Ya-Ting Yu, Bing Zhang, and Pei-Long Wang. 2023. "Photoredox-Catalyzed Synthesis of 3-Sulfonylated Pyrrolin-2-ones via a Regioselective Tandem Sulfonylation Cyclization of 1,5-Dienes" Molecules 28, no. 14: 5473. https://doi.org/10.3390/molecules28145473





