Computational Insights into Excited State Intramolecular Double Proton Transfer Behavior Associated with Atomic Electronegativity for Bis(2′-benzothiazolyl)hydroquinone
Abstract
:1. Introduction
2. Results and Discussion
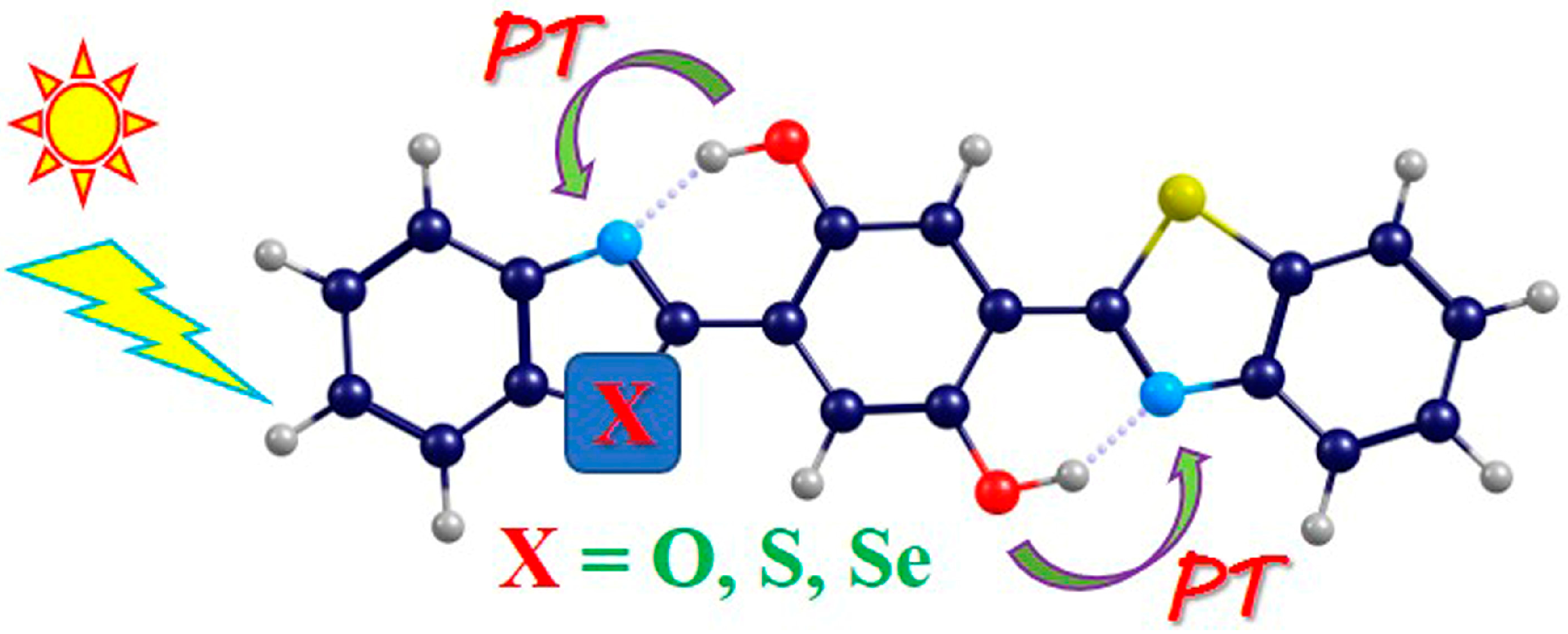
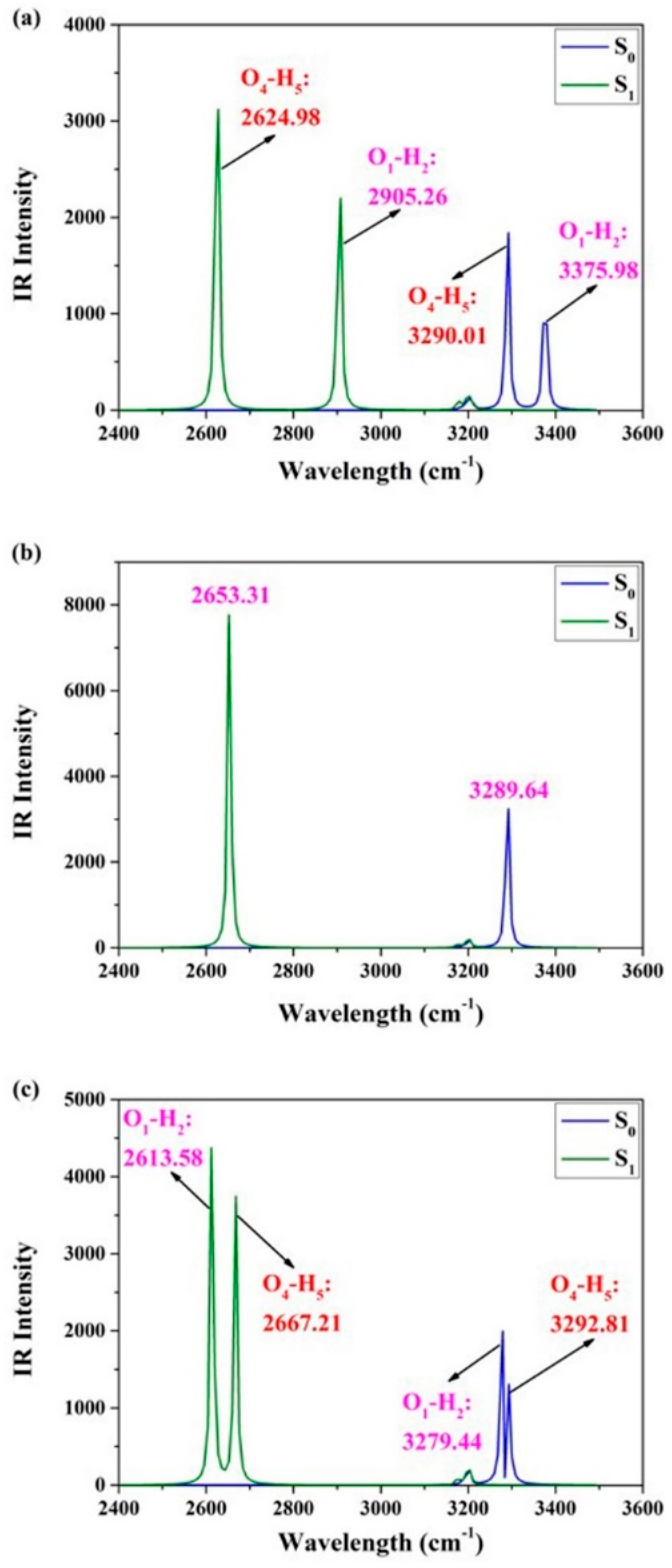
2.1. Photo-Induced Hydrogen Bonding Strengthening
2.2. Vertical Excitation
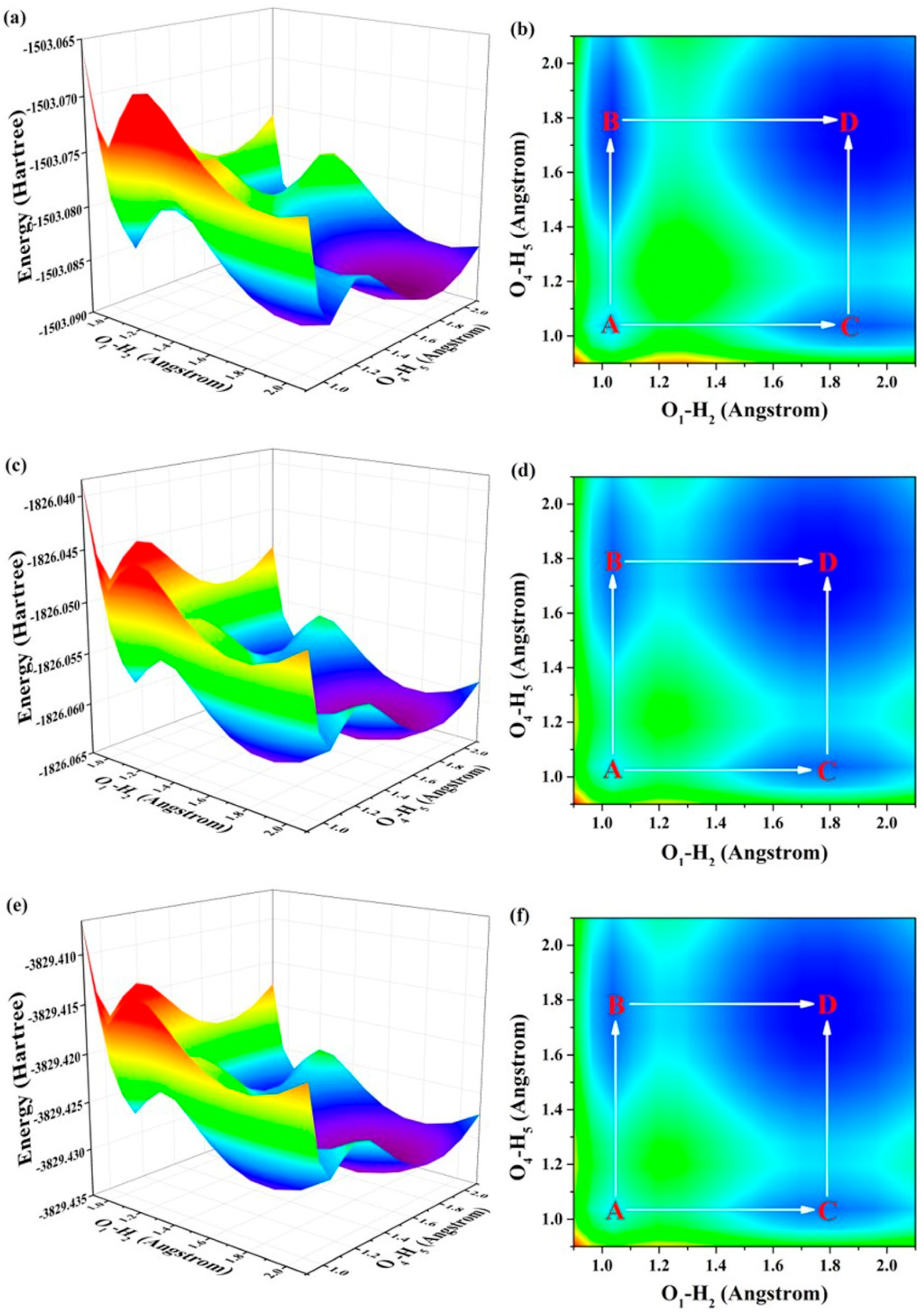
2.3. ESDPT Behaviors
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Demchenko, A.; Tang, K.; Chou, P. Excited-state proton coupled charge transfer modulated by molecular structure and media polarization. Chem. Soc. Rev. 2013, 42, 1379–1408. [Google Scholar] [CrossRef]
- Hammes-Schiffer, S.; Stuchebrukhov, A. Theory of coupled electron and proton transfer reactions. Chem. Rev. 2010, 110, 6939–6960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Han, K. Unraveling the detailed mechanism of excited-state proton transfer. Acc. Chem. Res. 2018, 51, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.; Mance, C.; Leutwyler, S. Probing the threshold to H atom transfer along a hydrogen-bonded ammonia wire. Science 2003, 302, 1736–1739. [Google Scholar] [CrossRef]
- Tomin, V.; Denchenko, A.; Chou, T. Thermodynamic vs. kinetic control of excited-state proton transfer reactions. J. Photochem. Photobiol. C 2015, 22, 1–18. [Google Scholar] [CrossRef]
- Murale, D.; Kim, H.; Choi, W.; Churchill, D. Highly selective excited state intramolecular proton transfer (ESIPT)-based superoxide probing. Org. Lett. 2013, 15, 3946–3949. [Google Scholar] [CrossRef]
- Kim, K.; Tsay, O.; Atwood, D.; Churchill, D. Destruction and detection of chemical warfare agents. Chem. Rev. 2011, 111, 5345–5403. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, J.; Huang, C.; Huang, T.; Chen, D.; Ho, S.; Chen, K.; Li, E.; Chou, P. Sulfur-based intramolecular hydrogen-bond: Excited-state hydrogen-bond on/off switch with dual room-temperature phosphorescence. J. Am. Chem. Soc. 2019, 141, 9885–9894. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Li, L.; Zhang, H. Theoretical insights into excited-state stepwise double proton transfer associated with solvent polarity for 2-bis(benzothia-zolyl)naphthalene-diol compound. ChemistrySelect 2023, 8, 202301202. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, K.; Chen, Y.; Shen, J.; Wu, Y.; Liu, S.; Lee, C.; Chen, C.; Lai, T.; Tung, S.; et al. Insight into the mechanism and outcoupling enhancement of excimer-associated white light generation. Chem. Sci. 2016, 7, 3556. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Chu, T. TD-DFT study on fluoride-sensing mechanism of 2-(2′-phenylureaphenyl)benzoxazole: The way to inhibit the ESIPT process. Phys. Chem. Chem. Phys. 2011, 13, 20766–20771. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Sonawane, P.; Liu, H.; Roychaudhury, A.; Lee, Y.; An, J.; Kim, D.; Kim, D.; Kim, Y.; Kim, Y.; et al. “Lighting up” fluoride: Cellular imaging and zebrafish model interrogations using a simple ESIPT-based mycophenolic acid precursor-based probe. Analyst 2023, 148, 2609–2615. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, M.; Seo, S.; Manjare, S.; Jon, S.; Churchill, D. A selective fluorescent probe for cysteine and its imaging in live cells. RSC Adv. 2014, 4, 64183–64186. [Google Scholar] [CrossRef]
- Li, G.; Han, K. The sensing mechanism studies of the fluorescent probes with electronically excited state calculations. WIREs Comput. Mol. Sci. 2018, 8, 1351. [Google Scholar] [CrossRef]
- Serdiuk, I.; Roshal, A. Exploring double proton transfer: A review on photochemical features of compounds with two proton-transfer sites. Dye. Pigment. 2017, 138, 223–244. [Google Scholar] [CrossRef]
- Crespo-Otero, R.; Kungwan, N.; Barbatti, M. Stepwise double excited-state proton transfer is not possible in 7-azaindole dimer. Chem. Sci. 2015, 6, 5762–5767. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Chen, J.; Cui, Y.; Wang, J.; Xia, L.; Dai, Y.; Song, P.; Ma, F. A questionable excited-state double-proton transfer mechanism for 3-hydroxyisoquinoline. Phys. Chem. Chem. Phys. 2015, 17, 1142–1150. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, H.; Zheng, Y. Elaborating the excited state multiple proton transfer mechanism for 9H-pyrido[3,4-b]indole. J. Lumin. 2018, 195, 228–233. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Zhu, C.; Lin, S. A TDDFT study on the excited-state double proton transfer reaction of 8-hydroxyquinoline along a hydrogen-bonded bridge. New J. Chem. 2017, 41, 8437–8442. [Google Scholar] [CrossRef]
- Shang, C.; Sun, C. Substituent effects on photophysical properties of ESIPT-based fluorophores bearing the 4-dithylaminosalicylaldehyde core. J. Mol. Liq. 2022, 367, 120477. [Google Scholar] [CrossRef]
- Yang, Y.; Li, D.; Li, C.; Liu, Y.; Jiang, K. Photoexcitation effect on the absorption of hazardous gases on silica surface. J. Hazard. Mater. 2018, 341, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Li, P.; Zhao, Y.; Han, K.K. Restriction of flip-flop motion as a mechanism for aggregation-induced emission. J. Phys. Chem. Lett. 2019, 10, 6929–6935. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Shi, W.; Zhao, Y.; Zhao, G.; Li, Y. Theoretical insights into the excited-state single and double proton transfer processes of DEASH in water. Chem. Phys. 2023, 570, 111882. [Google Scholar] [CrossRef]
- Han, J.; Liu, X.; Sun, C.; Li, Y.; Yin, H.; Shi, Y. Ingenious modification of molecular structure effectively regulates excited-state intramolecular proton and charge transfer: A theoretical study based on 3-hydroxyflavone. RSC Adv. 2018, 8, 29589–29597. [Google Scholar] [CrossRef] [PubMed]
- Manjare, S.; Kim, Y.; Churchill, D. Selenium- and tellurium- containing fluorescent molecular probes for the detection of biologically important analytes. Acc. Chem. Res. 2014, 47, 2985–2998. [Google Scholar] [CrossRef]
- An, J.; Chung, A.; Churchill, D. Nontoxic levels of Se-containing compounds increase survival by blocking oxidative and inflammoatory stresses via signal pathways whereas high level of Se induce apoptosis. Molecules 2023, 28, 5234. [Google Scholar] [CrossRef]
- Lou, Z.; Li, P.; Han, K. Redox-responsive fluorescent probes with different design strategies. Acc. Chem. Res. 2015, 48, 1358–1368. [Google Scholar] [CrossRef]
- Meng, F.; Chen, Y.; Chen, C.; Chou, P. Syntheses and excited-state intramolecular proton transfer of 3-hydroxythioflavone and its sulfone analogue. ChemPhotoChem 2018, 2, 475–480. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, H.; Liu, X.; Yin, H.; Shi, Y. Tunable ESIPT reaction and antioxidant activities of 3-hydroxyflavone and its derivatives by altering atomic electronegativity. Org. Chem. Front. 2018, 5, 3435–3442. [Google Scholar] [CrossRef]
- Cao, B.; Li, Y.; Zhou, Q.; Li, B.; Su, X.; Yin, H.; Shi, Y. Synergistically improving myricetin ESIPT and antioxidant activity via dexterously trimming atomic electronegativity. J. Mol. Liq. 2021, 325, 115272. [Google Scholar] [CrossRef]
- Lee, A.; Mercader, A.; Duchowicz, P.; Castro, E.; Pomilio, A. QSAR study of the DPPH radical scavenging activity of di(hetero)arylamines derivatives of benzo[b]thiophenes, halophenols and caffeic acid analogues. Chemom. Intell. Lab. Syst. 2012, 116, 33–40. [Google Scholar] [CrossRef]
- Chaihan, K.; Bui, T.; Goubard, F.; Kungwan, N. Tunable keto emission of 2-(2′-hydroxyphenyl)benzothiazole derivatives with π-expansion, substitution and additional proton transfer site for excited-state proton transfer-based fluorescent probes: Theoretical insights. J. Photochem. Photobiol. A 2021, 419, 113450. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [Green Version]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (Cam-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Truhlar, D. Comparative DFT study of van der Waals complexes: Rare-gas dimers, alkaline-earth dimers, zinc dimers, and zinc-rare-gas dimers. J. Phys. Chem. A 2006, 110, 5121–5129. [Google Scholar] [CrossRef] [PubMed]
- Lynch, B.; Fast, P.; Harris, M.; Truhlar, D. Adiabatic connection for kinetics. J. Phys. Chem. A 2000, 104, 4811–4815. [Google Scholar] [CrossRef]
- Chai, J.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Han, K. Hydrogen bonding in the electronic excited state. Acc. Chem. Res. 2012, 45, 404–413. [Google Scholar] [CrossRef]
- Tang, Z.; Bai, T.; Zhou, P. Sensing mechanism of a fluorescent probe for cysteine: Photoinduced electron transfer and invalidity of excited-state intramolecular proton transfer. J. Phys. Chem. A 2020, 124, 6920–6927. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, Z.; Liu, Y.; Guan, T.; Zhang, Q.; Qin, C.; Jiang, K.; Liu, Y. Transient absorption spectroscopy of a carbazole-based room-temperature phosphorescent molecule: Real-time monitoring of single-triplet transitions. J. Phys. Chem. Lett. 2022, 13, 9381–9389. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Liu, Y.; Jiang, Z.; Qin, C.; Jiang, K.; Liu, Y. Ultrafast dynamics of dual fluorescence of 2-(2’-hydroxyphenyl) benzothiazole and its derivatives by femtosecond transient absorption spectroscopy. J. Lumin. 2022, 248, 118922. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Y.; Yang, Y.; Wang, Y.; Tian, J.; Fei, X. Theoretical investigation of twisted charge-transfer-promoted intramolecular proton transfer in the excited state of 4′-dimethylaminoflavonol in a highly polar solvent. J. Lumin. 2018, 194, 785–790. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Feng, B.; Zhang, H.; Qin, C.; Yu, K.; Jiang, K.; Liu, Y. Discerning the multi-color fluorescence in donor-π-acceptor molecules by femtosecond transient absorption spectroscopy. J. Lumin. 2022, 242, 118591. [Google Scholar] [CrossRef]
- Zhou, P.; Han, K. ESIPT-based AIE luminogens: Design strategies, applications, and mechanisms. Aggregate 2022, 3, 160. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Zhai, H.; Jia, X.; He, Y.; Ma, Q.; Zhang, R.; Liu, Y.; Jiang, K. Fluorescent behaviors and reaction mechanism of 10-hydroxybenzo[h]quinoline on the detection of phenylboronic acid. J. Lumin. 2020, 223, 117224. [Google Scholar] [CrossRef]
- Fuster, F.; Silvi, B. Does the topological approach characterize the hydrogen bond? Theor. Chem. Acc. 2000, 104, 13–21. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Dommett, M.; Crespo-Otero, R. Excited state proton transfer in 2′-hydroxychalcone derivatives. Phys. Chem. Chem. Phys. 2017, 19, 2409. [Google Scholar] [CrossRef]
- Shekhovtsov, N.; Nikolaenkova, E.; Ryadun, A.; Samsonenko, D.; Tikhonov, A.; Bushuev, M. ESIPT-capable 4-(2-hydroxyphenyl)-2-(pyridine-2-yl)-1H-imidazoles with single and double proton transfer: Synthesis, selective reduction of the imidazolic OH group and luminescence. Molecules 2023, 28, 1793. [Google Scholar] [CrossRef]
- Dommett, M.; Rivera, M.; Smith, M.; Crespo-Otero, R. Molecular and crystalline requirements for solid state fluorescence exploiting excited state intramolecular proton transfer. J. Mater. Chem. C 2020, 8, 2558–2568. [Google Scholar] [CrossRef]
- Tang, Z.; Wei, H.; Zhou, P. Effects of solvents on the excited state intramolecular proton transfer and hydrogen bond mechanisms of alizarin and its isomers. J. Mol. Liq. 2020, 301, 112415. [Google Scholar] [CrossRef]
- Zhou, P.; Tang, Z.; Li, P.; Liu, J. Unraveling the mechanism for tuning the fluorescence of fluorescein derivatives: The role of the conical intersection and nπ* state. J. Phys. Chem. Lett. 2021, 12, 6478–6485. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, Y.; Yang, Y.; Shi, Y.; Li, Y. Fluorescence deactivation mechanism for a new probe detecting phosgene based on ESIPT and TICT. Org. Chem. Front. 2019, 6, 597–602. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, C.; Cao, Y.; Sun, C. Quantum mechanics/molecular mechanics studies on the photoprotection mechanisms of three chalcones. J. Mol. Liq. 2023, 372, 121165. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, X.; Liu, R.; Zhou, P. Theoretical investigations on the sensing mechanism of phenanthroimidazole fluorescent probes for the detection of selenocysteine. Molecules 2022, 27, 8444. [Google Scholar] [CrossRef]
- Wu, X.; Shi, W.; Yang, Y.; Zhao, D.; Li, Y. Multi-targeted fluorescent probes for detection of Zn(II) and Cu(II) ions based on ESIPT mechanism. Spectrochim. Acta Part A 2023, 287, 122051. [Google Scholar] [CrossRef]
- Peng, C.; Shen, J.; Chen, Y.; Wu, P.; Hung, W.; Hu, W.; Chou, P. Optically triggered stepwise double-proton transfer in an intramolecular proton relay: A case study of 1,8-dihydroxy-2-naphthaldehyde. J. Am. Chem. Soc. 2015, 137, 14349–14357. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, J.; Liu, J.; Hoffmann, M. Competitive excited-state single or double proton transfer mechanisms for bis-2,5-(2-benzoxazolyl)-hydroquinone and its derivatives. Phys. Chem. Chem. Phys. 2015, 17, 11990–11999. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, B.; Tang, Z. Solvent-polarity-dependent conformation and ESIPT behaviors for 2-(benzimidazole-2-yl)-3-hydroxychromone: A novel dynamical mechanism. Phys. Chem. Chem. Phys. 2022, 24, 27660–27669. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yu, K.; Ma, H.; Chai, S.; Dong, B. Theoretical investigation of excited-state single and double proton transfer mechanisms for 2,5-bis(benzoxazole-2-yl)thiophene-3,4-diol. Dye. Pigment. 2017, 141, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Becke, A. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Feller, D. The role of databases in support computational chemistry calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effects of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Cammi, R.; Tomasi, J. Remarks on the use of the apparent surface charges (ASC) methods in solvation problems: Iterative versus matrix-inversion procedures and renormalization of the apparent charges. J. Comput. Chem. 1995, 16, 1449–1458. [Google Scholar] [CrossRef]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]


| BBTHQ-SO | BBTHQ-SS | BBTHQ-SSe | ||||
|---|---|---|---|---|---|---|
| S0 | S1 | S0 | S1 | S0 | S1 | |
| O1-H2 | 0.98488 | 1.01016 | 0.98858 | 1.02371 | 0.98914 | 1.02617 |
| H2⋯N3 | 1.80813 | 1.68964 | 1.75430 | 1.61607 | 1.74982 | 1.60720 |
| O4-H5 | 0.98870 | 1.02551 | 0.98858 | 1.02371 | 0.98865 | 1.02306 |
| H5⋯N6 | 1.75141 | 1.60963 | 1.75430 | 1.61607 | 1.75384 | 1.61770 |
| Δ(O1H2N3) | 145.17 | 148.19 | 146.01 | 149.76 | 146.14 | 150.03 |
| Δ(O4H5N6) | 146.04 | 150.09 | 146.01 | 149.76 | 146.01 | 149.71 |
| BBTHQ-SO | BBTHQ-SS | BBTHQ-SSe | |||||
|---|---|---|---|---|---|---|---|
| S0 | S1 | S0 | S1 | S0 | S1 | ||
| O1-H2⋯N3 | ELF(C-V,D) | 0.0993 | 0.1010 | 0.1010 | 0.1035 | 0.1011 | 0.1038 |
| ELF(DH-A) | 0.1592 | 0.2240 | 0.1888 | 0.2826 | 0.1932 | 0.2929 | |
| CVB index | −0.0599 | −0.1230 | −0.0878 | −0.1791 | −0.0921 | −0.1891 | |
| O4-H5⋯N6 | ELF(C-V,D) | 0.1010 | 0.1036 | 0.1010 | 0.1035 | 0.1010 | 0.1034 |
| ELF(DH-A) | 0.1901 | 0.2876 | 0.1888 | 0.2826 | 0.1891 | 0.2811 | |
| CVB index | −0.0891 | −0.1840 | −0.0878 | −0.1791 | −0.0881 | −0.1777 | |
| Mulliken’s Charge | NPA Charge | ||||
|---|---|---|---|---|---|
| Atoms | S0 | S1 | S0 | S1 | |
| BBTHQ-SO | O1 | −0.282 | −0.276 | −0.652 | −0.650 |
| H2 | 0.297 | 0.297 | 0.505 | 0.503 | |
| N3 | −0.148 | −0.151 | −0.492 | −0.496 | |
| O4 | −0.283 | −0.279 | −0.653 | −0.651 | |
| H5 | 0.305 | 0.305 | 0.502 | 0.497 | |
| N6 | −0.109 | −0.121 | −0.471 | −0.477 | |
| BBTHQ-SS | O1 | −0.284 | −0.280 | −0.654 | −0.652 |
| H2 | 0.304 | 0.305 | 0.503 | 0.497 | |
| N3 | −0.112 | −0.123 | −0.471 | −0.478 | |
| O4 | −0.284 | −0.280 | −0.654 | −0.652 | |
| H5 | 0.304 | 0.305 | 0.503 | 0.497 | |
| N6 | −0.112 | −0.123 | −0.471 | −0.478 | |
| BBTHQ-SSe | O1 | −0.284 | −0.280 | −0.654 | −0.653 |
| H2 | 0.305 | 0.305 | 0.503 | 0.497 | |
| N3 | −0.122 | −0.135 | −0.471 | −0.479 | |
| O4 | −0.285 | −0.280 | −0.653 | −0.652 | |
| H5 | 0.304 | 0.304 | 0.502 | 0.496 | |
| N6 | −0.113 | −0.124 | −0.478 | −0.485 | |
| BBTHQ-SO | BBTHQ-SS | BBTHQ-SSe | |
|---|---|---|---|
| A → C | 3.5574 | 1.8323 | 1.5976 |
| C → D | 1.9277 | 1.9971 | 2.0237 |
| A → B | 1.7520 | 1.8323 | 1.9976 |
| B → D | 3.8121 | 1.9971 | 1.7131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Liu, C. Computational Insights into Excited State Intramolecular Double Proton Transfer Behavior Associated with Atomic Electronegativity for Bis(2′-benzothiazolyl)hydroquinone. Molecules 2023, 28, 5951. https://doi.org/10.3390/molecules28165951
Zhao J, Liu C. Computational Insights into Excited State Intramolecular Double Proton Transfer Behavior Associated with Atomic Electronegativity for Bis(2′-benzothiazolyl)hydroquinone. Molecules. 2023; 28(16):5951. https://doi.org/10.3390/molecules28165951
Chicago/Turabian StyleZhao, Jinfeng, and Chang Liu. 2023. "Computational Insights into Excited State Intramolecular Double Proton Transfer Behavior Associated with Atomic Electronegativity for Bis(2′-benzothiazolyl)hydroquinone" Molecules 28, no. 16: 5951. https://doi.org/10.3390/molecules28165951






