Bitter Almond Albumin ACE-Inhibitory Peptides: Purification, Screening, and Characterization In Silico, Action Mechanisms, Antihypertensive Effect In Vivo, and Stability
Abstract
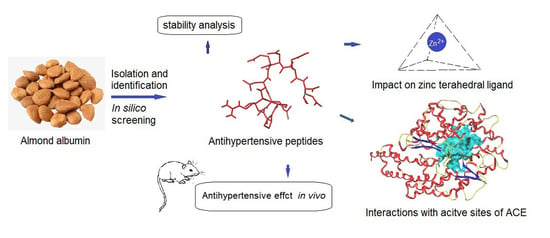
:1. Introduction
2. Results
2.1. Chromatographic Purification of BAAHs
2.2. Identification, Screening, and Inhibitory Effect on ACE of BAAH-4-C Peptides
2.3. Chelating Capacity toward Zinc Ions and Physicochemical Characteristics
2.4. Inhibitory Mechanisms of BAAH Peptides against ACE
2.4.1. Docking Modes of BAAH Peptides toward ACE Molecule
2.4.2. Inhibition Kinetics on ACE
2.4.3. The Binding Mode of RPPSEDEDQE to Zinc Ion
2.5. Stability of RPPSEDEDQE
2.6. Antihypertension In Vivo of RPPSEDEDQE and RPPSEDEDQE–Zn Complexes
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Extraction and Proteolysis of Bitter Almond Albumin
4.3. Chromatographic Separation of BAAHs
4.4. Inhibition Activity and Kinetics against ACE
4.5. Chelating Ability toward Zinc Ions
4.6. Amino Acid Sequence Analysis, Validation, and Screening
4.7. Chemical Synthesis, Chelating Capacity toward Zinc Ions, and Physicochemical Characteristics of Potential Antihypertensive Sequences
4.8. Docking Modes of BAAH Peptides toward ACE Molecule
4.9. Ligand Patterns of Zinc Ions with BAAH Peptides
4.10. Stabilities of BAAH Peptides
4.11. Antihypertension In Vivo
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Duan, X.; Dong, Y.; Zhang, M.; Li, Z.; Bu, G.; Chen, F. Identification and molecular interactions of novel ACE inhibitory peptides from rapeseed protein. Food Chem. 2023, 422, 136085. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; He, H.; Zhou, W.; Li, M.; Lu, A.; Che, T.; Shen, S. Purification identification and function analysis of ACE inhibitory peptide from Ulva prolifera protein. Food Chem. 2023, 345, 134127. [Google Scholar] [CrossRef]
- Wu, J.; Liao, W.; Udenigwe, C.C. Revisiting the mechanisms of ACE inhibitory peptides from food proteins. Trends Food Sci. Technol. 2017, 69, 214–219. [Google Scholar] [CrossRef]
- Pina, A.; Roque, A. Studies on the molecular recognition between bioactive peptides and angiotensin-converting enzyme. J. Mol. Recognit. 2009, 22, 162–168. [Google Scholar] [CrossRef]
- Li, R.; Zhou, X.; Sun, L.; Zhuang, Y. Identification, in silico screening, and molecular docking of novel ACE inhibitory peptides isolated from the edible symbiot Boletus griseus-Hypomyces chrysospermus. LWT-Food Sci. Technol. 2022, 169, 114008. [Google Scholar] [CrossRef]
- Yin, Z.; Yan, R.; Jiang, Y.; Feng, S.; Sun, H.; Sun, J.; Zhao, D.; Li, H.; Wang, B.; Zhang, N. Identification of peptides in Qingke baijiu and evaluation of its angiotensin converting enzyme (ACE) inhibitory activity and stability. Food Chem. 2022, 395, 133551. [Google Scholar] [CrossRef]
- Ruan, S.Y.; Sun, L.P.; Sun, X.D.; He, J.L.; Zhuang, Y.L. Novel umami peptides from tilapia lower jaw and molecular docking to the taste receptor T1R1/T1R3. Food Chem. 2021, 362, 130249. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, N.P.; Rajput, M.S.; Rathod, N.B.; Mudgil, P.; Pati, S.; Bono, G.; Nalinanon, S.; Li, L.; Maqsood, S. Structural characteristic and molecular docking simulation of fish protein-derived peptides: Recent updates on antioxidant, anti-hypertensive and anti-diabetic peptides. Food Chem. 2023, 405, 134737. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Vij, S. In vitro stability of bioactive peptides derived from fermented soy milk against heat treatment, pH and gastrointestinal enzymes. LWT-Food Sci. Technol. 2018, 91, 303–307. [Google Scholar] [CrossRef]
- Wongngam, W.; Hamzeh, A.; Tian, F.; Roytrakul, S.; Yongsawatdigul, J. Purification and molecular docking of angiotensin converting enzyme-inhibitory peptides derived from corn gluten meal hydrolysate and from in silico gastrointestinal digestion. Process Biochem. 2023, 129, 113–120. [Google Scholar] [CrossRef]
- Sahni, P.; Sharma, S.; Surasani, V.K.R. Influence of processing and pH on amino acid profile, morphology, electrophoretic pattern, bioactive potential and functional characteristics of alfalfa protein isolates. Food Chem. 2020, 333, 127503. [Google Scholar] [CrossRef] [PubMed]
- Magouz, O.; Mehanna, N.; Khalifa, M.; Sakr, H.; Gensberger-Reigl, S.; Dalabasmaz, S.; Pischetsrieder, M. Profiles, antioxidative and ACE inhibitory activity of peptides released from fermented buttermilk before and after simulated gastrointestinal digestion. Innov. Food Sci. Emerg. 2023, 84, 103266. [Google Scholar] [CrossRef]
- Tang, H.; Wang, C.; Cao, S.; Wang, F. Novel angiotensin I-converting enzyme (ACE) inhibitory peptides from walnut protein isolate: Separation, identification and molecular docking study. J. Food Biochem. 2022, 46, e14411. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Miao, X.; Dan, C.; Li, J.; Min, W. Purification and identification of ACE-inhibiting peptides from wild pine nut peptide fractions (PNPF). Eur. Food Res. Technol. 2018, 244, 979–988. [Google Scholar] [CrossRef]
- Mirzapour, M.; Rezaei, K.; Sentandreu, M.A. Identification of Potent ACE Inhibitory Peptides from Wild Almond Proteins. J. Food Sci. 2017, 82, 2421–2431. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Fu, J.; Zeng, Q.; Lu, H.; Wang, J.; Fang, L.; Liu, X.; Min, W.; Liu, C. Improving ACE inhibitory activity of hazelnut peptide modified by plastein: Physicochemical properties and action mechanism. Food Chem. 2023, 402, 134498. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.S.P.; Dias, F.F.G.; Koblitz, M.G.B.; Bell, J.M.L.N.M. Effects of enzymatic extraction of oil and protein from almond cake on the physicochemical and functional properties of protein extracts. Food Bioprod. Process. 2020, 122, 280–290. [Google Scholar] [CrossRef]
- Dias, F.F.G.; de Moura Bell, J.M.L.N. Understanding the impact of enzyme-assisted aqueous extraction on the structural, physicochemical, and functional properties of protein extracts from full-fat almond flour. Food Hydrocolloid. 2020, 127, 107534. [Google Scholar] [CrossRef]
- Li, S.G.; Chu, S.; Lu, J.K.; Wang, P.; Ma, M.H. Molecular and structural properties of three major protein components from almond kernel. J. Food Process. Pres. 2018, 42, e13536. [Google Scholar] [CrossRef]
- Ganesh, S.; Ningtyas, D.W.; Prakash, S. Investigating the functionality of enzymatically (transglutaminase and alcalase) treated almond protein isolate. Food Biosci. 2020, 49, 101914. [Google Scholar] [CrossRef]
- Civera, A.; Galan-Malo, P.; Segura-Gil, I.; Mata, L.; Tobajas, A.P.; Sánchez, L.; Pérez, M.D. Development of sandwich ELISA and lateral flow immunoassay to detect almond in processed food. Food Chem. 2022, 371, 131338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, J.; Zhao, H.; Zhao, X.; Xue, H.; Sun, Y.; Xue, W. Isolation and structural characterization of antioxidant peptides from degreased apricot seed kernels. J. AOAC Inter. 2018, 101, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Gao, T.; Hou, Y.; Li, D.; Fu, L. Identification and characterization of two novel α-glucosidase inhibitory peptides from almond (Armeniaca sibirica) oil manufacture residue. LWT-Food Sci. Technol. 2020, 134, 110215. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, Z. Pentapeptide-Zinc Chelate from Sweet Almond Expeller Amandin Hydrolysates: Structural and Physicochemical Characteristics, Stability and Zinc Transport Ability In Vitro. Molecules 2022, 27, 7936. [Google Scholar] [CrossRef]
- Zarei, M.; Ghanbari, R.; Zainal, N.; Ovissipour, R.; Saari, N. Inhibition kinetics, molecular docking, and stability studies of the effect of papain-generated peptides from palm kernel cake proteins on angiotensin-converting enzyme (ACE). Food Chem. Mol. Sci. 2022, 5, 100147. [Google Scholar] [CrossRef]
- Kumar, R.; Chaudhary, K.; Sharma, M.; Nagpal, G.; Chauhan, J.S.; Singh, S.; Gautam, A.; Raghava, G.P. AHTPDB: A comprehensive platform for analysis and presentation of antihypertensive peptides. Nucleic Acids Res. 2015, 43, D956–D962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khakhariya, R.; Basaiawmoit, B.; Sakure, A.A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Padhi, S.; Rai, A.K.; Liu, Z.; Hati, S. Production and Characterization of ACE Inhibitory and Anti-Diabetic Peptides from Buffalo and Camel Milk Fermented with Lactobacillus and Yeast: A Comparative Analysis with In Vitro, In Silico, and Molecular Interaction Study. Foods 2023, 12, 2006. [Google Scholar] [CrossRef]
- Lin, Z.; Lai, J.; He, P.; Pan, L.; Zhang, Y.; Zhang, M.; Wu, H. Screening, ACE-inhibitory mechanism and structure-activity relationship of a novel ACE-inhibitory peptide from Lepidium meyenii (Maca) protein hydrolysate. Food Biosci. 2023, 52, 102374. [Google Scholar] [CrossRef]
- Urbizo-Reyes, U.; Liceaga, A.M.; Reddivari, L.; Kim, K.-H.; Anderson, J.M. Enzyme kinetics, molecular docking, and in silico characterization of canary seed (Phalaris canariensis L.) peptides with ACE and pancreatic lipase inhibitory activity. J. Funct. Foods 2022, 88, 104892. [Google Scholar] [CrossRef]
- Abedin, M.M.; Chourasia, R.; Phukon, L.C.; Singh, S.P.; Rai, A.K. Characterization of ACE inhibitory and antioxidant peptides in yak and cow milk hard chhurpi cheese of the Sikkim Himalayan region. Food Chem. X 2022, 13, 100231. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Liu, X.; Yu, Y.; Miao, J.; Leng, K.; Gao, H. Preparation process optimization, structural characterization and in vitro digestion stability analysis of Antarctic krill (Euphausia superba) peptides-zinc chelate. Food Chem. 2021, 340, 128056. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, P.; Zheng, Y.; Guo, M.; Zhuang, Y.; Huo, X. Millet bran protein hydrolysates derived peptides-zinc chelate: Structural characterization, security prediction in silico, zinc transport capacity and stability against different food processing conditions. J. Food Sci. 2023, 88, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Udechukwu, M.C.; Downey, B.; Udenigwe, C.C. Influence of structural and surface properties of whey-derived peptides on zinc-chelating capacity, and in vitro gastric stability and bioaccessibility of the zinc-peptide complexes. Food Chem. 2018, 240, 1227–1232. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Shi, P.Q.; Li, Y.; Zhuang, Y.L.; Zhang, Y.L.; Liu, L.; Wang, W. A novel ACE-inhibitory hexapeptide from camellia glutelin-2 hydrolysates: Identification, characterization and stability profiles under different food processing conditions. LWT-Food Sci. Technol. 2021, 147, 111682. [Google Scholar] [CrossRef]
- Ke, X.; Hu, X.; Li, L.; Yang, X.; Chen, S.; Wu, Y.; Xue, C. A novel zinc-binding peptide identified from tilapia (Oreochromis niloticus) skin collagen and transport pathway across Caco-2 monolayers. Food Biosci. 2022, 42, 101127. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, F.; Liu, X.; Zhao, M. Particulate nanocomposite from oyster (Crassostrea rivularis) hydrolysates via zinc chelation improves zinc solubility and peptide activity. Food Chem. 2018, 258, 269–277. [Google Scholar] [CrossRef]
- Chen, M.; Wang, L.; Zheng, C.; Ma, A.; Hu, K.; Xiang, A.; Sun, Z.; Xie, B.; Xiong, G.; Shi, L.; et al. Novel ACE inhibitory peptides derived from bighead carp (Aristichthys nobilis) hydrolysates: Screening, inhibition mechanisms and the bioconjugation effect with graphene oxide. Food Biosci. 2023, 52, 102399. [Google Scholar] [CrossRef]
- Sonklin, G.; Alashi, A.M.; Laohakunjit, N.; Kerdchoechuen, O.; Aluko, R.E. Identification of antihypertensive peptides from mung bean protein hydrolysate and their effects in spontaneously hypertensive rats. J. Funct. Foods 2020, 64, 103635. [Google Scholar] [CrossRef]
- Dong, J.; Wang, S.; Yin, X.; Fang, M.; Gong, Z.; Wu, Y. Angiotensin I converting enzyme (ACE) inhibitory activity and antihypertensive effects of rice peptides. Food Sci. Hum. Wellness 2022, 11, 1539–1543. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved method for determining food protein degree of hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Zaharuddin, N.D.; Barkia, I.; Ibadullah, W.Z.W.; Zarei, M.; Saari, N. Identification, molecular docking, and kinetic studies of six novel angiotensin-I-converting enzyme (ACE) inhibitory peptides derived from Kenaf (Hibiscus cannabinus L.) seed. Int. J. Biol. Macromol. 2022, 220, 1512–1522. [Google Scholar] [CrossRef] [PubMed]









| Sequences | RPPSEDEDQE | KTETQP | TCGAS | SPPTAAAAGD | QPPAAAAAAGAG |
|---|---|---|---|---|---|
| Molecular mass (Da) | 1201.29 | 535.61 | 437.52 | 857.00 | 766.96 |
| Matched sequence in Semen Armeniacae Amarum a | P.RPPSEDEDQE.Y | R.KTETQP.- | S.TCGAS.S | F.SPPTAAAAGD.M | G.QPPAAAAAAGAG.R |
| Vector machine software score b | 1.35 | 0.90 | −0.58 | −0.96 | −0.52 |
| Antihypertension predict c | AHP | Non-AHP | Non-AHP | Non-AHP | Non-AHP |
| Inhibitory effect against ACE (IC50: μmol·L−1) | 205.50 | ND | ND | ND | ND |
| Inhibitory effect against ACE after gastrointestinal digestion (IC50: μmol·L−1) | 213.48 | ND | ND | ND | ND |
| Chelating ability toward zinc ions (mg·g−1) | 20.67 ± 3.58 d | 19.30 ± 1.46 e | 6.44 ± 0.64 f | 2.16 ± 0.45 g | 1.14 ± 0.01 g |
| Chelating ability toward zinc ions after gastrointestinal digestion (mg·g−1) | 18.55 ± 0.95 | ND | ND | ND | ND |
| Peptides | RPPSEDEDQE | KTETQP | TCGAS | SPPTAAAAGD | QPPAAAAAAGAG |
|---|---|---|---|---|---|
| Acidic amino acid content (%) | 50.00% | 16.67% | 0.00% | 10.00% | 0.00% |
| Hydrophobic amino acid content (%) | 20.00% | 16.67% | 20.00% | 60.00% | 75.00% |
| Hydrophobicity | −0.61 | −0.09 | 0.00 | −0.01 | 0.18 |
| Amphiphilicity | 0.75 | 1.59 | 0.00 | 0.00 | 0.00 |
| Hydrophilicity | 1.85 | −0.66 | −0.32 | 0.09 | −0.35 |
| Isoelectric point | 3.84 | 7.25 | 5.85 | 3.80 | 5.88 |
| Ligand | T-Score | C-Score | Interaction Force | Active Sites of ACE and Hydrogen Bond Length |
|---|---|---|---|---|
| RPPSEDEDQE | 8.06 | 5 | Hydrogen bond | Lys368: 1.94 Å; Asp377: 2.91 Å; Glu376: 2.47 Å; Pro508: 2.03 Å; Thr282: 1.97 Å; Arg522: 2.12 Å; Lys454: 2.81 Å |
| Hydrophobic interactions | His353, Cys352, Tyr523, Ala354, Ser355, Phe457, Phe527, Asp415, Pro163, Gln369, Cys370, Glu376, Asn374, Ala170, Asn167 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, N.; Chen, C.; Zhang, N.; Song, L.; Li, Y.; Guo, L.; Liu, R.; Zhang, W. Bitter Almond Albumin ACE-Inhibitory Peptides: Purification, Screening, and Characterization In Silico, Action Mechanisms, Antihypertensive Effect In Vivo, and Stability. Molecules 2023, 28, 6002. https://doi.org/10.3390/molecules28166002
Qin N, Chen C, Zhang N, Song L, Li Y, Guo L, Liu R, Zhang W. Bitter Almond Albumin ACE-Inhibitory Peptides: Purification, Screening, and Characterization In Silico, Action Mechanisms, Antihypertensive Effect In Vivo, and Stability. Molecules. 2023; 28(16):6002. https://doi.org/10.3390/molecules28166002
Chicago/Turabian StyleQin, Nan, Chao Chen, Najun Zhang, Lulu Song, Yunfei Li, Lili Guo, Rui Liu, and Wenfang Zhang. 2023. "Bitter Almond Albumin ACE-Inhibitory Peptides: Purification, Screening, and Characterization In Silico, Action Mechanisms, Antihypertensive Effect In Vivo, and Stability" Molecules 28, no. 16: 6002. https://doi.org/10.3390/molecules28166002