The Amoebicidal Activity of Diferrocenyl Derivatives: A Significant Dependence on the Electronic Environment
Abstract
:1. Introduction
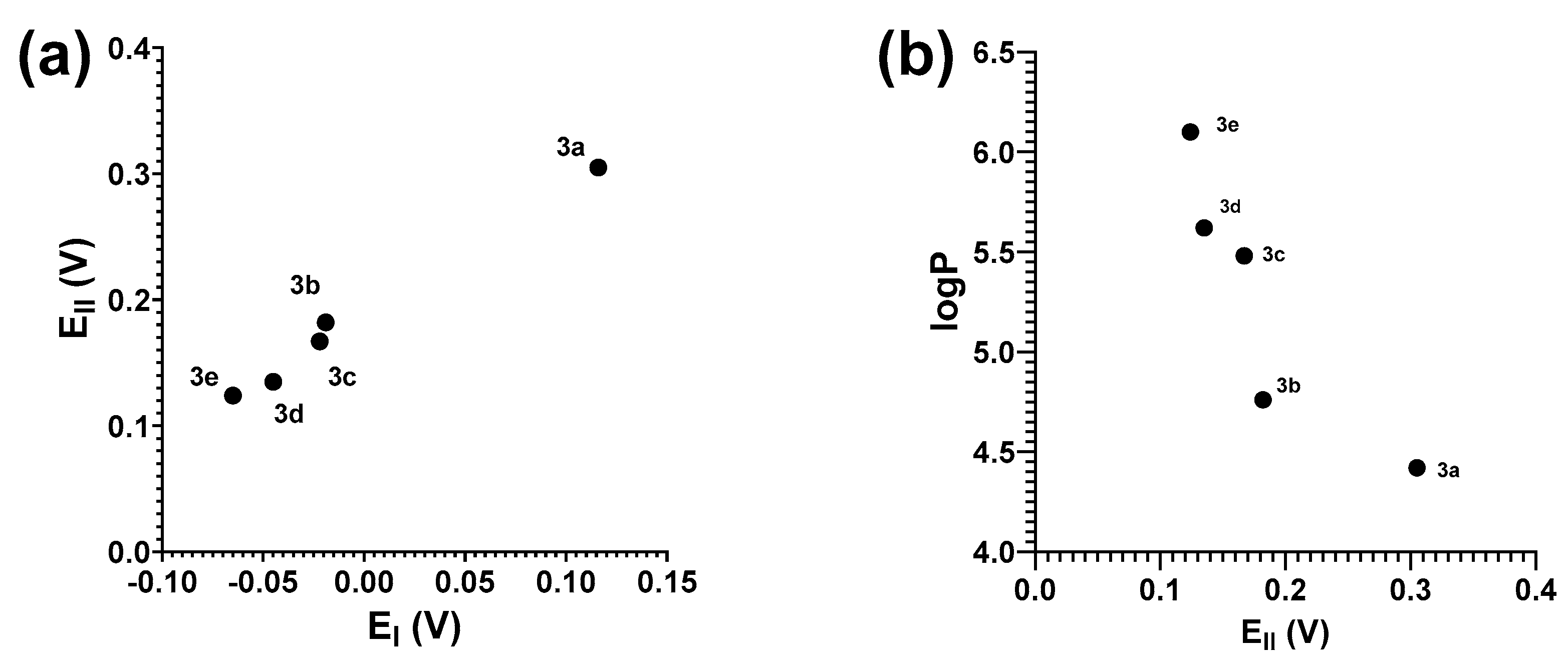
2. Results and Discussion
2.1. Characterization
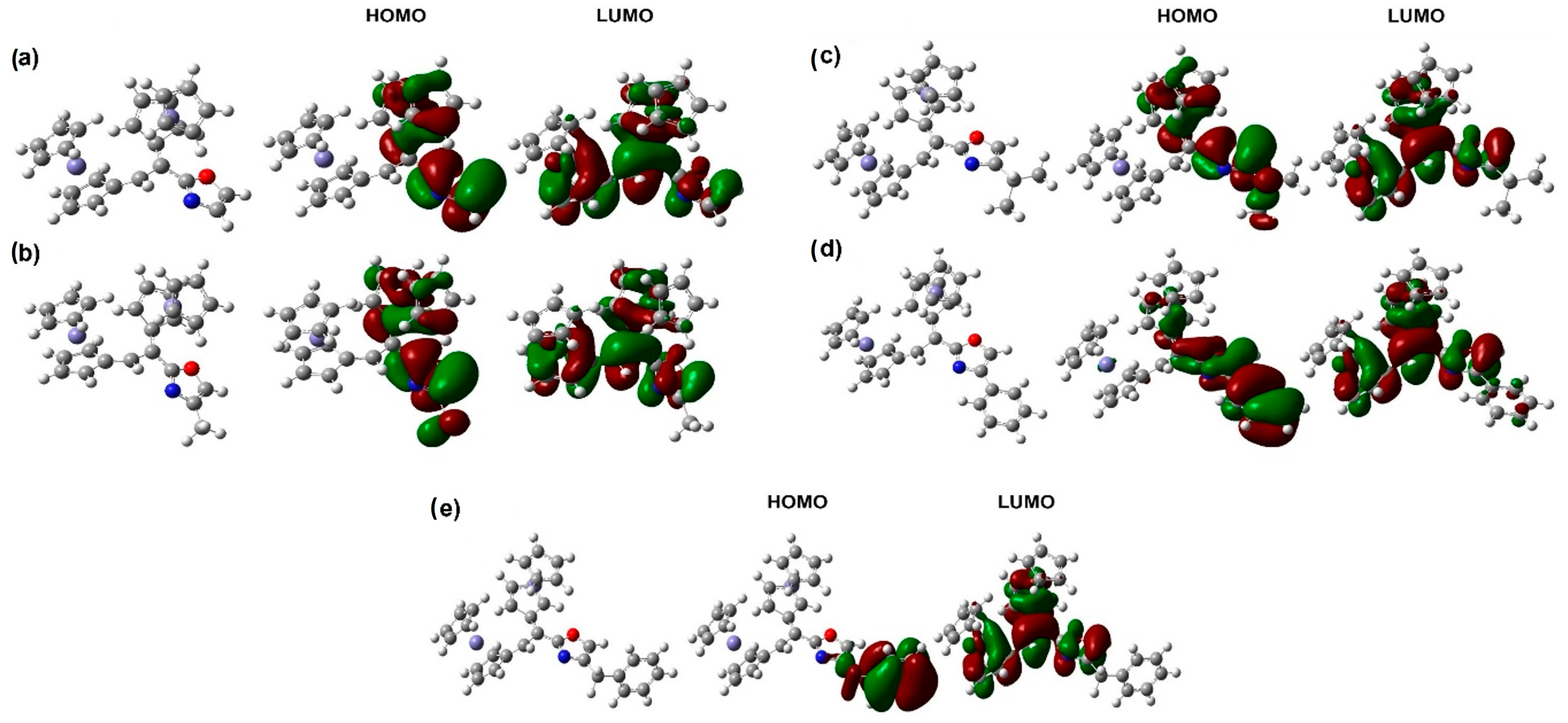
2.2. Computational Chemistry
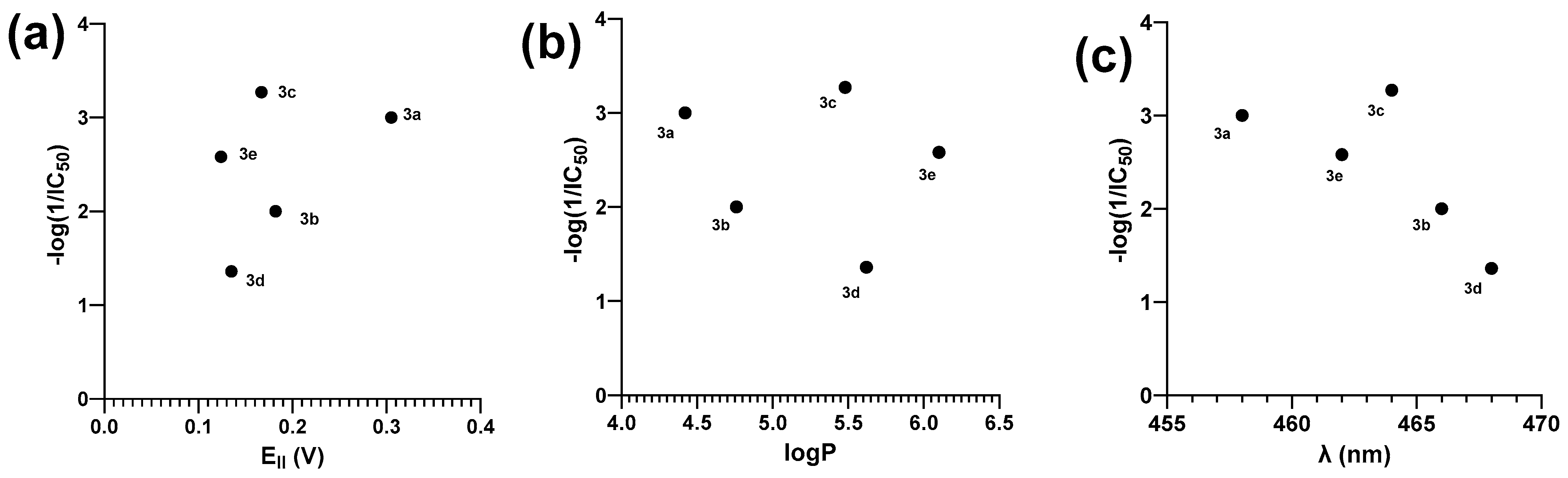
2.3. Amoebicidal Activity
3. Materials and Methods
3.1. Synthesis and Characterization
3.2. Reaction of 2,3-Diferrocenyl-1-morpholinocyclopropenylium Tetrafluoroborates (1a) with 1,2-Amino Alcohols (2a–e) (General Procedure)
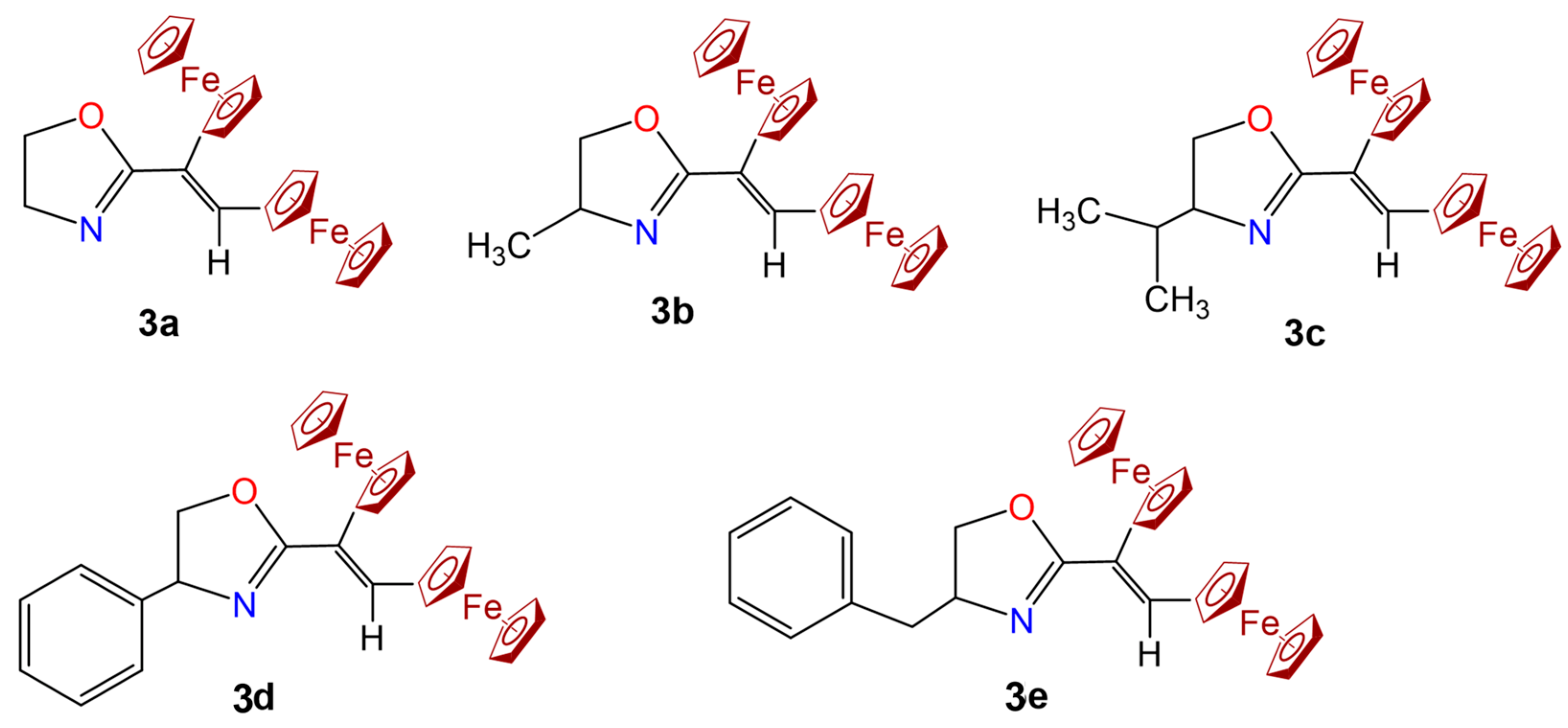
- 2-(Z-2,3-Diferrocenylvinyl-4,5-dihydrooxazole (3a) orange crystals, m.p. 123–124 °C. IR (KBr): ν 481, 535, 587, 659, 730, 813, 891, 827, 858, 947, 999, 1039, 1027, 1105, 1179, 1242, 1306, 1357, 1348, 1477, 1630, 1762, 2870, 2893, 3087, 3150 cm−1. 1H NMR [300 MHz, CDCl3]: δ 4.05 (5H, s, C5H5), 4.06 (2H, t, CH2, J = 9.0 Hz), 4.07 (5H, s, C5H5), 4.19 (4H, m, C5H4), 4.23 (2H, m, C5H4), 4.44 (2H, t, CH2, J = 9.0 Hz), 4.48 (2H, m, C5H4), 7.15 (1H, s, CH=). 13C NMR [75 MHz, CDCl3]: δ 50.42, 63.78 (2CH2), 69.14, 69.34 (2C5H5), 67.84, 69.19, 69.51, 70.50 (2C5H4), 79.60, 79.41 (2CipsoFc), 136.94 (CH=), 126.30, 167.62 (2C). Anal. calcd. for C25H23Fe2NO: C, 64.55; H, 4.99; N, 3.01. Found: C, 64.17; H, 4.93; N, 3.01%. MS (El, 70 eV): m/z 465 [M]+. UV–vis (λ, nm): 330, 368, 458. E(I) = 116 mV, E(II) = 305 mV.
- 2-(Z-1,2-Diferrocenylvinyl)-4-methyl-4,5-dihydrooxazole (3b) orange powder, m.p. 127–129 °C. IR (KBr): ν 471, 645, 732, 749, 810, 849, 971, 1006, 1041, 1163, 1241, 1277, 1315, 1340, 1410, 11,457, 1475, 1588, 1617, 1724, 2248, 2852, 2922, 3086, 3462 cm−1. 1H NMR [300 MHz, CDCl3]: δ 1.42 (3H, d, CH3, J = 6.6 Hz), 3.93 (1H, t, CH2, J = 7.8 Hz), 4.08 (10H, s, 2C5H5), 4.19 (4H, m, C5H4), 4.20 (1H, m, C5H4), 4.23 (4H, m, C5H4), 4.36 (1H, m, CH), 4.47 (2H, m, C5H4), 4.54 (1H, dd, CH2, J = 7.8, 9.3 Hz),7.14 (1H, s, CH=). 13C NMR [75 MHz, CDCl3]: δ 21.73 (CH3), 61.82 (CH), 67.75 (CH2), 69.27, 69.35 (2C5H5), 69.06 (2C), 69.58, 70.21, 70.44, 70.62, 70.79, 73.68 (2C5H4), 80.55, 80.59 (2CipsoFc), 134.20 (CH=), 123.78, 165.35 (2C). Anal. calcd. for C26H25Fe2NO: C, 65.17; H, 5.26; N, 2.92. Found: C, 65.36; H, 5.35; N, 3.16%. MS (El, 70 eV): m/z 479 [M]+. UV–vis (λ, nm): 331, 372, 466. E(I) = −19 mV, E(II) = 182 mV.
- 2-(Z-1,2-Diferrocenylvinyl)-4-isopropyl-4,5-dihydrooxazoline (3c) orange powder, m.p. 85–87 °C. IR (KBr): ν 478, 707, 738, 800, 811, 886, 929, 972 998, 1038, 1056, 1105, 1174, 1189, 1244, 1295, 1348, 1381, 1449, 1468, 1607, 1630, 1703, 2823, 2905, 3085, 3092 cm−1. 1H NMR [300 MHz, C6D6]: δ 0.83 (3H, d, CH3, J = 6.6 Hz), 1.11 (3H, d, CH3, J = 6.6 Hz), 1.67 (1H, m, CH), 3.74 (1H, m, CH), 3.82 (1H, dd, CH2, J = 6.9, 8.7 Hz), 4.02 (1H, dd, CH2, J = 7.2, 8.7 Hz), 3.87 (5H, s, C5H5), 3.98 (2H, m, C5H4), 4.10 (1H, m, C5H4), 4.13 (5H, s, C5H5), 4.15 (1H, m, C5H4), 4.27 (2H, m, C5H4), 4.80 (1H, m, C5H4), 4.88 (1H, m, C5H4), 7.62 (1H, s, CH=). 13C NMR (75MHz, C6D6): δ 19.05, 19.73 (2CH3), 33.96, 68.0 (2CH), 68.05 (CH2), 69.73, 70.01 (2C5H5), 69.44, 69.46, 69.89, 70.56, 70.98, 71.33, 71.67, 73.97 (2C5H4), 81.55, 81.60 (2CipsoFc), 134.43 (CH=), 124.61, 164.51 (2C). Anal. calcd. for C28H29Fe2NO: C, 66.30; H, 5.76; N, 2.76. Found: C, 66.46; H, 5.54; N, 2.80. MS (El, 70 eV): m/z 507 [M]+. UV–vis (λ, nm): 329, 368, 464. E(I) = −22 mV, E(II) = 167 mV.
- 2-(Z-1,2-Diferrocenylvinyl)-4-phenyl-4,5-dihydrooxazoline (3d) orange powder, m.p. 153–154 °C. IR (KBr): ν 480, 494, 624, 698, 815, 898, 930, 999, 1031, 1049, 1105, 1183, 1184, 1242, 1256, 1281, 1331, 1377, 1449, 1477, 1491, 1603, 1711, 1741, 2851, 2923, 3092 cm−1. 1H NMR [300 MHz, CDCl3]: δ 4.04 (5H, s, C5H5), 4.07 (5H, s, C5H5), 4.18 (1H, t, CH, J = 8.7 Hz), 4.20 (3H, m, C5H4), 4.24 (3H, m, C5H4), 4.27 (1H, m, C5H4), 4.50 (1H, m, C5H4), 4.74 (1H, dd, CH2, J = 8.7, 9.9 Hz), 5.38 (1H, dd, CH2, J = 8.7, 9.9 Hz), 7.29 (1H, s, CH=), 7.38–7.40 (5H, m, C6H5). 13C NMR [75 MHz, (CD3)2CO)]: δ 67.83 (CH), 67.90 (CH2), 69.41, 69.48 (2C5H5), 69.28, 69.86, 70.16, 70.44, 70.55, 70.78, 70.88, 74.07 (2C5H4), 80.48, 80.64 (2CipsoFc), 126.87, 127.62, 128.84 (C6H5), 135.04 (CH=), 123.39, 142.66, 166.45 (3C). Anal. calcd. for C31H27Fe2NO: C, 68.79; H, 5.03; N, 2.59. Found: C, 68.53; H, 5.02; N, 2.35%. MS (El, 70 eV): m/z 541 [M]+. UV–vis (λ, nm): 334, 370, 468. E(I) = −45 mV, E(II) = 135 mV.
- 4-Benzyl-2-(Z-1,2-diferrocenylvinyl)-4,5-dihydrooxazoline (3e) orange powder, m.p. 119–120 °C. IR (KBr): ν 482, 644, 695, 709, 734, 803, 815, 832, 878, 911, 955, 997, 1027, 1035, 1047, 1104, 1185, 1213, 1267, 1306, 1356, 1410, 1454, 1480, 1497, 1602, 1635, 1711, 1775, 1948, 2087, 2200, 2853, 2923, 3029, 3086, 3106 cm−1. 1H NMR [300 MHz, C6D6]: δ 2.63 (1H, dd, CH2, J = 8.1, 13.8 Hz), 3.13 (1H, dd, CH2, J = 6.0, 13.8 Hz), 3.80 (1H, t, CH2, J = 8.7 Hz), 3.88 (5H, s, C5H5), 3.93 (1H, t, CH2, J = 8.7 Hz), 3.98 (2H, m, C5H4), 4.11 (5H, s, C5H5), 4.13 (1H, m, C5H4), 4.15 (1H, m, C5H4), 4.24 (1H, m, C5H4), 4.27 (1H, m, C5H4), 4.80 (1H, m, C5H4), 4.85 (1H, m, C5H4), 4.41 (1H, m, CH), 7.07–7.19 (5H, m,C6H5), 7.59 (1H, s, CH=). 13C NMR [75 MHz, C6D6]: δ 42.38, 68.22 (2CH2), 68.10 (CH), 69.74, 70.06 (2C5H5), 68.76, 69.50 (2C), 70.72, 70.88, 71.06, 71.45, 71.59 (2C5H4), 81.45, 81.47 (2CipsoFc), 126.68, 128.77, 129.63 (C6H5), 134.76 (CH=), 124.45, 138.87, 166.98 (3C). Anal. calcd. for C32H29Fe2NO: C, 69.22; H, 5.26; N, 2.52. Found: C, 69.43; H, 5.13; N, 2.65%. MS (El, 70 eV): m/z 555 [M]+. UV–vis (λ, nm): 328, 368, 462. E(I) = −65 mV, E(II) = 124 mV.
3.3. Computational Chemistry
3.4. Amoebicidal Activity
3.5. Determination of LogP by the TLC Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Carrero, J.C.; Reyes-López, M.; Serrano-Luna, J.; Shibayama, M.; Unzueta, J.; León-Sicairos, N.; de la Garza, M. Intestinal Amoebiasis: 160 Years of Its First Detection and Still Remains as a Health Problem in Developing Countries. Int. J. Med. Microbiol. 2020, 310, 151358. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, J.; Akhoundi, M.; Haouchine, D.; Marteau, A.; Mantelet, S.; Wind, P.; Benamouzig, R.; Bouchaud, O.; Dhote, R.; Izri, A. Updates on the Worldwide Burden of Amoebiasis: A Case Series and Literature Review. J. Infect. Public Health 2022, 15, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Guillén, N. Pathogenicity and Virulence of Entamoeba Histolytica, the Agent of Amoebiasis. Virulence 2023, 14, 2158656. [Google Scholar] [CrossRef]
- Shirley, D.A.T.; Watanabe, K.; Moonah, S. Significance of Amebiasis: 10 Reasons Why Neglecting Amebiasis Might Come Back to Bite Us in the Gut. PLoS Negl. Trop. Dis. 2019, 13, e0007744. [Google Scholar] [CrossRef] [PubMed]
- Kumanan, T.; Sujanitha, V.; Sreeharan, N. Amoebic Liver Abscess: A Neglected Tropical Disease. Lancet Infect. Dis. 2020, 20, 160–162. [Google Scholar] [CrossRef]
- WHO. Global Report on Neglected Tropical Diseases 2023; WHO: Geneva, Switzerland, 2023; ISBN 9789240067295.
- Shrivastav, M.T.; Malik, Z.; Somlata, Z. Revisiting Drug Development Against the Neglected Tropical Disease, Amebiasis. Front. Cell. Infect. Microbiol. 2021, 10, 628257. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, S.; Ankri, S. Target Identification and Intervention Strategies against Amebiasis. Drug Resist. Updat. 2019, 44, 1–14. [Google Scholar] [CrossRef]
- Jeelani, G.; Nozaki, T. Entamoeba Thiol-Based Redox Metabolism: A Potential Target for Drug Development. Mol. Biochem. Parasitol. 2016, 206, 39–45. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Salas, P.F.; Herrmann, C.; Orvig, C. Metalloantimalarials. Chem. Rev. 2013, 113, 3450–3492. [Google Scholar] [CrossRef]
- Scalese, G.; Kostenkova, K.; Crans, D.C.; Gambino, D. Metallomics and Other Omics Approaches in Antiparasitic Metal-Based Drug Research. Curr. Opin. Chem. Biol. 2022, 67, 102127. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M. Gold Complexes as Potential Anti-Parasitic Agents. Coord. Chem. Rev. 2009, 253, 1619–1626. [Google Scholar] [CrossRef]
- Navarro, M.; Justo, R.M.S.; Delgado, G.Y.S.; Visbal, G. Metallodrugs for the Treatment of Trypanosomatid Diseases: Recent Advances and New Insights. Curr. Pharm. Des. 2021, 27, 1763–1789. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uyama, H. Polymerization of Cyclic Imino Ethers: From Its Discovery to the Present State of the Art. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 192–209. [Google Scholar] [CrossRef]
- Adhikary, S.; Mukherjee, K.; Banerji, B. Cell-Imaging Studies of Highly Substituted Oxazole Derivatives as Organelle Targeting Fluorophores (OTFPs). Sci. Rep. 2022, 12, 16555. [Google Scholar] [CrossRef] [PubMed]
- Amino, Y.; Tahara, Y.K.; Yamada, K.; Nakazawa, M.; Tagami, U.; Tajima, T.; Kuroda, M. Design, Synthesis, and Taste Evaluation of a High-Intensity Umami-Imparting Oxazole-Based Compound. Biosci. Biotechnol. Biochem. 2017, 81, 1690–1698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Aty, A.S. Pesticidal Effects of Some Imidazolidine and Oxazolone Derivatives. World J. Agric. Sci. 2009, 5, 105–113. [Google Scholar]
- Kakkar, S.; Narasimhan, B. A Comprehensive Review on Biological Activities of Oxazole Derivatives. BMC Chem. 2019, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Averina, E.B.; Vasilenko, D.A.; Gracheva, Y.A.; Grishin, Y.K.; Radchenko, E.V.; Burmistrov, V.V.; Butov, G.M.; Neganova, M.E.; Serkova, T.P.; Redkozubova, O.M.; et al. Synthesis and Biological Evaluation of Novel 5-Hydroxylaminoisoxazole Derivatives as Lipoxygenase Inhibitors and Metabolism Enhancing Agents. Bioorganic Med. Chem. 2016, 24, 712–720. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Prencipe, F.; Oliva, P.; Baraldi, S.; Salvador, M.K.; Lopez-Cara, L.C.; Brancale, A.; Ferla, S.; Hamel, E.; et al. Synthesis and Biological Evaluation of 2-Methyl-4,5-Disubstituted Oxazoles as a Novel Class of Highly Potent Antitubulin Agents. Sci. Rep. 2017, 7, 46356. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Ahmad, P.; Maurya, R.A.; Singh, A.B.; Srivastava, A.K. Novel 2-Aryl-Naphtho [1,2-d]Oxazole Derivatives as Potential PTP-1B Inhibitors Showing Antihyperglycemic Activities. Eur. J. Med. Chem. 2009, 44, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, D.; Uchida, R.; Ohtawa, M.; Arima, S.; Futamura, Y.; Katane, M.; Homma, H.; Nagamitsu, T.; Osada, H.; Tomoda, H. Synthesis and Biological Activity of 5-(4-Methoxyphenyl)-Oxazole Derivatives. Bioorganic Med. Chem. Lett. 2015, 25, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, H.R.; Guo, Y.; Liu, P.; Chioda, M.D.; Fung, S.; Lanza, T.J.; Chang, L.; Bakshi, R.K.; Dellureficio, J.P.; Hong, Q.; et al. Discovery of MK-4409, a Novel Oxazole FAAH Inhibitor for the Treatment of Inflammatory and Neuropathic Pain. ACS Med. Chem. Lett. 2014, 5, 717–721. [Google Scholar] [CrossRef] [Green Version]
- Wiley, R.H.; Bennett, L.L. The Chemistry of the Oxazolines. Chem. Rev. 1949, 44, 447–476. [Google Scholar] [CrossRef]
- Graham, T.H. A Direct Synthesis of Oxazoles from Aldehydes. Org. Lett. 2010, 12, 3614–3617. [Google Scholar] [CrossRef]
- Sharma, B.; Kumar, V. Has Ferrocene Really Delivered Its Role in Accentuating the Bioactivity of Organic Scaffolds? J. Med. Chem. 2021, 64, 16865–16921. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ayala, L.F.; Toledano-Magaña, Y.; Ortiz-Frade, L.; Flores-Alamo, M.; Galindo-Murillo, R.; Reina, M.; García-Ramos, J.C.; Ruiz-Azuara, L. Heteroleptic NiII Complexes: Synthesis, Structural Characterization, Computational Studies and Amoebicidal Activity Evaluation. J. Inorg. Biochem. 2020, 206, 111043. [Google Scholar] [CrossRef]
- Husain, K.; Abid, M.; Azam, A. Novel Pd(II) Complexes of 1-N-Substituted 3-Phenyl-2-Pyrazoline Derivatives and Evaluation of Antiamoebic Activity. Eur. J. Med. Chem. 2008, 43, 393–403. [Google Scholar] [CrossRef]
- Maurya, M.R.; Agarwal, S.; Abid, M.; Azam, A.; Bader, C.; Ebel, M.; Rehder, D. Synthesis, Characterisation, Reactivity and in Vitro Antiamoebic Activity of Hydrazone Based Oxovanadium(iv), Oxovanadium(v) and µ-Bis(Oxo)Bis{oxovanadium(v)} Complexes. Dalt. Trans. 2006, 7, 937–947. [Google Scholar] [CrossRef]
- Naeimi, H.; Karshenas, A. Highly Regioselective Conversion of Epoxides to β-Hydroxy Nitriles Using Metal(II) Schiff Base Complexes as New Catalysts under Mild Conditions. Polyhedron 2013, 49, 234–238. [Google Scholar] [CrossRef]
- Singh, S.; Athar, F.; Maurya, M.R.; Azam, A. Cyclooctadiene Ru(II) Complexes of Thiophene-2-Carboxaldehyde-Derived Thiosemicarbazones: Synthesis, Characterization and Antiamoebic Activity. Eur. J. Med. Chem. 2006, 41, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Parveen, H.; Mukhtar, S.; Azam, A. Novel Ferrocenyl Linked Pyrazoline Analogs as Potent Antiamoebic Agents. J. Heterocycl. Chem. 2016, 53, 473–478. [Google Scholar] [CrossRef]
- Parveen, H.; Alsharif, M.A.; Alahmdi, M.I.; Mukhtar, S.; Azam, A. Novel Pyrimidine-Based Ferrocenyl Substituted Organometallic Compounds: Synthesis, Characterization and Biological Evaluation. Appl. Organomet. Chem. 2018, 32, e4261. [Google Scholar] [CrossRef]
- Parveen, H.; Hayat, F.; Salahuddin, A.; Azam, A. Synthesis, Characterization and Biological Evaluation of Novel 6-Ferrocenyl-4-Aryl-2-Substituted Pyrimidine Derivatives. Eur. J. Med. Chem. 2010, 45, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, J.J.; Toledano-Magaña, Y.; Flores-Alamo, M.; Martínez-Klimova, E.; Galindo-Murillo, R.; Hernández-Ayala, L.F.; Ortiz-Frade, L.; García-Ramos, J.C.; Klimova, E.I. Polycyclic Ferrocenyl(Dihydro)Thiazepine Derivatives: Diastereo-Selective Synthesis, Characterization, Electrochemical Behavior, Theoretical and Biological Investigation. J. Inorg. Biochem. 2017, 166, 141–149. [Google Scholar] [CrossRef]
- Sánchez García, J.J.; Flores-Alamo, M.; Ortiz-Frade, L.; Klimova, E.I. Reactions of 2,3-Diferrocenylcyclopropenilium Salts with Bis-1,4-N,O-Nucleophiles: Novel Synthesis, Characterization, Chemical and Electrochemical Properties of the 2-(Z-1,2-Diferrocenylvinyl)- 4,5-Dihydrooxazole Derivatives. J. Organomet. Chem. 2017, 842, 21–31. [Google Scholar] [CrossRef]
- Kaur, S.; Kaur, M.; Kaur, P.; Clays, K.; Singh, K. Ferrocene Chromophores Continue to Inspire. Fine-Tuning and Switching of the Second-Order Nonlinear Optical Response. Coord. Chem. Rev. 2017, 343, 185–219. [Google Scholar] [CrossRef]
- Zanello, P. Inorganic Electrochemistry. Theory, Practice and Application; The Royal Society of Chemistry: Cambridge, UK, 2003; Volume 4, ISBN 0854046615. [Google Scholar]
- Klimova, E.I.; Flores-Alamo, M.; Maya, S.C.; García-Ramos, J.C.; Ortiz-Frade, L.; Stivalet, J.M.M. Novel Synthesis and Electrochemistry of 2-(1,2-Diferrocenylvinyl)-Imidazoline and -Imidazolidine Derivatives. J. Organomet. Chem. 2013, 743, 24–30. [Google Scholar] [CrossRef]
- Klimova, E.I.; Rubio, C.G.; García-Valdés, J.; Nuñez-Cruz, E.; Martínez-Klimova, E.; Beletskaya, I.P.; Flores-Álamo, M.; Sánchez García, J.J. Synthesis, Characterization and Electrochemistry of Diferrocenyl-1,3-Thiazoles, 1,3-Oxathiolanes, Benzo-[b]-1,4-Oxathiepines and Ethyl 2-Propenethioates. J. Organomet. Chem. 2020, 922, 121363. [Google Scholar] [CrossRef]
- Huber, I.; Pandur, E.; Sipos, K.; Barna, L.; Harazin, A.; Deli, M.A.; Tyukodi, L.; Gulyás-Fekete, G.; Kulcsár, G.; Rozmer, Z. Novel Cyclic C5-Curcuminoids Penetrating the Blood-Brain Barrier: Design, Synthesis and Antiproliferative Activity against Astrocytoma and Neuroblastoma Cells. Eur. J. Pharm. Sci. 2022, 173, 106184. [Google Scholar] [CrossRef]
- Huber, I.; Rozmer, Z.; Gyöngyi, Z.; Budán, F.; Horváth, P.; Kiss, E.; Perjési, P. Structure Activity Relationship Analysis of Antiproliferative Cyclic C5-Curcuminoids without DNA Binding: Design, Synthesis, Lipophilicity and Biological Activity. J. Mol. Struct. 2020, 1206, 127661. [Google Scholar] [CrossRef]
- Johnson, A.D.; Buhagiar, J.A.; Magri, D.C. 4-Amino-1,8-Naphthalimide-Ferrocene Conjugates as Potential Multi-Targeted Anticancer and Fluorescent Cellular Imaging Agents. RSC Med. Chem. 2021, 12, 2060–2064. [Google Scholar] [CrossRef]
- Abraham, M.H.; Benjelloun-Dakhama, N.; Gola, J.M.R.; Acree, J.; Cain, W.S.; Cometto-Muniz, J.E. Solvation Descriptors for Ferrocene, and the Estimation of Some Physicochemical and Biochemical Properties. New J. Chem. 2000, 24, 825–829. [Google Scholar] [CrossRef]
- Pinto, A.; Hoffmans, U.; Ott, M.; Fricker, G.; Metzler-Nolte, N. Modification with Organometallic Compounds Improves Crossing of the Blood-Brain Barrier of [Leu5]-Enkephalin Derivatives in an in Vitro Model System. ChemBioChem 2009, 10, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Perjési, P.; Takács-Novák, K.; Rozmer, Z.; Sohár, P.; Bozak, R.E.; Allen, T.M. Comparison of Structure, LogP and P388 Cytotoxicity of Some Phenyl and Ferrocenyl Cyclic Chalcone Analogues. Application of RP-TLC for LogP Determination of the Ferrocenyl Analogues. Cent. Eur. J. Chem. 2012, 10, 1500–1505. [Google Scholar] [CrossRef]
- Rodríguez, M.A.; García-Pérez, R.M.; Mendoza, L.; Sánchez, T.; Guillen, N.; Orozco, E. The Pyruvate:Ferredoxin Oxidoreductase Enzyme Is Located in the Plasma Membrane and in a Cytoplasmic Structure in Entamoeba. Microb. Pathog. 1998, 25, 1–10. [Google Scholar] [CrossRef]
- Edwards, D.I. Reduction of Nitroimidazoles in Vitro and DNA Damage. Biochem. Pharmacol. 1986, 35, 53–58. [Google Scholar] [CrossRef]
- Zhou, L.; Guan, Q.; Li, W.; Zhang, Z.; Li, Y.; Dong, Y. A Ferrocene-Functionalized Covalent Organic Framework for Enhancing Chemodynamic Therapy via Redox Dyshomeostasis. Small 2021, 17, 2101368. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhou, T.; Xia, Q.; Lu, X.; Xu, H.; Hu, M.; Jing, S. Design of Ferrocenylseleno-Dopamine Derivatives to Optimize the Fenton-like Reaction Efficiency and Antitumor Efficacy. RSC Adv. 2021, 11, 25477–25483. [Google Scholar] [CrossRef]
- de Avellar, I.G.J.; Magalhães, M.M.M.; Silva, A.B.; Souza, L.L.; Leitão, A.C.; Hermes-Lima, M. Reevaluating the Role of 1,10-Phenanthroline in Oxidative Reactions Involving Ferrous Ions and DNA Damage. Biochim. Biophys. Acta-Gen. Subj. 2004, 1675, 46–53. [Google Scholar] [CrossRef]
- Juneja, A.; Macedo, T.S.; Magalhaes Moreira, D.R.; Pereira Soares, M.B.; Lima Leite, A.C.; Kelle De Andrade Lemoine Neves, J.; Alves Pereira, V.R.; Avecilla, F.; Azam, A. Synthesis of 4′-(2-Ferrocenyl)-2,2′:6′2′′-Terpyridine: Characterization and Antiprotozoal Activity of Mn(II), Co(II), Ni(II), Cu(II) and Zn(II) Complexes. Eur. J. Med. Chem. 2014, 75, 203–210. [Google Scholar] [CrossRef]
- Enami, S.; Sakamoto, Y.; Colussi, A.J. Fenton Chemistry at Aqueous Interfaces. Proc. Natl. Acad. Sci. USA 2014, 111, 623–628. [Google Scholar] [CrossRef]
- Klimova, E.I.; Berestneva, T.K.; Ortega, S.H.; Iturbide, D.M.; Marquez, A.G.; García, M.M. Diferrocenylcyclopropenyl Cations. Synthesis, Structures, and Some Chemical Properties. J. Organomet. Chem. 2005, 690, 3333–3339. [Google Scholar] [CrossRef]
- Berestneva, T.K.; Klimova, E.I.; Méndez Stivalet, J.M.; Hernández-Ortega, S.; García, M.M. Diferrocenyl(Methylthio)Cyclopropenylium Iodide in the Synthesis of 2,3-Diferrocenyl-1-Methylthio-1,3-Dienes and -1,3,5-Trienes. Eur. J. Org. Chem. 2005, 2005, 4406–4413. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Hohenstein, E.G.; Chill, S.T.; Sherrill, C.D. Assessment of the Performance of the M05#2X and M06#2X Exchange Correlation Functionals for Noncovalent Interactions in Biomolecules. J. Chem. Theory Comput. 2008, 4, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03 2009. Available online: https://gaussian.com/ (accessed on 6 August 2022).
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView 2016. Available online: https://gaussian.com/gaussview6/ (accessed on 8 June 2022).



| H NMR | C NMR | E(I) | E(II) | Log Kcom | UV–vis | logP b | HOMO | LUMO | ΔHOMO–LUMO | |
|---|---|---|---|---|---|---|---|---|---|---|
| 3a | 7.15 | 136.94 | 0.116 a | 0.305 a | 3.19 a (1563) | 458, 368, 330 | 4.42 (0.2) | −0.526 | −0.357 | −0.169 |
| 3b | 7.14 | 134.20 | −0.019 a | 0.182 a | 3.39 a (2495) | 466, 372, 331 | 4.76 (0.1) | −0.515 | −0.358 | −0.156 |
| 3c | 7.62 | 134.43 | −0.022 | 0.167 | 1563 | 464, 368, 329 | 5.48 (0.1) | −0.511 | −0.358 | −0.152 |
| 3d | 7.29 | 135.04 | −0.045 a | 0.135 a | 1101 a | 468, 370, 334 | 5.62 (0.2) | −0.461 | −0.354 | −0.106 |
| 3e | 7.59 | 134.76 | −0.065 | 0.124 | 1557 | 462, 368, 328 | 6.1 (0.09) | −0.450 | −0.358 | −0.091 |
| Compound | 3a | 3b | 3c | 3d | 3e | Metronidazole |
|---|---|---|---|---|---|---|
| IC50 (µM) | 1000 ± 46 | 100 ± 9 | 1891 ± 28 | 23 ± 3 | 385 ± 11 | 7 ± 0.9 |
| −log (1/IC50) | 3 | 2 | 3.27 | 1.36 | 2.58 | −0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toledano-Magaña, Y.; Néquiz, M.; Valenzuela-Salas, L.M.; Sánchez-García, J.J.; Galindo-Murillo, R.; García-Ramos, J.C.; Klimova, E.I. The Amoebicidal Activity of Diferrocenyl Derivatives: A Significant Dependence on the Electronic Environment. Molecules 2023, 28, 6008. https://doi.org/10.3390/molecules28166008
Toledano-Magaña Y, Néquiz M, Valenzuela-Salas LM, Sánchez-García JJ, Galindo-Murillo R, García-Ramos JC, Klimova EI. The Amoebicidal Activity of Diferrocenyl Derivatives: A Significant Dependence on the Electronic Environment. Molecules. 2023; 28(16):6008. https://doi.org/10.3390/molecules28166008
Chicago/Turabian StyleToledano-Magaña, Yanis, Mario Néquiz, Lucía Margarita Valenzuela-Salas, Jessica J. Sánchez-García, Rodrigo Galindo-Murillo, Juan Carlos García-Ramos, and Elena I. Klimova. 2023. "The Amoebicidal Activity of Diferrocenyl Derivatives: A Significant Dependence on the Electronic Environment" Molecules 28, no. 16: 6008. https://doi.org/10.3390/molecules28166008






