Stability of Phenolic Compounds, Antioxidant Activity and Color Parameters in Colored-Flesh Potato Chips
Abstract
:1. Introduction
2. Results
2.1. Identification and Quantification of Anthocyanins and HCADs
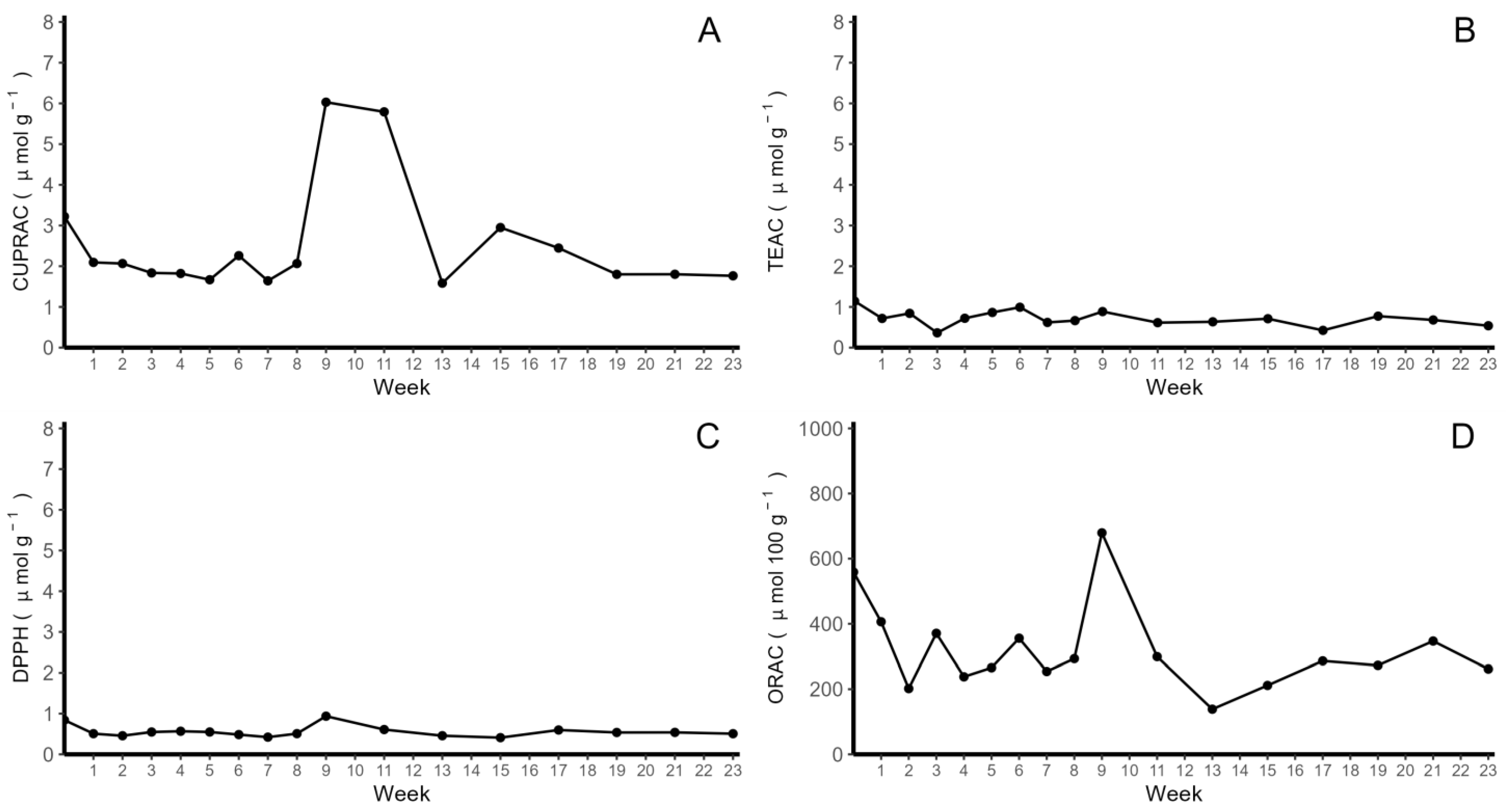
2.2. Determination of Antioxidant Activity
2.3. Color Determinations
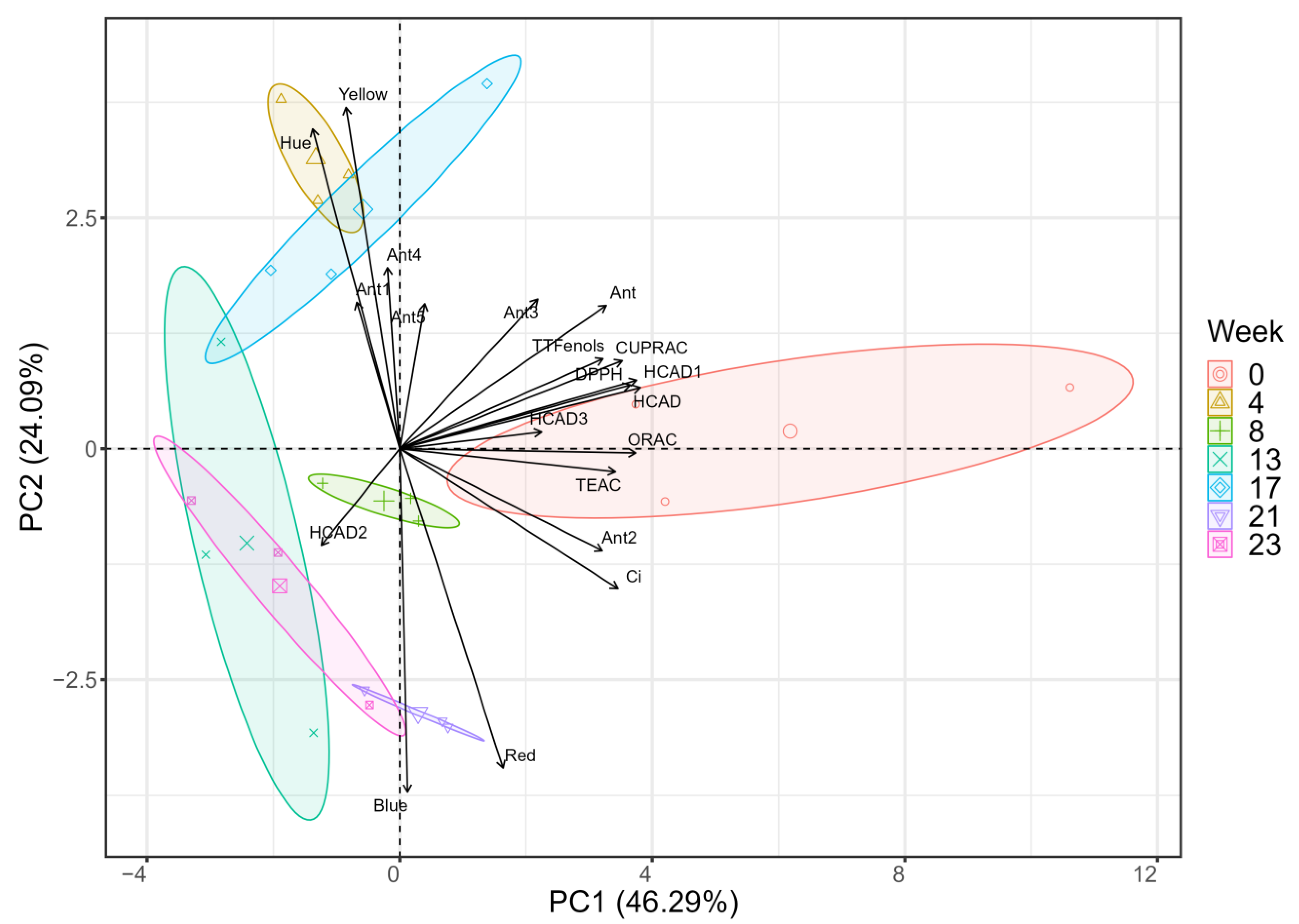
2.4. Principal Component Analysis of Initial and Final Conditions
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Samples
4.3. Extraction of Anthocyanins and HCAD
4.4. Identification and Quantification of Anthocyanins and HCADs
4.5. Determination of Total Phenols by the Folin–Ciocalteu Method
4.6. Determination of Antioxidant Activity
4.7. Color Determinations
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- FAOSTAT. Food and Agriculture Organization of the United Nations [Online]. 2019. Available online: http://www.fao.org/faostat/es/#data (accessed on 3 August 2022).
- Fogelman, E.; Oren-Shamir, M.; Hirschberg, J.; Mandolino, G.; Parisi, B.; Ovadia, R.; Tanami, Z.; Faigenboim, A.; Ginzberg, I. Nutritional value of potato (Solanum tuberosum) in hot climates: Anthocyanins, carotenoids, and steroidal glycoalkaloids. Planta 2019, 249, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Aguilera, A.; Ercoli, S.; Parada, J.; Winterhalter, P.; Contreras, B.; Cornejo, P. Effect of the frying process on the composition of hydroxycinnamic acid derivatives and antioxidant activity in flesh colored potatoes. Food Chem. 2018, 268, 577–584. [Google Scholar] [CrossRef]
- Ercoli, S.; Parada, J.; Bustamante, L.; Hermosín-Gutiérrez, I.; Contreras, B.; Cornejo, P.; Ruiz, A. Noticeable Quantities of Functional Compounds and Antioxidant Activities Remain after Cooking of Colored Fleshed Potatoes Native from Southern Chile. Molecules 2021, 26, 314. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Tausend, A.; Galan, J.; Groh, I.; Berry, D.; Tur, J.; Marko, D.; Richling, E. Antioxidative activity and health benefits of anthocyanin-rich fruit juice in healthy volunteers. Free Radic. Res. 2019, 53, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Fang, T.; Lin, Q.; Song, H.; Liu, B.; Chen, L. Red raspberry and its anthocyanins: Bioactivity beyond antioxidant capacity. Trends Food Sci. Technol. 2017, 66, 153–165. [Google Scholar] [CrossRef]
- Xie, L.; Su, H.; Sun, C.; Zheng, X.; Chen, W. Recent advances in understanding the anti-obesity activity of anthocyanins and their biosynthesis in microorganisms. Trends Food Sci. Technol. 2018, 72, 13–24. [Google Scholar] [CrossRef]
- Gamel, T.; Wright, A.; Pickard, M.; Abdel-Aal, E. Characterization of anthocyanin-containing purple wheat prototype products as functional foods with potential health benefits. Cereal Chem. 2019, 97, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Coman, V.; Vodnar, D. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxid. Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Candy & Snack Today. U.S. Potato Chips Market to See 4.4% Sales Gains By 2025. 2018. Available online: https://candyusa.com/cst/u-s-potato-chips-market-to-see-4-4-sales-gains-by-2025/ (accessed on 29 August 2022).
- Vaitkevičienė, N.; Jarienė, E.; Kulaitienė, J.; Levickienė, D. The Physico-Chemical and Sensory Characteristics of Coloured-Flesh Potato Chips: Influence of Cultivar, Slice Thickness and Frying Temperature. Appl. Sci. 2022, 12, 1211. [Google Scholar] [CrossRef]
- Saar-Reismaa, P.; Kotkas, K.; Rosenberg, V.; Kulp, M.; Kuhtinskaja, M.; Vaher, M. Analysis of Total Phenols, Sugars, and Mineral Elements in Colored Tubers of Solanum tuberosum L. Foods 2020, 9, 1862. [Google Scholar] [CrossRef] [PubMed]
- Vaitkevičienė, N. A comparative study on proximate and mineral composition of coloured potato peel and flesh. J. Sci. Food. Agric. 2019, 99, 6227–6233. [Google Scholar] [CrossRef] [PubMed]
- Nemś, A.; Pęksa, A.; Kucharska, A.; Sokół-Łętowska, A.; Kita, A.; Drożdż, W.; Hamouz, K. Anthocyanin and antioxidant activity of snacks with coloured potato. Food Chem. 2015, 172, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tajner-Czopek, A.; Rytel, E.; Kita, A.; Sokół-Łetowska, A.; Kucharska, A.Z. Content and Stability of Hydroxycinnamic Acids during the Production of French Fries Obtained from Potatoes of Varieties with Light-Yellow, Red and Purple Flesh. Antioxidants 2023, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, S.; Cartes, J.; Cornejo, P.; Tereucán, G.; Winterhalter, P.; Contreras, B.; Ruiz, A. Stability of phenolic compounds, antioxidant activity and colour parameters of a coloured extract obtained from coloured-flesh potatoes. LWT-Food Sci. Technol. 2021, 136, 110370. [Google Scholar] [CrossRef]
- Sharma, R.J.; Gupta, R.C.; Singh, S.; Bansal, A.K.; Singh, I.P. Stability of anthocyanins- and anthocyanidins-enriched extracts, and formulations of fruit pulp of Eugenia jambolana (‘jamun’). Food Chem. 2016, 190, 808–817. [Google Scholar] [CrossRef]
- Guan, Y.; Zhong, Q. The improved thermal stability of anthocyanins at pH 5.0 by gum arabic. LWT-Food Sci. Technol. 2015, 64, 706–712. [Google Scholar] [CrossRef]
- Sui, X.; Dong, X.; Zhou, W. Combined effect of pH and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014, 163, 163–170. [Google Scholar] [CrossRef]
- Tereucán, G.; Ercoli, S.; Cornejo, P.; Winterhalter, P.; Contreras, B.; Ruiz, A. Stability of antioxidant compounds and activities of a natural dye from coloured-flesh potatoes in dairy foods. LWT 2021, 144, 111252. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kirkpatrick, J.; Kuhnert, N.; Roozendaal, H.; Salgado, P.R. LC–MSn analysis of the cis isomers of chlorogenic acids. Food Chem. 2008, 106, 379–385. [Google Scholar] [CrossRef]
- Ertan, K.; Türkyılmaz, M.; Özkan, M. Color and stability of anthocyanins in strawberry nectars containing various co-pigment sources and sweeteners. Food Chem. 2020, 310, 125856. [Google Scholar] [CrossRef] [PubMed]
- Ertan, K.; Türkyılmaz, M.; Özkan, M. Effects of natural copigment sources in combination with sweeteners on the stability of anthocyanins in sour cherry nectars. Food Chem. 2019, 294, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Özkan, M. Degradation of anthocyanins in sour cherry and pomegranate juices by hydrogen peroxide in the presence of added ascorbic acid. Food Chem. 2002, 78, 499–504. [Google Scholar] [CrossRef]
- Chen, J.Y.; Du, J.; Li, M.L.; Li, C.M. Degradation kinetics and pathways of red raspberry anthocyanins in model and juice systems and their correlation with color and antioxidant changes during storage. LWT 2020, 128, 109448. [Google Scholar] [CrossRef]
- Oyarzún, P.; Cornejo, P.; Gómez-Alonso, S.; Ruiz, A. Influence of Profiles and Concentrations of Phenolic Compounds in the Coloration and Antioxidant Properties of Gaultheria poeppigii Fruits from Southern Chile. Plant Foods Hum. Nutr. 2020, 75, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, Y.; Xu, X.; Wu, J.; Liao, X.; Chen, F. Degradation Kinetics of Malvidin-3-glucoside and Malvidin-3,5-diglucoside Exposed to Microwave Treatment. J. Agric. Food Chem. 2013, 61, 373–378. [Google Scholar] [CrossRef]
- Mozetič, B.; Simčič, M.; Trebše, P. Anthocyanins and hydroxycinnamic acids of Lambert Compact cherries (Prunus avium L.) after cold storage and 1-methylcyclopropene treatment. Food Chem. 2006, 97, 302–309. [Google Scholar] [CrossRef]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and Storage Effects on Procyanidin Composition and Concentration of Processed Blueberry Products. J. Agric. Food Chem. 2009, 57, 1896–1902. [Google Scholar] [CrossRef]
- de la Torre-Robles, A.; Monteagudo, C.; Mariscal-Arcas, M.; Lorenzo-Tovar, M.L.; Olea-Serrano, F.; Rivas, A. Effect of Light Exposure on the Quality and Phenol Content of Commercial Extra Virgin Olive Oil during 12-Month Storage. J. Am. Oil Chem. Soc. 2019, 96, 381–389. [Google Scholar] [CrossRef]
- Cinquanta, L.; Esti, M.; Notte, E.L. Evolution of phenolic compounds in virgin olive oil during storage. J. Amr. Oil Chem. Soc. 1997, 74, 1259–1264. [Google Scholar] [CrossRef]
- Gargouri, B.; Zribi, A.; Bouaziz, M.A. Effect of containers on the quality of Chemlali olive oil during storage. J. Food Sci. Technol. 2015, 52, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Bustamante, L.; Vergara, C.; von Baer, D.; Hermosín-Gutiérrez, I.; Obando, L.; Mardones, C. Hydroxycinnamic acids and flavonols in native edible berries of South Patagonia. Food Chem. 2015, 167, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; Aiello, A.; De Luca, L.; Pizzolongo, F.; Durazzo, A.; Lucarini, M.; Severino, P.; Souto, E.B.; Santini, A. Deep-frying purple potato Purple Majesty using sunflower oil: Effect on the polyphenols, anthocyanins and antioxidant activity. Heliyon 2022, 8, e09337. [Google Scholar] [CrossRef] [PubMed]
- Ouerghemmi, S.; Sebei, H.; Siracusa, L.; Ruberto, G.; Saija, A.; Cimino, F.; Cristani, M. Comparative study of phenolic composition and antioxidant activity of leaf extracts from three wild Rosa species grown in different Tunisia regions: Rosa canina L., Rosa moschata Herrm. and Rosa sempervirens L. Ind. Crops Prod. 2016, 94, 167–177. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Cerretani, L.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Distribution of phenolic compounds and other polar compounds in the tuber of Solanum tuberosum L. by HPLC-DAD-q-TOF and study of their antioxidant activity. J. Food Compost. Anal. 2014, 36, 1–11. [Google Scholar] [CrossRef]
- Jaffe, R.; Mani, J. Polyphenolics Evoke Healing Responses: Clinical Evidence and Role of Predictive Biomarkers. In Polyphenols: Mechanisms of Action in Human Health and Disease, 2nd ed.; Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 5, pp. 403–413. [Google Scholar] [CrossRef]
- Nemś, A.; Pęksa, A. Polyphenols of coloured-flesh potatoes as native antioxidants in stored fried snacks. LWT-Food Sci. Technol. 2018, 97, 597–602. [Google Scholar] [CrossRef]
- García-Viguera, C.; Zafrilla, P. Changes in Anthocyanins during Food Processing: Influence on Color. In Chemistry and Physiology of Selected Food Colorants, 1st ed.; Ames, J.M., Hofmann, T., Eds.; American Chemical Society: Washington, DC, USA, 2001; Volume 775, pp. 56–65. [Google Scholar] [CrossRef]
- Ścibisz, I.; Ziarno, M.; Mitek, M. Color stability of fruit yogurt during storage. J. Food Sci. Technol. 2019, 56, 1997–2009. [Google Scholar] [CrossRef] [Green Version]
- Vukoja, J.; Pichler, A.; Kopjar, M. Stability of Anthocyanins, Phenolics and Color of Tart Cherry Jams. Foods 2019, 8, 255. [Google Scholar] [CrossRef] [Green Version]
- Brownmiller, C.; Howard, L.R.; Prior, R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blueberry products. J. Food Sci. 2008, 73, H72–H79. [Google Scholar] [CrossRef]
- Howard, L.R.; Castrodale, C.; Brownmiller, C.; Mauromoustakos, A. Jam processing and storage effects on blueberry polyphenolics and antioxidant capacity. J. Agric. Food Chem. 2010, 58, 4022–4029. [Google Scholar] [CrossRef] [PubMed]
- Carrera, C.; Zelaya-Medina, C.F.; Chinchilla, N.; Ferreiro-González, M.; Barbero, G.F.; Palma, M. How Different Cooking Methods Affect the Phenolic Composition of Sweet Potato for Human Consumption (Ipomea batata (L.) Lam). Agronomy 2021, 11, 1636. [Google Scholar] [CrossRef]
- Nova-Baza, D.; Olivares-Caro, L.; Bustamante, L.; Pérez, A.; Vergara, C.; Fuentealba, J.; Mardones, C. Metabolic profile and antioxidant capacity of five Berberis leaves species: A comprehensive study to determine their potential as natural food or ingredient. Food Res. Int. 2022, 160, 111642. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Orthofer, R.; Lamuela-Raventos, R. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology, 1st ed.; Packer, L., Ed.; Elsevier S&T: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
- Pastrana-Bonilla, E.; Akoh, C.; Sellappan, S.; Krewer, G. Phenolic Content and Antioxidant Capacity of Muscadine Grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef]
- Ribeiro, J.; Magalhães, L.; Reis, S.; Lima, J.; Segundo, M. High-throughput total cupric ion reducing antioxidant capacity of biological samples determined using flow injection analysis and microplate-based methods. Anal. Sci. 2011, 27, 483–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado, P.D.; Rivero-Cruz, I.; Mata, R.; Pedraza-Chaverrí, J. Antioxidant Activity of A-Type Proanthocyanidins from Geranium niveum (Geraniaceae). J. Agric. Food Chem. 2005, 53, 1996–2001. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and Prooxidant Behavior of Flavonoids: Structure-Activity Relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Prediction of red and rosé wine CIELab parameters from simple absorbance measurements. J. Sci. Food Agric. 2002, 82, 1319–1324. [Google Scholar] [CrossRef]



| Peak Number | tR (min) | Tentative Identification | [M]+ | [M − H]− | Product Ions | ^max (nm) |
|---|---|---|---|---|---|---|
| 1 | 8.2 | 5-caffeoylquinic acid | - | 353.1 | 191.1 | 325 |
| 2 | 9.1 | n.i | - | - | - | 323 |
| 3 | 10.5 | Caffeoylquinic acid isomer | - | 353.1 | 191.1 | 319 |
| 4 | 19.5 | Petunidin-3-p-coumaroylrutinoside-5-glucoside | 933.2 | - | 771.2; 479.0; 317.0 | 530 |
| 5 | 21.0 | Petunidin-3-feruloylrutinoside-5-glucoside | 963.3 | - | 479.0; 317.0 | - |
| 6 | 21.5 | Pelargonidin-3-p-coumaroylrutinoside-5-glucoside | 887.3 | - | 271.3; 725.2; 433.1 | 504 |
| 7 | 22.0 | Peonidin-3-p-coumaroylrutinoside-5-glucoside | 917.1 | - | 755.3; 463.2; 301.2 | 528 |
| 8 | 22.5 | n.i | - | - | 504 |
| HPLC, HCADs (mg kg−1) | HPLC, Anthocyanins (mg kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Week | Peak 1 | Peak 2 | Peak 3 | Peak 4 | Peak 5 | Peak 6 | Peak 7 | Peak 8 |
| 1 | 97.82 ± 34.47 a | nd | 1.79 ± 0.54 ab | nd | 3.24 ± 0.94 a | 1.61 ± 1.56 ab | nd | nd |
| 2 | 52.47 ± 8.42 bcd | nd | 1.24 ± 1.07 bc | 1.04 ± 0.91 bc | 0.46 ± 0.80 c | nd | nd | nd |
| 3 | 40.27 ± 8.96 cd | nd | 0.,47 ± 0.81 bc | 1.62 ± 1.41 ab | nd | nd | nd | nd |
| 4 | 51.6 ± 20.98 bcd | nd | 0.93 ± 0.81 bc | 1.94 ± 0.47 ab | nd | nd | nd | nd |
| 5 | 46.24 ± 6.58 bcd | nd | 1.00 ± 0.11 bc | 1.98 ± 0.07 ab | nd | nd | nd | nd |
| 6 | 54.12 ± 5.65 bcd | nd | nd | 2.14 ± 0.29 ab | nd | nd | nd | nd |
| 7 | 69.93 ± 9.85 abc | nd | nd | 2.10 ± 0.22 ab | nd | nd | nd | nd |
| 8 | 39.21 ± 8.32 cd | nd | nd | 1.53 ± 0.07 ab | nd | nd | nd | nd |
| 9 | 45.83 ± 6.49 bcd | nd | 1.20 ± 1.04 bc | 1.73 ± 0.26 ab | nd | nd | nd | nd |
| 12 | 21.20 ± 1.12 d | 23.02 ± 0.48 a | nd | nd | 1.52 ± 0.14 b | nd | nd | nd |
| 14 | 25.08 ± 3.75 d | 5.20 ± 0.78 b | nd | nd | nd | 0.69 ± 0.62 ab | nd | nd |
| 16 | 23.13 ± 3.38 d | 4.30 ± 0.30 b | nd | nd | nd | 1.17 ± 0.24 ab | 0.28 ± 0.48 b | nd |
| 18 | 39.20 ± 9.74 cd | 5.63 ± 2.14 b | 1.89 ± 0.49 ab | nd | nd | 1.89 ± 1.18 a | 1.21 ± 0.24 a | 0.34 ± 0.59 a |
| 20 | 35.88 ± 14.78 cd | 5.37 ± 2.06 b | 1.89 ± 0.60 ab | nd | nd | 1.20 ± 1.10 ab | 0.42 ± 0.74 b | nd |
| 22 | 36.87 ± 5.93 cd | 5.19 ± 0.63 b | 1.86 ± 0.20 ab | nd | 2.14 ± 0.28 b | nd | nd | nd |
| 24 | 26.08 ± 8.20 d | 4.02 ± 1.07 b | 1.33 ± 0.28 bc | nd | nd | nd | nd | nd |
| Method | Standard | Equation | R2 | DL | QL | LR | CV (%) |
|---|---|---|---|---|---|---|---|
| HPLC | Petunidin-3-glucoside; Chlorogenic acid | y = 55,324x − 44,878; y = 73,284x + 6553.5 | 0.998; 1.000 | 0.332 mg L−1; 0.042 mg L−1 | 1.108 mg L−1; 0.140 mg L−1 | 1.108–100 mg L−1; 0.140–100 mg L−1 | 0.32%; 0.39% |
| Folin | Gallic acid | y = 0.0008x + 0.0492 | 0.999 | 4.535 mg L−1 | 15.116 mg L−1 | 15.116–100 mg L−1 | 2.41% |
| CUPRAC | Trolox | y = 3.158x + 0.1822 | 0.993 | 0.032 mmol L−1 | 0.105 mmol L−1 | 0.105–0.7 mmol L−1 | 4.28% |
| TEAC | Trolox | y = 0.4249x + 0.0193 | 0.998 | 0.022 mmol L−1 | 0.074 mmol L−1 | 0.074–0.7 mmol L−1 | 13.35% |
| DPPH | Trolox | y = 0.4031x + 0.0769 | 0.996 | 0.011 mmol L−1 | 0.037 mmol L−1 | 0.037–0.7 mmol L−1 | 4.49% |
| ORAC | Trolox | y = 0.4296x + 4.2862 | 0.995 | 0.532 umol L−1 | 1.774 umol L−1 | 1.774–80 umol L−1 | 13.96% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo, C.; Peña, F.; Nahuelcura, J.; Vidal, C.; González, F.; Jiménez-Aspee, F.; Bustamante, L.; Contreras, B.; Ruiz, A. Stability of Phenolic Compounds, Antioxidant Activity and Color Parameters in Colored-Flesh Potato Chips. Molecules 2023, 28, 6047. https://doi.org/10.3390/molecules28166047
Bravo C, Peña F, Nahuelcura J, Vidal C, González F, Jiménez-Aspee F, Bustamante L, Contreras B, Ruiz A. Stability of Phenolic Compounds, Antioxidant Activity and Color Parameters in Colored-Flesh Potato Chips. Molecules. 2023; 28(16):6047. https://doi.org/10.3390/molecules28166047
Chicago/Turabian StyleBravo, Catalina, Fabiola Peña, Javiera Nahuelcura, Catalina Vidal, Felipe González, Felipe Jiménez-Aspee, Luis Bustamante, Boris Contreras, and Antonieta Ruiz. 2023. "Stability of Phenolic Compounds, Antioxidant Activity and Color Parameters in Colored-Flesh Potato Chips" Molecules 28, no. 16: 6047. https://doi.org/10.3390/molecules28166047





