Synthesis of a Series of Trimeric Branched Glycoconjugates and Their Applications for Supramolecular Gels and Catalysis
Abstract
:1. Introduction
2. Results and Discussions
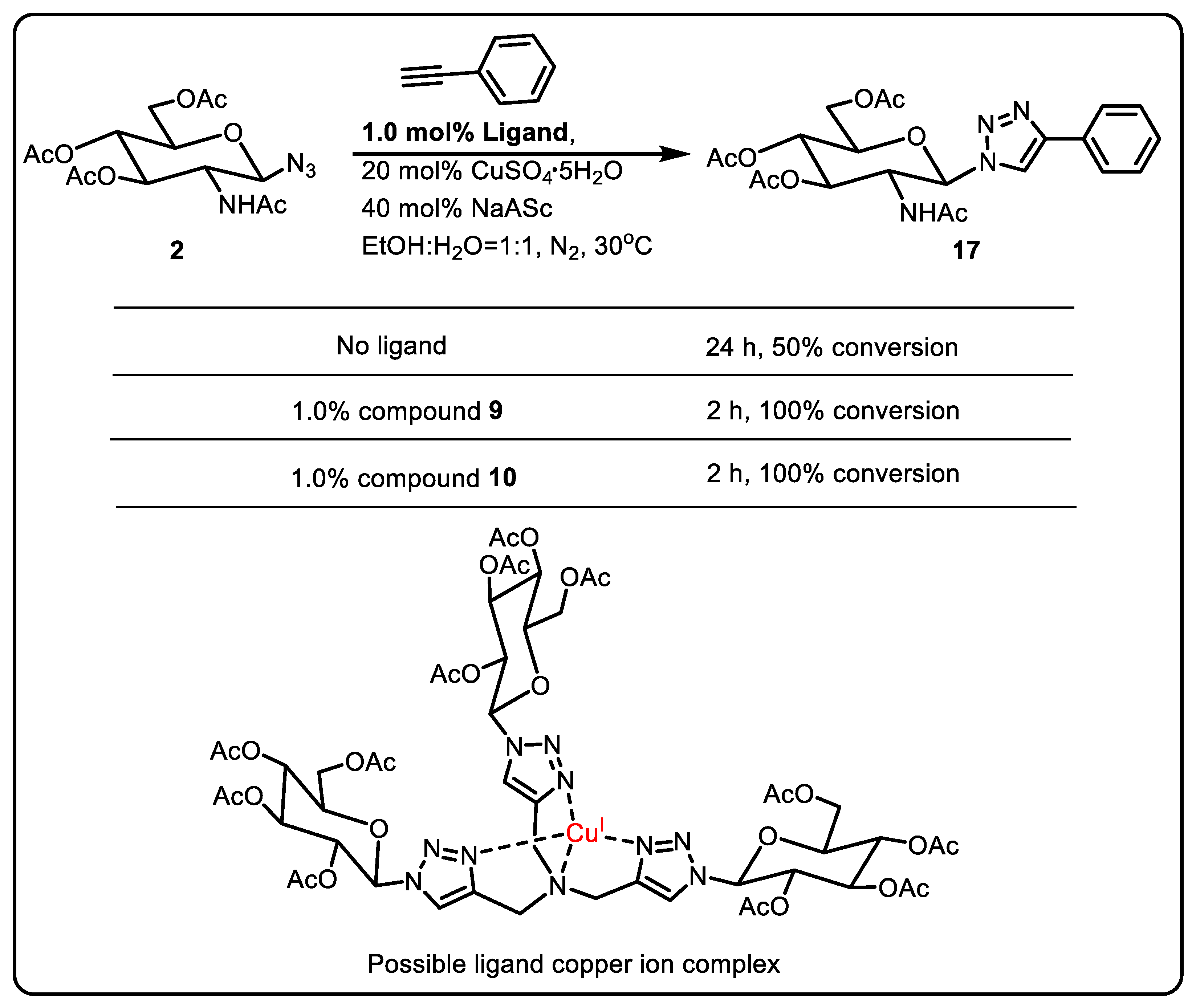
Metallogels Formation and Applications as Catalytic Gels
3. Experimental Section
3.1. General Methods
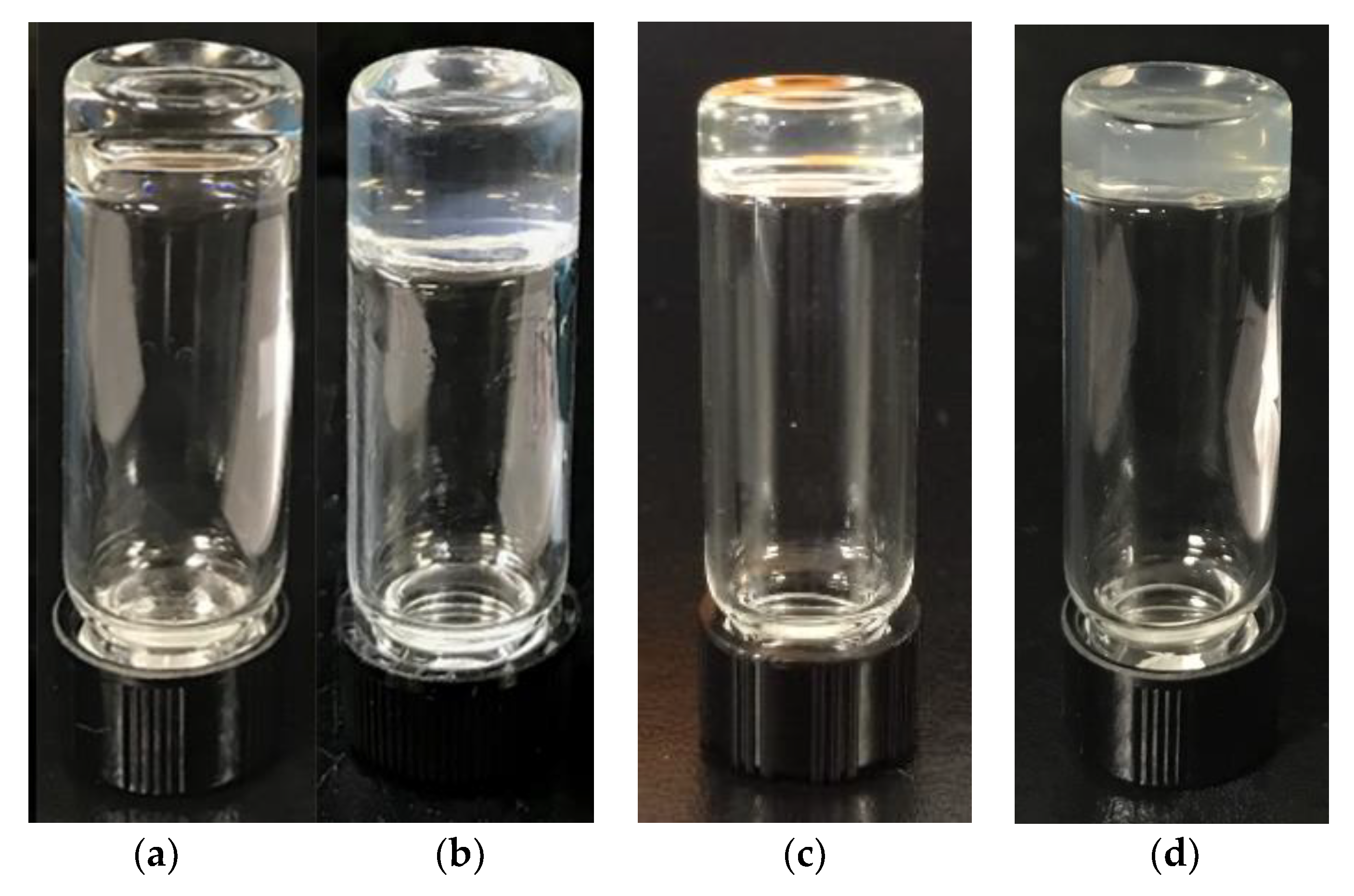
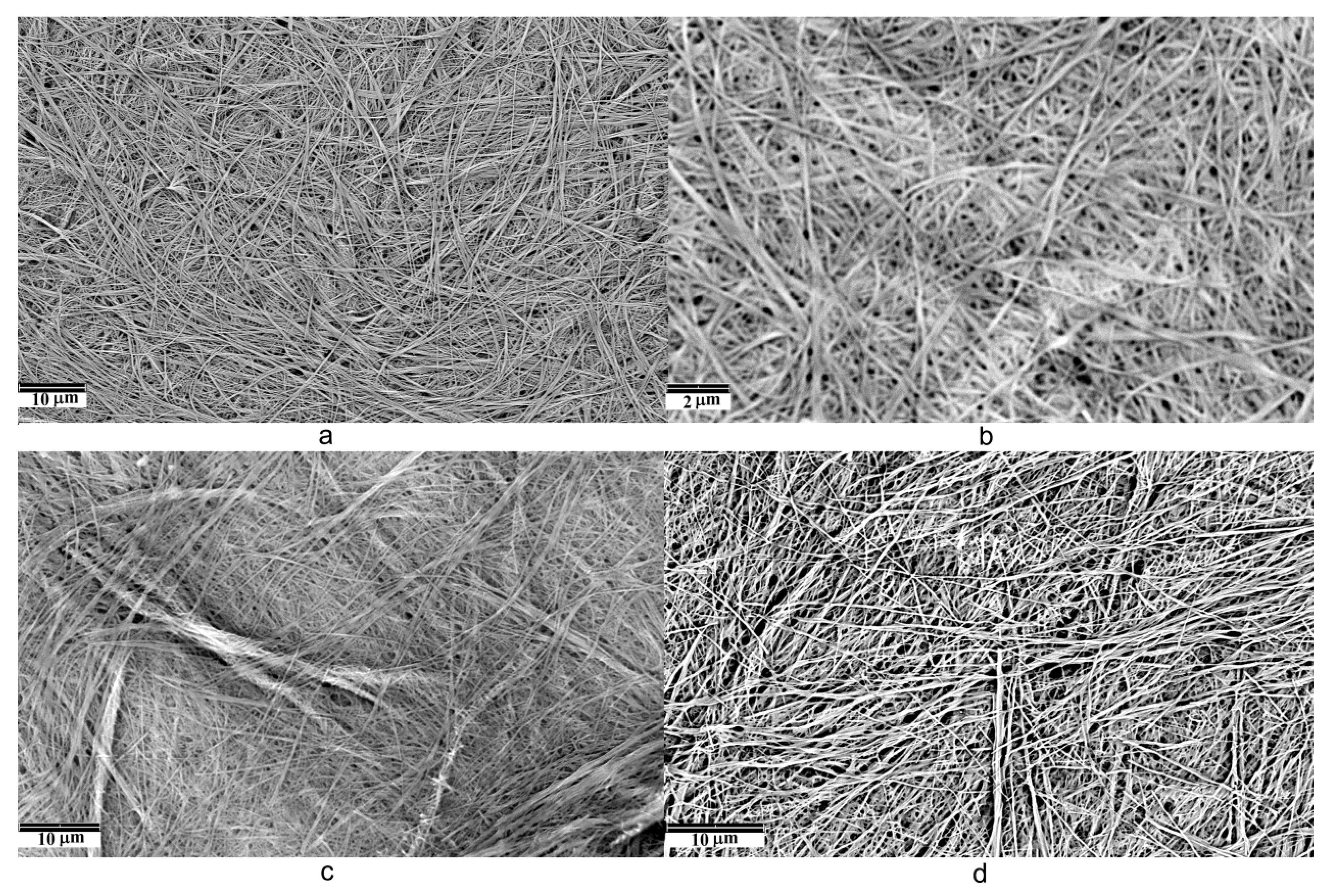
3.2. Scanning Electronic Microscopy
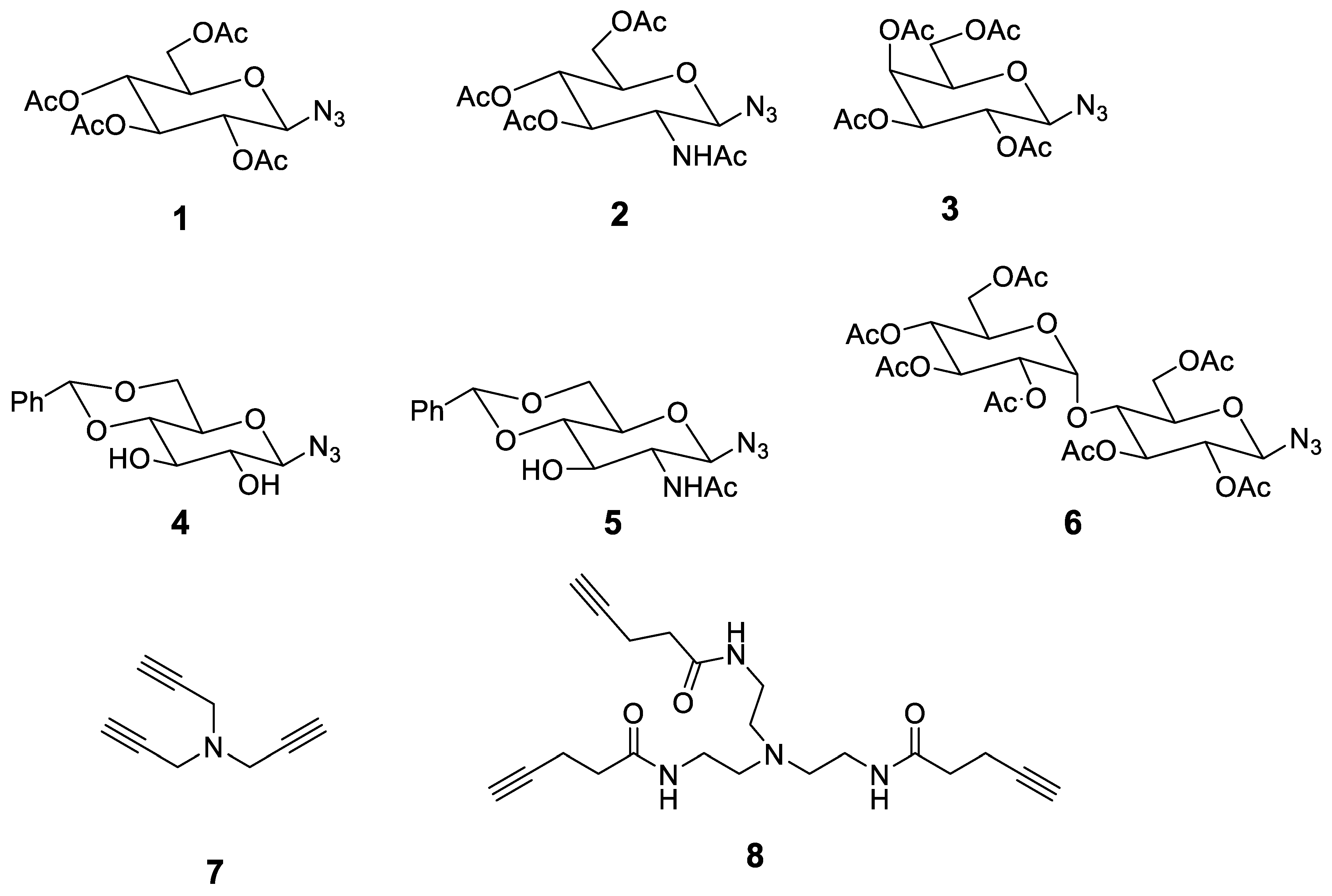
3.3. Synthesis and Characterization of Compounds 8–16
3.4. Synthesis of Compounds 19 and 20 by Catalytic Gel Column
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [PubMed]
- Datta, S.; Bhattacharya, S. Multifarious facets of sugar-derived molecular gels: Molecular features, mechanisms of self-assembly and emerging applications. Chem. Soc. Rev. 2015, 44, 5596–5637. [Google Scholar]
- Morris, J.; Bietsch, J.; Bashaw, K.; Wang, G. Recently Developed Carbohydrate Based Gelators and Their Applications. Gels 2021, 7, 24. [Google Scholar] [PubMed]
- Tian, R.; Chen, J.; Niu, R. The development of low-molecular weight hydrogels for applications in cancer therapy. Nanoscale 2014, 6, 3474–3482. [Google Scholar] [CrossRef]
- Dong, R.; Pang, Y.; Su, Y.; Zhu, X. Supramolecular hydrogels: Synthesis, properties and their biomedical applications. Biomater. Sci. 2015, 3, 937–954. [Google Scholar]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting-self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar]
- Vibhute, A.M.; Muvvala, V.; Sureshan, K.M. A Sugar-Based Gelator for Marine Oil-Spill Recovery. Angew. Chem. Int. Ed. 2016, 55, 7782–7785. [Google Scholar]
- Escuder, B.; Rodriguez-Llansola, F.; Miravet, J.F. Supramolecular gels as active media for organic reactions and catalysis. New J. Chem. 2010, 34, 1044–1054. [Google Scholar]
- Diaz Diaz, D.; Kuhbeck, D.; Koopmans, R.J. Stimuli-responsive gels as reaction vessels and reusable catalysts. Chem. Soc. Rev. 2011, 40, 427–448. [Google Scholar] [CrossRef]
- Doring, A.; Birnbaum, W.; Kuckling, D. Responsive hydrogels—Structurally and dimensionally optimized smart frameworks for applications in catalysis, micro-system technology and material science. Chem. Soc. Rev. 2013, 42, 7391–7420. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhang, Y.; Wu, J.; Liu, C.; Zhu, H.; Tu, T. Recent Advances in Supramolecular Gels and Catalysis. Chem. Asian J. 2018, 13, 712–729. [Google Scholar]
- Rizzo, C.; Marullo, S.; Billeci, F.; D’Anna, F. Catalysis in Supramolecular Systems: The Case of Gel Phases. Eur. J. Org. Chem. 2021, 2021, 3148–3169. [Google Scholar]
- Hawkins, K.; Patterson, A.K.; Clarke, P.A.; Smith, D.K. Catalytic Gels for a Prebiotically Relevant Asymmetric Aldol Reaction in Water: From Organocatalyst Design to Hydrogel Discovery and Back Again. J. Am. Chem. Soc. 2020, 142, 4379–4389. [Google Scholar]
- Singh, N.; Zhang, K.; Angulo-Pachon, C.A.; Mendes, E.; van Esch, J.H.; Escuder, B. Tandem reactions in self-sorted catalytic molecular hydrogels. Chem. Sci. 2016, 7, 5568–5572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Llansola, F.; Escuder, B.; Miravet, J.F. Switchable performance of an L-proline-derived basic catalyst controlled by supramolecular gelation. J. Am. Chem. Soc. 2009, 131, 11478–11484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, C.-Y. Metal-organic gels: From discrete metallogelators to coordination polymers. Coord. Chem. Rev. 2013, 257, 1373–1408. [Google Scholar]
- Tam, A.Y.-Y.; Yam, V.W.-W. Recent advances in metallogels. Chem. Soc. Rev. 2013, 42, 1540–1567. [Google Scholar]
- Zheng, Y.; Li, G.; Zhang, Y. Organometallic Hydrogels. ChemNanoMat 2016, 2, 364–375. [Google Scholar] [CrossRef]
- Haring, M.; Diaz, D.D. Supramolecular metallogels with bulk self-healing properties prepared by in situ metal complexation. Chem. Commun. 2016, 52, 13068–13081. [Google Scholar]
- Liu, Z.; Zhao, X.; Chu, Q.; Feng, Y. Recent Advances in Stimuli-Responsive Metallogels. Molecules 2023, 28, 2274. [Google Scholar] [CrossRef]
- Yuan, J.; Fang, X.; Zhang, L.; Hong, G.; Lin, Y.; Zheng, Q.; Xu, Y.; Ruan, Y.; Weng, W.; Xia, H.; et al. Multi-responsive self-healing metallo-supramolecular gels based on “click” ligand. J. Mater. Chem. 2012, 22, 11515–11522. [Google Scholar] [CrossRef]
- Liu, H.; Feng, J.; Zhang, J.; Miller, P.W.; Chen, L.; Su, C.-Y. A catalytic chiral gel microfluidic reactor assembled via dynamic covalent chemistry. Chem. Sci. 2015, 6, 2292–2296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, V.K.; Mishra, B.B.; Mishra, K.B.; Mishra, N.; Singh, A.S.; Chen, X. Cu-Catalyzed Click Reaction in Carbohydrate Chemistry. Chem. Rev. 2016, 116, 3086–3240. [Google Scholar] [CrossRef]
- Kushwaha, D.; Dwivedi, P.; Kuanar, S.K.; Tiwari, V.K. Click reaction in carbohydrate chemistry: Recent developments and future perspective. Curr. Org. Synth. 2013, 10, 90–135. [Google Scholar]
- Percec, V.; Leowanawat, P.; Sun, H.-J.; Kulikov, O.; Nusbaum, C.D.; Tran, T.M.; Bertin, A.; Wilson, D.A.; Peterca, M.; Zhang, S.; et al. Modular Synthesis of Amphiphilic Janus Glycodendrimers and Their Self-Assembly into Glycodendrimersomes and Other Complex Architectures with Bioactivity to Biomedically Relevant Lectins. J. Am. Chem. Soc. 2013, 135, 9055–9077. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Wang, D.; Bietsch, J.; Wang, G. Synthesis and characterization of pentaerythritol derived glycoconjugates as supramolecular gelators. Org. Biomol. Chem. 2019, 17, 6043–6056. [Google Scholar]
- Cano, M.E.; Di Chenna, P.H.; Lesur, D.; Wolosiuk, A.; Kovensky, J.; Uhrig, M.L. Chirality inversion, supramolecular hydrogelation and lectin binding of two thiolactose amphiphiles constructed on a di-lauroyl-L-tartaric acid scaffold. New J. Chem. 2017, 41, 14754–14765. [Google Scholar] [CrossRef]
- De la Torre, A.F.; Ali, A.; Westermann, B.; Schmeda-Hirschmann, G.; Walter Pertino, M. An efficient cyclization of lapachol to new benzo[h]chromene hybrid compounds: A stepwise vs. one-pot esterification-click (CuAAC) study. New J. Chem. 2018, 42, 19591–19599. [Google Scholar]
- Chen, A.; Samankumara, L.P.; Dodlapati, S.; Wang, D.; Adhikari, S.; Wang, G. Syntheses of Bis-Triazole Linked Carbohydrate Based Macrocycles and Their Applications for Accelerating Copper Sulfate Mediated Click Reaction. Eur. J. Org. Chem. 2019, 2019, 1189–1194. [Google Scholar] [CrossRef]
- Ozkal, E.; Oezcubukcu, S.; Jimeno, C.; Pericas, M.A. Covalently immobilized tris(triazolyl)methanol-Cu(I) complexes: Highly active and recyclable catalysts for CuAAC reactions. Catal. Sci. Technol. 2012, 2, 195–200. [Google Scholar]
- Krishnan, B.P.; Sureshan, K.M. Topochemical azide-alkyne cycloaddition reaction in gels: Size-tunable synthesis of triazole-linked polypeptides. J. Am. Chem. Soc. 2017, 139, 1584–1589. [Google Scholar]
- Araujo, M.; Diaz-Oltra, S.; Escuder, B. Triazolyl-Based Molecular Gels as Ligands for Autocatalytic ‘Click’ Reactions. Chem. Eur. J. 2016, 22, 8676–8684. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, D.; Bietsch, J.; Chen, A.; Sharma, P. Synthesis of Dendritic Glycoclusters and Their Applications for Supramolecular Gelation and Catalysis. J. Org. Chem. 2020, 85, 16136–16156. [Google Scholar] [CrossRef]
- Chabre, Y.M.; Roy, R. Multivalent glycoconjugate syntheses and applications using aromatic scaffolds. Chem. Soc. Rev. 2013, 42, 4657–4708. [Google Scholar] [PubMed]
- Sherman, S.E.; Xiao, Q.; Percec, V. Mimicking Complex Biological Membranes and Their Programmable Glycan Ligands with Dendrimersomes and Glycodendrimersomes. Chem. Rev. 2017, 117, 6538–6631. [Google Scholar] [PubMed]
- Bojarova, P.; Kren, V. Sugared biomaterial binding lectins: Achievements and perspectives. Biomater. Sci. 2016, 4, 1142–1160. [Google Scholar]
- Tyagi, M.; Kartha, K.P.R. Synthesis of glycotriazololipids and observations on their self-assembly properties. Carbohydr. Res. 2015, 413, 85–92. [Google Scholar] [CrossRef]
- Ligeour, C.; Vidal, O.; Dupin, L.; Casoni, F.; Gillon, E.; Meyer, A.; Vidal, S.; Vergoten, G.; Lacroix, J.-M.; Souteyrand, E.; et al. Mannose-centered aromatic galactoclusters inhibit the biofilm formation of Pseudomonas aeruginosa. Org. Biomol. Chem. 2015, 13, 8433–8444. [Google Scholar]
- Buzzacchera, I.; Xiao, Q.; Han, H.; Rahimi, K.; Li, S.; Kostina, N.Y.; Toebes, B.J.; Wilner, S.E.; Moller, M.; Rodriguez-Emmenegger, C.; et al. Screening Libraries of Amphiphilic Janus Dendrimers Based on Natural Phenolic Acids to Discover Monodisperse Unilamellar Dendrimersomes. Biomacromolecules 2019, 20, 712–727. [Google Scholar] [CrossRef]
- Feng, Y.; He, Y.-M.; Fan, Q.-H. Supramolecular Organogels Based on Dendrons and Dendrimers. Chem. Asian J. 2014, 9, 1724–1750. [Google Scholar]
- Mangunuru, H.P.R.; Yerabolu, J.R.; Wang, G. Synthesis and study of N-acetyl D-glucosamine triazole derivatives as effective low molecular weight gelators. Tetrahedron Lett. 2015, 56, 3361–3364. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Okafor, I.S.; Garcia, C.; Wang, G. Synthesis and self-assembling properties of 4,6-O-benzylidene acetal protected D-glucose and D-glucosamine β-1,2,3-triazole derivatives. Carbohydr. Res. 2018, 461, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Okafor, I.S.; Wang, G. Synthesis and gelation property of a series of disaccharide triazole derivatives. Carbohydr. Res. 2017, 451, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Berg, R.; Straub, B.F. Advancements in the mechanistic understanding of the copper-catalyzed azide-alkyne cycloaddition. Beilstein J. Org. Chem. 2013, 9, 2715–2750. [Google Scholar] [CrossRef] [Green Version]
- Diez-Gonzalez, S. Well-defined copper(i) complexes for Click azide-alkyne cycloaddition reactions. One Click beyond. Catal. Sci. Technol. 2011, 1, 166–178. [Google Scholar]
- Eltepu, L.; Jayaraman, M.; Rajeev, K.G.; Manoharan, M. An immobilized and reusable Cu(I) catalyst for metal ion-free conjugation of ligands to fully deprotected oligonucleotides through click reaction. Chem. Commun. 2013, 49, 184–186. [Google Scholar]
- Chen, H.; Soubra-Ghaoui, C.; Zhu, Z.; Li, S.; Albright, T.A.; Cai, C. Isolation of an acetylide-CuI3-tris(triazolyl methyl)amine complex active in the CuAAC reaction. J. Catal. 2018, 361, 407–413. [Google Scholar] [CrossRef]
- Bong, D.T.; Ghadiri, M.R. Chemoselective Pd(0)-catalyzed peptide coupling in water. Org. Lett. 2001, 3, 2509–2511. [Google Scholar]





| Compound | Tol | EtOH | i-PrOH | n-PrOH | n-BuOH | Glycerol | EtOH: H2O (1:1) | EtOH: H2O (1:2) | DMSO: H2O (1:1) | DMSO: H2O (1:2) | H2O | EG | TG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | GC 4.0 | GT 4.0 | GT 3.3 | GT 3.3 | GT 3.3 | Gp 20.0 | GT 6.7 | GT 5.0 | P | P | P | GC 1.8 | GC 10.0 |
| 10 | I | P | I | GT 3.3 | GT 3.3 | Gc 20.0 | GO 20.0 | GO 2.8 | S | I | I | S | S |
| 11 | S | S | S | S | P | Gp 20.0 | S | S | P | P | P | P | S |
| 12 | I | P | P | P | P | GO 20.0 | P | P | P | GO 10.0 | P | GO 20.0 | GO 20.0 |
| 13 | I | I | I | I | I | I | Gp 5.0 | GO 5.0 | GO 6.7 | GO 6.7 | I | GO 10.0 | GO 20.0 |
| 14 | S | P | P | P | P | S | P | P | P | P | P | P | S |
| 15 | Gc 3.3 | S | GC 4.0 | GC 5.0 | GC 5.0 | Gp 20.0 | S | P | P | P | I | S | S |
| 16 | I | S | GT,P 20.0 | GC 20.0 | GC 20.0 | Gp 20.0 | S | P | S | P | I | S | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bietsch, J.; Chen, A.; Wang, D.; Wang, G. Synthesis of a Series of Trimeric Branched Glycoconjugates and Their Applications for Supramolecular Gels and Catalysis. Molecules 2023, 28, 6056. https://doi.org/10.3390/molecules28166056
Bietsch J, Chen A, Wang D, Wang G. Synthesis of a Series of Trimeric Branched Glycoconjugates and Their Applications for Supramolecular Gels and Catalysis. Molecules. 2023; 28(16):6056. https://doi.org/10.3390/molecules28166056
Chicago/Turabian StyleBietsch, Jonathan, Anji Chen, Dan Wang, and Guijun Wang. 2023. "Synthesis of a Series of Trimeric Branched Glycoconjugates and Their Applications for Supramolecular Gels and Catalysis" Molecules 28, no. 16: 6056. https://doi.org/10.3390/molecules28166056







