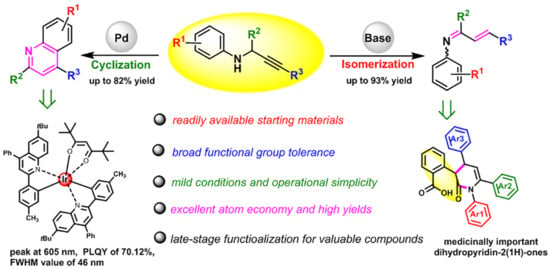
Highly Selective Cyclization and Isomerization of Propargylamines to Access Functionalized Quinolines and 1-Azadienes
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Experimental Section
3.2.1. Synthesis of Propargylamines following Reported Procedures (J. Org. Chem. 2006, 71, 2064–2070; Org. Lett. 2006, 8, 2405–2408; Tetrahedron 2014, 70, 3134–3140)
3.2.2. General Procedure for the Preparation of Quinolines through Palladium-Catalyzed Cyclization
3.2.3. General Procedure for the Preparation of 1-Azadienes via Bu4NOAc-Promoted Isomerization
3.2.4. General Procedure for the Preparation of Ir-2m
3.2.5. General Procedure for the Preparation of Dihydropyridin-2(1H)-Ones via Cycloaddition Reaction with 1-Azadienes and Homophthalic Anhydride
3.3. Characterization of Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Synthesis and Reactivity of Propargylamines in Organic Chemistry. Chem. Rev. 2017, 117, 14091–14200. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Li, C.-J. Modern Alkyne Chemistry; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Trost, B.M.; Lumb, J.-P.; Azzarelli, J.M. An Atom-Economic Synthesis of Nitrogen Heterocycles from Alkynes. J. Am. Chem. Soc. 2011, 133, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.-K.; Wu, Z.-G.; Wang, Y.; Pan, Y. Solvent controlled radical cyclization of propargylamines for multi-iodinated quinoline formation. Org. Biomol. Chem. 2017, 15, 6901–6904. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sun, S.; Xia, M.; Gu, N.; Cheng, J. Copper-catalyzed radical 1,2-cyclization of indoles with arylsulfonyl hydrazides: Access to 2-thiolated 3H-pyrrolo [1,2-a] indoles. Org. Chem. Front. 2017, 4, 2153–2155. [Google Scholar] [CrossRef]
- Sheng, X.; Chen, K.; Shi, C.; Huang, D. Recent Advances in Reactions of Propargylamines. Synthesis 2020, 52, 1–20. [Google Scholar] [CrossRef]
- Sadamitsu, Y.; Saito, K.; Yamada, T. Stereoselective amination via vinyl-silver intermediates derived from silver-catalyzed carboxylative cyclization of propargylamine. Chem. Commun. 2020, 56, 9517–9520. [Google Scholar] [CrossRef]
- Han, L.; Li, S.-J.; Zhang, X.-T.; Tian, S.-K. Aromatic Aza-Claisen Rearrangement of Arylpropargylammonium Salts Generated in situ from Arynes and Tertiary Propargylamines. Chem. Eur. J. 2021, 27, 3091–3097. [Google Scholar] [CrossRef]
- Budi, H.S.; Mustafa, Y.F.; Al-Hamdani, M.M.; Surendar, A.; Ramezani, M. Synthesis of heterocycles from propargylamines. Synth. Commun. 2021, 51, 3694–3716. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, L.; Qiu, K.; Liu, B.; Li, H.; Yu, F. Enantioselective Peroxidation of C-alkynyl imines enabled by chiral BINOL calcium phosphate. Chem. Commun. 2022, 58, 3035–3038. [Google Scholar] [CrossRef]
- He, X.; Li, R.; Choy, P.Y.; Duan, J.; Yin, Z.; Xu, K.; Tang, Q.; Zhong, R.-L.; Shang, Y.; Kwong, F.Y. An expeditious FeCl3-catalyzed cascade 1,4-conjugate addition/annulation/1,5-H shift sequence for modular access of all-pyrano-moiety-substituted chromenes. Chem. Sci. 2022, 13, 13617–13622. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.-F.; Tan, S.-Z.; Ouyang, Q.; Chen, Z.-C.; Du, W.; Chen, Y.-C. Formal nucleophilic pyrrolylmethylation via palladium-based auto-tandem catalysis: Switchable regiodivergent synthesis and remote chirality transfer. Chem. Sci. 2022, 13, 12433–12439. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-G.; Liang, X.; Zhou, J.; Yu, L.; Wang, Y.; Zheng, Y.-X.; Li, Y.-F.; Zuo, J.-L.; Pan, Y. Photocatalyzed cascade oxidative annulation of propargylamines and phosphine oxides. Chem. Commun. 2017, 53, 6637–6640. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.S.; Nallapati, S.B.; Sri Saranya, P.S.V.K.; Sridhar, B.; Giliyaru, V.B.; Hariharapura, R.C.; Shenoy, G.G.; Pal, M. Propargylamine (secondary) as a building block in indole synthesis involving ultrasound assisted Pd/Cu-catalyzed coupling-cyclization method: Unexpected formation of (pyrazole) imine derivatives. Tetrahedron Lett. 2018, 59, 4587–4592. [Google Scholar] [CrossRef]
- Shen, J.; Zhao, J.; Hu, B.; Chen, Y.; Wu, L.; You, Q.; Zhao, L. Base-catalysed [3 + 2] cycloaddition of propargylamines and aldehydes to substituted furans. Green Chem. 2018, 20, 600–603. [Google Scholar] [CrossRef]
- Lo, V.K.-Y.; Chan, Y.-M.; Zhou, D.; Toy, P.H.; Che, C.-M. Highly Enantioselective Synthesis Using Prolinol as a Chiral Auxiliary: Silver-Mediated Synthesis of Axially Chiral Vinylallenes and Subsequent (Hetero)-Diels–Alder Reactions. Org. Lett. 2019, 21, 7717–7721. [Google Scholar] [CrossRef]
- Jiang, X.-L.; Jiao, Y.-E.; Hou, S.-L.; Geng, L.-C.; Wang, H.-Z.; Zhao, B. Green Conversion of CO2 and Propargylamines Triggered by Triply Synergistic Catalytic Effects in Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2021, 60, 20417–20423. [Google Scholar] [CrossRef] [PubMed]
- Holsbeeck, K.V.; Elsocht, M.; Ballet, S. Propargylamine Amino Acids as Constrained Nε-Substituted Lysine Mimetics. Org. Lett. 2023, 25, 130–133. [Google Scholar] [CrossRef]
- Schmidt, E.Y.; Bidusenko, I.A.; Protsuk, N.I.; Demyanov, Y.V.; Ushakov, I.A.; Vashchenko, A.V.; Trofimov, B.A. Transition-Metal-Free Superbase-Catalyzed C–H Vinylation of Aldimines with Acetylenes to 1-Azadienes. J. Org. Chem. 2020, 85, 3417–3425. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, Y.; Liu, Y.; Wang, J. Sequential catalytic process: Synthesis of quinoline derivatives by AuCl3/CuBr-catalyzed three-component reaction of aldehydes, amines, and alkynes. Tetrahedron 2008, 64, 2755–2761. [Google Scholar] [CrossRef]
- Zhu, M.; Fu, W.; Xun, C.; Zou, G. An Efficient Synthesis of Substituted Quinolines via Indium (III) Chloride Catalyzed Reaction of Imines with Alkynes. Bull. Korean Chem. Soc. 2012, 33, 43–47. [Google Scholar] [CrossRef]
- Cao, K.; Zhang, F.-M.; Tu, Y.-Q.; Zhuo, X.-T.; Fan, C.-A. Iron (III)-Catalyzed and Air-Mediated Tandem Reaction of Aldehydes, Alkynes and Amines: An Efficient Approach to Substituted Quinolines. Chem. Eur. J. 2009, 15, 6332–6334. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, N.; Suresh, P. Iron-MOF-Catalyzed Domino Cyclization and Aromatization Strategy for the Synthesis of 2,4-Diarylquinolines. Asian. J. Org. Chem. 2020, 9, 437–444. [Google Scholar] [CrossRef]
- Cho, H.; Torok, F.; Torok, B. Energy efficiency of heterogeneous catalytic microwave-assisted organic reactions. Green Chem. 2014, 16, 3623–3634. [Google Scholar] [CrossRef]
- Jiang, K.-M.; Kang, J.-A.; Jin, Y.; Lin, J. Synthesis of substituted 4-hydroxyalkyl-quinoline derivatives by a three-component reaction using CuCl/AuCl as sequential catalysts. Org. Chem. Front. 2018, 5, 434–441. [Google Scholar] [CrossRef]
- McNulty, J.; Vemula, R.; Bordón, C.; Yolken, R.; Jones-Brando, L. Synthesis and anti-toxoplasmosis activity of 4-arylquinoline-2-carboxylate derivatives. Org. Biomol. Chem. 2014, 12, 255–260. [Google Scholar] [CrossRef]
- Zhu, M.; Fu, W.; Zou, G.; Xun, C.; Deng, D.; Ji, B. An efficient synthesis of 2-trifluoromethyl quinolines via gold-catalyzed cyclization of trifluoromethylated propargylamines. J. Fluor. Chem. 2012, 135, 195–199. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, T.; Campo, M.A.; Larock, R.C. Synthesis of substituted quinolines by the electrophilic cyclization of n-(2-alkynyl) anilines. Tetrahedron 2010, 66, 1177–1187. [Google Scholar] [CrossRef]
- Su, Y.; Lu, M.; Dong, B.; Chen, H.; Shi, X. Silver-Catalyzed Alkyne Activation: The Surprising Ligand Effect. Adv. Synth. Cat. 2014, 356, 692–696. [Google Scholar] [CrossRef]
- Kuninobu, Y.; Inoue, Y.; Takai, K. Copper (I)- and Gold (I)-catalyzed Synthesis of 2,4-Disubstituted Quinoline Derivatives from N-Aryl-2-propynylamines. Chem. Lett. 2007, 36, 1422–1423. [Google Scholar] [CrossRef]
- Wei, C.; Li, C.-J. Enantioselective Direct-Addition of Terminal Alkynes to Imines Catalyzed by Copper (I) pybox Complex in Water and in Toluene. J. Am. Chem. Soc. 2002, 124, 5638–5639. [Google Scholar] [CrossRef]
- Bisai, A.; Singh, V.K. Enantioselective one-pot three-component synthesis of propargylamines. Org. Lett. 2006, 8, 2405–2408. [Google Scholar] [CrossRef]
- Ghosh, S.; Biswas, K. Metal-free multicomponent approach for the synthesis of propargylamine: A review. RSC Adv. 2021, 11, 2047–2065. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-R.; Seo, J.-S.; Ahn, Y.; Lee, J.-H.; Suh, M.C. Thermally stable benzo [f] quinoline based bipolar host materials for green phosphorescent OLEDs. Org. Electron. 2018, 63, 194–199. [Google Scholar] [CrossRef]
- Yu, F.; Liu, Q.; Sheng, Y.; Chen, Y.; Zhang, Y.; Sun, Z.; Zhang, C.; Xia, Q.; Li, H.; Hang, X.-C.; et al. Solution-Processable Csp3-Annulated Hosts for High-Efficiency Deep Red Phosphorescent OLEDs. ACS Appl. Mater. Interfaces 2020, 12, 33960–33967. [Google Scholar] [CrossRef]
- Lou, S.-J.; Zhang, L.; Luo, Y.; Nishiura, M.; Luo, G.; Luo, Y.; Hou, Z. Regiodivergent C–H Alkylation of Quinolines with Alkenes by Half-Sandwich Rare-Earth Catalysts. J. Am. Chem. Soc. 2020, 142, 18128–18137. [Google Scholar] [CrossRef] [PubMed]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef] [PubMed]
- Attygalle, A.B.; Hearth, K.B.; Iyengar, V.K.; Morgan, R.C. Biosynthesis of Quinoline by a Stick Insect. J. Nat. Prod. 2021, 84, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Mao, W.; Jia, H.; Sun, J.; Lu, G.; Jiang, H.; Zhang, M. Reductive electrophilic C–H alkylation of quinolines by a reusable iridium nanocatalyst. Chem. Sci. 2021, 12, 13802–13808. [Google Scholar] [CrossRef]
- Wang, G.; Jia, J.; Liu, G.; Yu, M.; Chu, X.; Liu, X.; Zhao, X. Copper (i)-catalyzed tandem synthesis of 2-acylquinolines from 2-ethynylanilines and glyoxals. Chem. Commun. 2021, 57, 11811–11814. [Google Scholar] [CrossRef]
- Jayakumar, S.; Ishar, M.P.S.; Mahajan, M.P. Recent advances in synthetic applications of azadienes. Tetrahedron 2002, 58, 379–471. [Google Scholar] [CrossRef]
- Groenendaal, B.; Ruijtera, E.; Orru, R.V.A. 1-Azadienes in cycloaddition and multicomponent reactions towards N-heterocycles. Chem. Commun. 2008, 43, 5474–5489. [Google Scholar] [CrossRef] [PubMed]
- Monbaliu, J.-C.M.; Masscheleina, K.G.R.; Stevens, C.V. Electron-deficient 1- and 2-azabuta-1,3-dienes: A comprehensive survey of their synthesis and reactivity. Chem. Soc. Rev. 2011, 40, 4708–4739. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Xu, Z.; Yu, S.; Wu, Q.; Zhu, D. Imine Reductase-Catalyzed Enantioselective Reduction of Bulky α,β-Unsaturated Imines en Route to a Pharmaceutically Important Morphinan Skeleton. Adv. Synth. Catal. 2019, 361, 556–561. [Google Scholar] [CrossRef]
- Li, T.-Y.; Wu, J.; Wu, Z.-G.; Zheng, Y.-X.; Zuo, J.-L.; Pan, Y. Rational design of phosphorescent iridium (III) complexes for emission color tunability and their applications in OLEDs. Coord. Chem. Rev. 2018, 374, 55–92. [Google Scholar] [CrossRef]
- Ou, C.; Qiu, Y.-C.; Cao, C.; Zhang, H.; Qin, J.; Tu, Z.-L.; Shi, J.; Wu, Z.-G. Modulating the peripheral large steric hindrance of iridium complexes for achieving narrowband emission and pure red OLEDs with an EQE up to 32.0%. Inorg. Chem. Front. 2023, 10, 1018–1026. [Google Scholar] [CrossRef]
- Nantermet, P.G.; Barrow, J.C.; Selnick, H.G.; Homnick, C.F.; Freidinger, R.M.; Chang, R.S.L.; O’Malley, S.S.; Reiss, D.R.; Broten, T.P.; Ransom, R.W.; et al. Selective α1a Adrenergic Receptor Antagonists Based on 4-Aryl-3,4-dihydropyridine-2-ones. Bioorg. Med. Chem. Lett. 2000, 10, 1625–1628. [Google Scholar] [CrossRef] [PubMed]
- Guranova, N.; Golubev, P.; Bakulina, O.; Dar’in, D.; Kantin, G.; Krasavin, M. Unexpected formal [4 + 2]-cycloaddition of chalcone imines and homophthalic anhydrides: Preparation of dihydropyridin-2 (1H)-ones. Org. Biomol. Chem. 2021, 19, 3829–3833. [Google Scholar] [CrossRef]





| Entry | Additive (Catalyst/Base) | Solvent | Yield b |
|---|---|---|---|
| 1 | Pd(dppf)Cl2 | DMF | 20%/0 |
| 2 | Pd(PPh3)2Cl2 | DMF | 10%/0 |
| 3 | PdCl2 | DMF | 31%/0 |
| 4 | Pd(OAc)2 | DMF | 65%/0 |
| 5 | Pd(TFA)2 | DMF | 45%/0 |
| 6 | CuCl | DMF | trace/0 |
| 7 | Cu(OAc)2 | DMF | 15%/0 |
| 8 | Fe(OTf)3 | DMF | NR/0 |
| 9 | Ni(acac)2 | DMF | trace/0 |
| 10 | Pd(OAc)2 | DMSO | 45%/0 |
| 11 | Pd(OAc)2 | NMP | 60%/0 |
| 12 | Pd(OAc)2 | DCE | 68%/0 |
| 13 | Pd(OAc)2 | dioxane | 25%/0 |
| 14 | Pd(OAc)2 | CH3CN | 58%/0 |
| 15 | Pd(OAc)2 | toluene | 80%/0 |
| 16 | Pd(OAc)2/TBAI | toluene | 20%/25% |
| 17 | Pd(OAc)2/KOAc | toluene | 41%/trace |
| 18 | Pd(OAc)2/Na2CO3 | toluene | 34%/trace |
| 19 | Pd(OAc)2/Cs2CO3 | toluene | 15%/30% |
| 20 | Cs2CO3 | toluene | 0/31% |
| 21 | Cs2CO3 | DCE | 0/11% |
| 22 | Cs2CO3 | dioxane | 0/35% |
| 23 | Cs2CO3 | CH3CN | 0/81% |
| 24 | Cs2CO3 | DMF | 0/79% |
| 25 | Na2CO3 | CH3CN | 0/21% |
| 26 | K2CO3 | CH3CN | 0/50% |
| 27 | KOAc | CH3CN | 0/40% |
| 28 | Bu4NOAc | CH3CN | 0/91% |
| 29 | DABCO | CH3CN | 0/61% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-G.; Zhang, H.; Cao, C.; Lu, C.; Jiang, A.; He, J.; Zhao, Q.; Tang, Y. Highly Selective Cyclization and Isomerization of Propargylamines to Access Functionalized Quinolines and 1-Azadienes. Molecules 2023, 28, 6259. https://doi.org/10.3390/molecules28176259
Wu Z-G, Zhang H, Cao C, Lu C, Jiang A, He J, Zhao Q, Tang Y. Highly Selective Cyclization and Isomerization of Propargylamines to Access Functionalized Quinolines and 1-Azadienes. Molecules. 2023; 28(17):6259. https://doi.org/10.3390/molecules28176259
Chicago/Turabian StyleWu, Zheng-Guang, Hui Zhang, Chenhui Cao, Chaowu Lu, Aiwei Jiang, Jie He, Qin Zhao, and Yanfeng Tang. 2023. "Highly Selective Cyclization and Isomerization of Propargylamines to Access Functionalized Quinolines and 1-Azadienes" Molecules 28, no. 17: 6259. https://doi.org/10.3390/molecules28176259
APA StyleWu, Z.-G., Zhang, H., Cao, C., Lu, C., Jiang, A., He, J., Zhao, Q., & Tang, Y. (2023). Highly Selective Cyclization and Isomerization of Propargylamines to Access Functionalized Quinolines and 1-Azadienes. Molecules, 28(17), 6259. https://doi.org/10.3390/molecules28176259