High-Performance Ag2Se Film by a Microwave-Assisted Synthesis Method for Flexible Thermoelectric Generators
Abstract
:1. Introduction
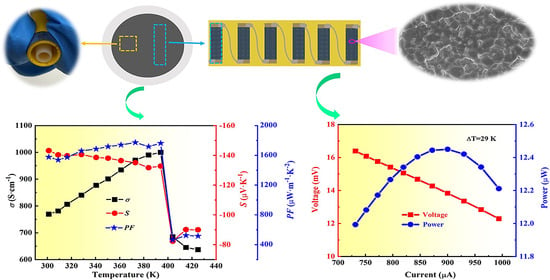
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, X.; Cai, K.; Gao, M.; Du, Y.; Shen, S. Recent advances in flexible thermoelectric films and devices. Nano Energy 2021, 89, 106309. [Google Scholar] [CrossRef]
- Cao, T.; Shi, X.-L.; Chen, Z.-G. Advances in the design and assembly of flexible thermoelectric device. Prog. Mater. Sci. 2023, 131, 101003. [Google Scholar] [CrossRef]
- Yang, S.; Qiu, P.; Chen, L.; Shi, X. Recent Developments in Flexible Thermoelectric Devices. Small Sci. 2021, 1, 2100005. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, X.-L.; Yang, Y.-L.; Chen, Z.-G. Flexible thermoelectric materials and devices: From materials to applications. Mater. Today 2021, 46, 62–108. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, Z.; Sun, Z.; Zhang, Q.; Wei, P.; Mu, X.; Zhou, H.; Li, C.; Ma, S.; He, D.; et al. Superparamagnetic enhancement of thermoelectric performance. Nature 2017, 549, 247–251. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Shi, X.-L.; Shi, X.; Chen, L.; Dargusch, M.S.; Zou, J.; Chen, Z.-G. Flexible Thermoelectric Materials and Generators: Challenges and Innovations. Adv. Mater. 2019, 31, 1807916. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Li, P.; Liu, Y.; He, C. Recent Advances in Polyaniline-Based Thermoelectric Composites. CCS Chem. 2021, 3, 2547–2560. [Google Scholar] [CrossRef]
- Song, H.; Meng, Q.; Lu, Y.; Cai, K. Progress on PEDOT:PSS/Nanocrystal Thermoelectric Composites. Adv. Electron. Mater. 2019, 5, 1800822. [Google Scholar] [CrossRef]
- Xu, S.; Shi, X.-L.; Dargusch, M.; Di, C.; Zou, J.; Chen, Z.-G. Conducting polymer-based flexible thermoelectric materials and devices: From mechanisms to applications. Prog. Mater. Sci. 2021, 121, 100840. [Google Scholar] [CrossRef]
- Zheng, Z.-H.; Shi, X.-L.; Ao, D.-W.; Liu, W.-D.; Li, M.; Kou, L.-Z.; Chen, Y.-X.; Li, F.; Wei, M.; Liang, G.-X.; et al. Harvesting waste heat with flexible Bi2Te3 thermoelectric thin film. Nat. Sustain. 2023, 6, 180–191. [Google Scholar] [CrossRef]
- Ferhat, M.; Nagao, J. Thermoelectric and transport properties of β-Ag2Se compounds. J. Appl. Phys. 2000, 88, 813–816. [Google Scholar] [CrossRef]
- Lee, C.; Park, Y.-H.; Hashimoto, H. Effect of nonstoichiometry on the thermoelectric properties of a Ag2Se alloy prepared by a mechanical alloying process. J. Appl. Phys. 2007, 101, 024920. [Google Scholar] [CrossRef]
- Wang, P.; Chen, J.-L.; Zhou, Q.; Liao, Y.T.; Peng, Y.; Liang, J.S.; Miao, L. Enhancing the thermoelectric performance of Ag2Se by non-stoichiometric defects. Appl. Phys. Lett. 2022, 120, 193902. [Google Scholar] [CrossRef]
- Wang, H.; Han, G.; Zhang, B.; Chen, Y.; Liu, X.; Zhang, K.; Lu, X.; Wang, G.; Zhou, X. AgSbSe2 inclusions enabling high thermoelectric and mechanical performance in n-type Ag2Se-based composites. Acta Mater. 2023, 248, 118753. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Q.; Bao, D.; Tian, B.-Z.; Wang, Z.; Tang, J.; Zhou, D.; Yang, L.; Chen, Z.-G. Simultaneously enhanced strength and plasticity of Ag2Se-based thermoelectric materials endowed by nano-twinned CuAgSe secondary phase. Acta Mater. 2021, 220, 117335. [Google Scholar] [CrossRef]
- Tee, S.Y.; Tan, X.Y.; Wang, X.; Lee, C.J.J.; Win, K.Y.; Ni, X.P.; Teo, S.L.; Seng, D.H.L.; Tanaka, Y.; Han, M.-Y. Aqueous Synthesis, Doping, and Processing of n-Type Ag2Se for High Thermoelectric Performance at Near-Room-Temperature. Inorg. Chem. 2022, 61, 6451–6458. [Google Scholar] [CrossRef]
- Jood, P.; Ohta, M. Temperature-Dependent Structural Variation and Cu Substitution in Thermoelectric Silver Selenide. ACS Appl. Energy Mater. 2020, 3, 2160–2167. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Z.; Sun, C.; Deng, H.; Fu, Q. Biomimetic Approach to Facilitate the High Filler Content in Free-Standing and Flexible Thermoelectric Polymer Composite Films Based on PVDF and Ag2Se Nanowires. ACS Appl. Mater. Inter. 2020, 12, 51506–51516. [Google Scholar] [CrossRef]
- Ding, Y.; Qiu, Y.; Cai, K.; Yao, Q.; Chen, S.; Chen, L.; He, J. High performance n-type Ag2Se film on nylon membrane for flexible thermoelectric power generator. Nat. Commun. 2019, 10, 841. [Google Scholar] [CrossRef]
- Lei, Y.; Qi, R.; Chen, M.; Chen, H.; Xing, C.; Sui, F.; Gu, L.; He, W.; Zhang, Y.; Baba, T.; et al. Microstructurally Tailored Thin β-Ag2Se Films toward Commercial Flexible Thermoelectrics. Adv. Mater. 2022, 34, e2104786. [Google Scholar] [CrossRef]
- Gao, J.; Miao, L.; Lai, H.; Zhu, S.; Peng, Y.; Wang, X.; Koumoto, K.; Cai, H. Thermoelectric Flexible Silver Selenide Films: Compositional and Length Optimization. iScience 2020, 23, 100753. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-H.; Li, Y.-L.; Niu, J.-Y.; Wei, M.; Zhang, D.-L.; Zhong, Y.-m.; Nisar, M.; Abbas, A.; Chen, S.; Li, F.; et al. Significantly (00l)-textured Ag2Se thin films with excellent thermoelectric performance for flexible power applications. J. Mater. Chem. A 2022, 10, 21603–21610. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Y.; Wang, Z.; Li, J.; Wei, P.; Zhao, W.; Chen, L.; Cai, K. High performance Ag2Se films by a one-pot method for a flexible thermoelectric generator. J. Mater. Chem. A 2022, 10, 25644–25651. [Google Scholar] [CrossRef]
- Jiang, C.; Ding, Y.; Cai, K.; Tong, L.; Lu, Y.; Zhao, W.; Wei, P. Ultrahigh Performance of n-Type Ag2Se Films for Flexible Thermoelectric Power Generators. ACS Appl. Mater. Int. 2020, 12, 9646–9655. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, Y.; Cai, K.; Ding, Y.; Wang, M.; Jiang, C.; Yao, Q.; Huang, C.; Chen, L.; He, J. Ultrahigh power factor and flexible silver selenide-based composite film for thermoelectric devices. Energy Environ. Sci. 2020, 13, 1240–1249. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, Y.; Cai, K.; Li, X.; Gao, M.; Jiang, C.; He, J. Ultrahigh performance PEDOT/Ag2Se/CuAgSe composite film for wearable thermoelectric power generators. Mater. Today Phys. 2020, 14, 100223. [Google Scholar] [CrossRef]
- Jiang, C.; Wei, P.; Ding, Y.; Cai, K.; Tong, L.; Gao, Q.; Lu, Y.; Zhao, W.; Chen, S. Ultrahigh performance polyvinylpyrrolidone/Ag2Se composite thermoelectric film for flexible energy harvesting. Nano Energy 2021, 80, 105488. [Google Scholar] [CrossRef]
- Li, Y.; Lou, Q.; Yang, J.; Cai, K.; Liu, Y.; Lu, Y.; Qiu, Y.; Lu, Y.; Wang, Z.; Wu, M.; et al. Exceptionally High Power Factor Ag2Se/Se/Polypyrrole Composite Films for Flexible Thermoelectric Generators. Adv. Funct. Mater. 2021, 32, 2106902. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, Q.; Wang, W.; Lu, Y.; Cai, K.; Li, Y.; Wu, M.; He, J. High performance Ag2Se/Ag/PEDOT composite films for wearable thermoelectric power generators. Mater. Today Phys. 2021, 21, 100553. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, W.; Lu, Y.; Cai, K.; Li, Y.; Wang, Z.; Wu, M.; Huang, C.; He, J. High Power Factor Ag/Ag2Se Composite Films for Flexible Thermoelectric Generators. ACS Appl. Mater. Inter. 2021, 13, 14327–14333. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Li, Y.; Cai, K. The influence of Ga doping on preparation and thermoelectric properties of flexible Ag2Se films. Compos. Commun. 2021, 27, 100895. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Cai, K.; Gao, M.; Li, Y.; Wang, Z.; Wu, M.; Wei, P.; Zhao, W.; Du, Y.; et al. Exceptional power factor of flexible Ag/Ag2Se thermoelectric composite films. Chem. Eng. J. 2022, 434, 134739. [Google Scholar] [CrossRef]
- Chen, D.; Tang, K.; Shen, G.; Sheng, J.; Fang, Z.; Liu, X.; Zheng, H.; Qian, Y. Microwave-assisted synthesis of metal sulfides in ethylene glycol. Mater. Chem. Phys. 2003, 82, 206–209. [Google Scholar] [CrossRef]
- Ni, D.; Chen, Y.; Yang, X.; Liu, C.; Cai, K. Microwave-assisted synthesis method for rapid synthesis of tin selenide electrode material for supercapacitors. J. Alloys Compd. 2018, 737, 623–629. [Google Scholar] [CrossRef]
- Pei, J.; Chen, G.; Jia, D.; Jin, R.; Xu, H.; Chen, D. Rapid synthesis of Ag2Se dendrites with enhanced electrical performance by microwave-assisted solution method. N. J. Chem. 2013, 37, 323–328. [Google Scholar] [CrossRef]
- Kojda, D.; Mitdank, R.; Handwerg, M.; Mogilatenko, A.; Albrecht, M.; Wang, Z.; Ruhhammer, J.; Kroener, M.; Woias, P.; Fischer, S.F. Temperature-dependent thermoelectric properties of individual silver nanowires. Phys. Rev. B 2015, 91, 024302. [Google Scholar] [CrossRef]
- Kishimoto, S.; Hasegawa, T.; Kinto, H.; Matsumoto, O.; Iida, S. Effect and comparison of co-doping of Ag, Ag+In, and Ag+Cl in ZnS: N/GaAs layers prepared by vapor-phase epitaxy. J. Cryst. Growth 2000, 214/215, 556–561. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liu, Y.; Li, J.; Huang, C.; Cai, K. High-Performance Ag2Se Film by a Microwave-Assisted Synthesis Method for Flexible Thermoelectric Generators. Molecules 2023, 28, 6397. https://doi.org/10.3390/molecules28176397
Wang Z, Liu Y, Li J, Huang C, Cai K. High-Performance Ag2Se Film by a Microwave-Assisted Synthesis Method for Flexible Thermoelectric Generators. Molecules. 2023; 28(17):6397. https://doi.org/10.3390/molecules28176397
Chicago/Turabian StyleWang, Zixing, Ying Liu, Jiajia Li, Changjun Huang, and Kefeng Cai. 2023. "High-Performance Ag2Se Film by a Microwave-Assisted Synthesis Method for Flexible Thermoelectric Generators" Molecules 28, no. 17: 6397. https://doi.org/10.3390/molecules28176397