Biocatalysis in Water or in Non-Conventional Media? Adding the CO2 Production for the Debate
Abstract
:1. Introduction
2. Results and Discussion
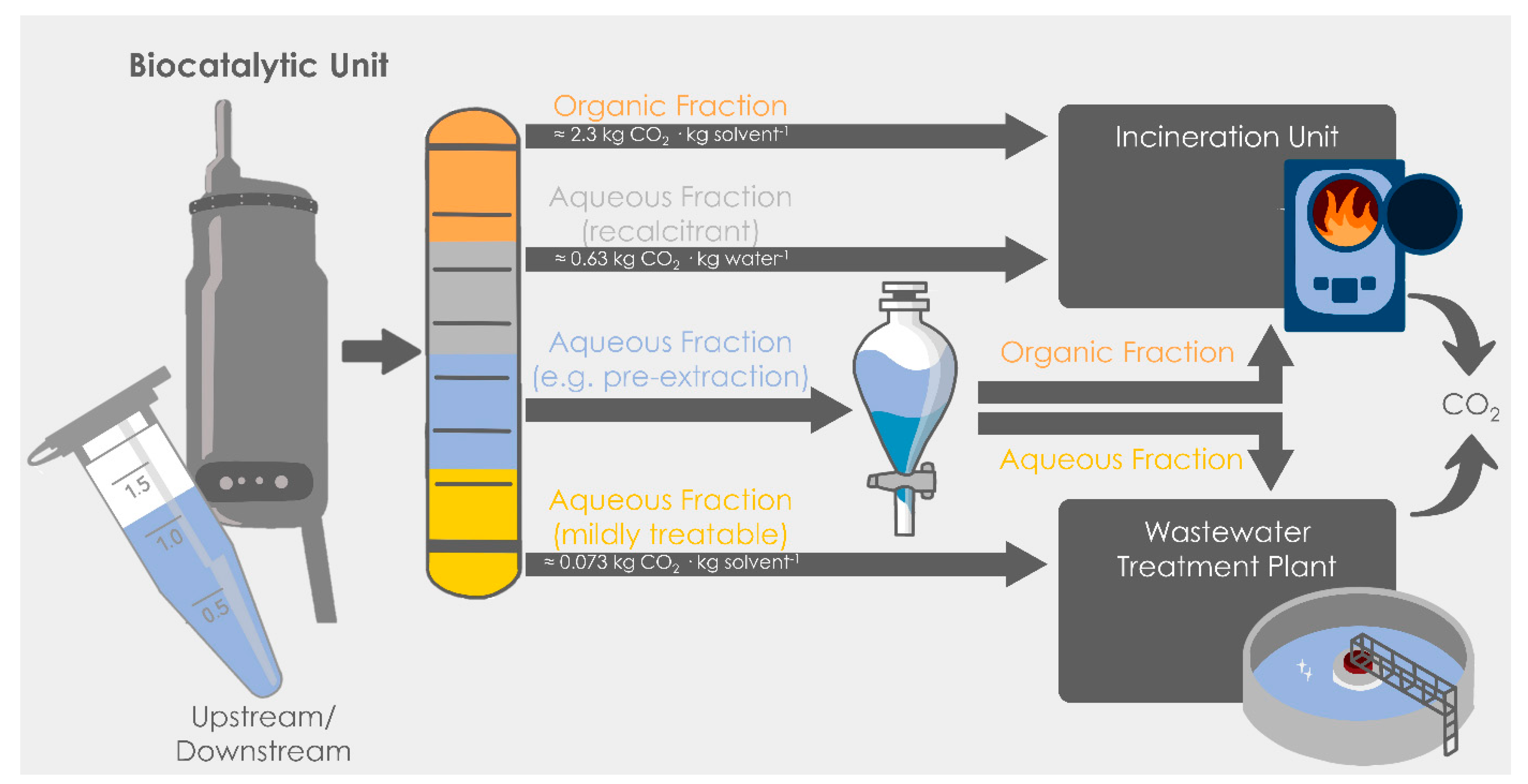
2.1. Using the Same Currency: Converting Waste Effluents into Carbon Dioxide Production
2.2. The Water Hazard Class Concept (WGK)
2.3. Metrics Based on CO2 for Different Scenarios
3. Materials and Methods
Calculation of the TCR
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M. The atom economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, D.; Hollmann, F.; Bouchaut, B. Contribution of enzyme catalysis to the achievement of the United Nation’s Sustainable Development Goals. Molecules 2023, 28, 4125. [Google Scholar] [CrossRef]
- Alcántara, A.R.; Domínguez de María, P.; Littlechild, J.; Schürmann, M.; Sheldon, R.; Wohlgemuth, R. Biocatalysis as key to sustainable industrial chemistry. ChemSusChem 2022, 15, e202102709. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of green chemistry and sustainability: Past, present and future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E-factor at 30: Passion for pollution prevention. Green Chem. 2023, 25, 1704–1728. [Google Scholar] [CrossRef]
- Domínguez de María, P.; Hollmann, F. On the (un)greenness of biocatalysis: Some challenging figures and some promising options. Front. Microbiol. 2015, 6, 1257. [Google Scholar] [CrossRef]
- Domínguez de María, P. Biocatalysis, sustainability and industrial applications: Show me the metrics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100514. [Google Scholar] [CrossRef]
- Ni, Y.; Holtmann, D.; Hollmann, F. How green is biocatalysis? To calculate is to know. ChemCatChem 2014, 6, 930–943. [Google Scholar] [CrossRef]
- Tieves, F.; Tonin, F.; Fernández-Fueyo, E.; Robbins, J.M.; Bommarius, B.; Bommarius, A.S.; Alcalde, M.; Hollmann, F. Energising the E-Factor: The E+-factor. Tetrahedron 2019, 75, 1311–1314. [Google Scholar] [CrossRef]
- Tulus, V.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. Planetary metrics for the absolute environmental sustainability of chemicals. Green. Chem. 2021, 23, 9881–9893. [Google Scholar] [CrossRef]
- Galán-Martín, A.; Tulus, V.; Díaz, I.; Pozo, C.; Pérez-Ramírez, J.; Guillén-Gosálbez, G. Sustainability footprints of a renewable carbon transition for the petrochemical sector within planetary boundaries. One Earth 2021, 4, 565–583. [Google Scholar] [CrossRef]
- García Calvo-Flores, F. Sustainable Chemistry Metrics. ChemSusChem 2009, 2, 905–919. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of Green Chemistry: Waste minimization. Curr. Opin. Green Sustain. Chem. 2022, 33, 100569. [Google Scholar] [CrossRef]
- Onken, U.; Koettgen, A.; Scheidat, H.; Schuepp, P.; Gallou, F. Environmental metrics to drive a cultural change: Our green eco-label. Chimia 2019, 73, 730–736. [Google Scholar] [CrossRef]
- Domínguez de María, P. On the need for gate-to-gate environmental metrics in biocatalysis: Fatty acid hydration catalyzed by oleate hydratases as a case study. Green Chem. 2022, 24, 9620–9628. [Google Scholar] [CrossRef]
- Domínguez de María, P. Green solvents and biocatalysis: A bigger picture. EFB Bioeconomy J. 2023, 3, 100056. [Google Scholar] [CrossRef]
- van Schie, M.; Spöring, J.D.; Bocola, M.; Domínguez de María, P.; Rother, D. Applied biocatalysis beyond just buffers—From aqueous to unconventional media. Options and guidelines. Green Chem. 2021, 23, 3191–3206. [Google Scholar] [CrossRef] [PubMed]
- Gallou, F.; Gröger, H.; Lipshutz, B.H. Status check: Biocatalysis; it’s use with and without chemocatalysis. How does the fine chemicals industry view this area? Green Chem. 2023, 25, 6092–6107. [Google Scholar] [CrossRef]
- Gröger, H.; Gallou, F.; Lipshutz, B.H. Where chemocatalysis meets biocatalysis: In water. Chem. Rev. 2023, 123, 5262–5296. [Google Scholar] [CrossRef]
- Lipshutz, B. Nanomicelle-enabled chemoenzymatic catalysis: Clean chemistry in “dirty” water. Chem. Catal. 2023, 3, 100458. [Google Scholar] [CrossRef]
- Cortes-Clerget, M.; Yu, J.; Kincaid, J.R.A.; Walde, P.; Gallou, F.; Lipshutz, B. Water as the reaction medium in organic chemistry: From our worst enemy to our best friend. Chem. Sci. 2021, 12, 4237–4266. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, J.R.A.; Wong, M.J.; Akporji, N.; Gallou, F.; Fialho, D.M.; Lipscutz, B.H. Introducing Savie: A biodegradable surfactant enabling chemo- and biocatalysis and related reactions in recyclable water. J. Am. Chem. Soc. 2023, 145, 4266–4278. [Google Scholar] [CrossRef]
- Holtmann, D.; Hollmann, F. Is water the best solvent for biocatalysis? Mol. Catal. 2022, 517, 112035. [Google Scholar] [CrossRef]
- Burek, B.; Dawood, A.; Hollmann, F.; Liese, A.; Holtmann, D. Process intensification as game changer in enzyme catalysis. Front. Catal. 2022, 2, 858706. [Google Scholar] [CrossRef]
- Guajardo, N.; Domínguez de María, P. Continous biocatalysis in environmentally-friendly media: A triple synergy for future sustainable processes. ChemCatChem 2019, 11, 3128–3137. [Google Scholar] [CrossRef]
- Kourist, R.; González-Sabín, J. Non-conventional media as strategy to overcome the solvent dilemma in chemoenzymatic tandem catalysis. ChemCatChem 2020, 12, 1903–1912. [Google Scholar] [CrossRef]
- Krell, C.; Schreiber, R.; Hueber, L.; Sciascera, L.; Zheng, X.; Clarke, Z.; Haenggi, R.; Parmentier, M.; Baguia, H.; Rodde, S.; et al. Strategies to tackle the waste water from α-tocopherol-derived surfactant chemistry. Org. Process Res. Dev. 2021, 25, 900–915. [Google Scholar] [CrossRef]
- Available online: https://webrigoletto.uba.de/rigoletto/public/welcome.do (accessed on 8 August 2023).
- Meyer, L.E.; Hobisch, M.; Kara, S. Process intensification in continuous flow biocatalysis by up and downstream processing strategies. Curr. Opin. Biotechnol. 2022, 78, 102835. [Google Scholar] [CrossRef]
- Crotti, M.; Robescu, M.S.; Bolivar, J.M.; Ubiali, D.; Wilson, L.; Contente, M.L. What´s new in flow biocatalysis? A snapshot of 2020–2022. Front. Catal. 2023, 3, 1154452. [Google Scholar] [CrossRef]
- Gkantzou, E.; Weinhart, M.; Kara, S. 3D printing for flow biocatalysis. RSC Sustain. 2023. advance article. [Google Scholar] [CrossRef]
- Fleck, N.; Roschangar, F.; Haydl, A.M. API syntheses in aqueous media: Assessing the environmental footprint en route from academic discovery to industrial applications as “green opportunity” for process chemistry. Org. Process Res. Dev. 2023, 27, 822–830. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez de María, P.; Kara, S.; Gallou, F. Biocatalysis in Water or in Non-Conventional Media? Adding the CO2 Production for the Debate. Molecules 2023, 28, 6452. https://doi.org/10.3390/molecules28186452
Domínguez de María P, Kara S, Gallou F. Biocatalysis in Water or in Non-Conventional Media? Adding the CO2 Production for the Debate. Molecules. 2023; 28(18):6452. https://doi.org/10.3390/molecules28186452
Chicago/Turabian StyleDomínguez de María, Pablo, Selin Kara, and Fabrice Gallou. 2023. "Biocatalysis in Water or in Non-Conventional Media? Adding the CO2 Production for the Debate" Molecules 28, no. 18: 6452. https://doi.org/10.3390/molecules28186452



