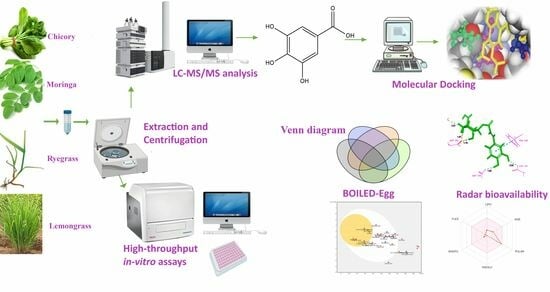
Optimized Extraction of Polyphenols from Unconventional Edible Plants: LC-MS/MS Profiling of Polyphenols, Biological Functions, Molecular Docking, and Pharmacokinetics Study
Abstract
:1. Introduction
2. Results and Discussions
2.1. Extraction Process Optimization for Total Phenolic Content in Selected Unconventional Edible Plants
2.2. Quantification of Total Polyphenols in Selected Plant Extracts
2.3. Antioxidant and Antidiabetic Activities of Selected Plant Extracts
2.4. Correlation Analysis
2.5. Characterization of Phenolic Compounds Using LC-MS
2.5.1. Phenolic Acids
2.5.2. Flavonoids
Flavonols
2.5.3. Isoflavonoids
2.5.4. Stilbenes and Lignans
2.5.5. Other Compounds
2.6. Venn Distribution of Polyphenols in Plant Extracts
2.7. Quantification/Semiquantification of Selected Phenolic Compounds
2.8. In Silico Molecular Docking of Phenolic Compounds
2.9. ADMET Properties of Abundant Phenolic Compounds
2.9.1. Absorption and Distribution
2.9.2. Metabolism, Excretion, and Toxicity
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation and Method Optimization for Extraction of Phenolic Compounds
3.3. Total Phenolic Content and Antioxidant Potential
3.3.1. Determination of Total Phenolic Content
3.3.2. Total Flavonoid Content
3.3.3. Total Tannin Content
3.4. Antioxidant Activities of Edible Plants
3.4.1. ABTS Radical Scavenging Assay
3.4.2. DPPH Radical Scavenging Assay
3.4.3. Hydroxyl Radical Scavenging Assay
3.4.4. Fe2+ Chelating Activity (FICA)
3.5. Alpha-Glucosidase Inhibition Activity
3.6. LC-MS/MS Characterization of Phenolic Compounds
3.7. In Silico Molecular Docking and Simulated Pharmacokinetics Study of the Most Abundant Phenolic Compounds
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heinrich, M. Quality and safety of herbal medical products: Regulation and the need for quality assurance along the value chains. Br. J. Clin. Pharmacol. 2015, 80, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Poswal, F.S.; Russell, G.; Mackonochie, M.; MacLennan, E.; Adukwu, E.C.; Rolfe, V. Herbal Teas and their Health Benefits: A Scoping Review. Plant Foods Hum. Nutr. 2019, 74, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Kiloni, S.M.; Cáceres-Vélez, P.R.; Jusuf, P.R.; Cottrell, J.J.; Dunshea, F.R. Phytochemicals, Antioxidant Activities, and Toxicological Screening of Native Australian Fruits Using Zebrafish Embryonic Model. Foods 2022, 11, 4038. [Google Scholar] [CrossRef]
- Rodriguez-Casado, A. The Health Potential of Fruits and Vegetables Phytochemicals: Notable Examples. Crit. Rev. Food Sci. Nutr. 2016, 56, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Tahira, R.; Naeemullah, M.; Akbar, F.; Masood, M.S. Major phenolic acids of local and exotic mint germplasm grown in Islamabad. Pak. J. Bot. 2011, 43, 151–154. [Google Scholar]
- Sharopov, F.S.; Sulaimonova, V.A.; Setzer, W.N. Essential oil composition of Mentha longifolia from wild populations growing in Tajikistan. J. Med. Act. Plants 2012, 1, 76–84. [Google Scholar]
- Londonkar, R.L.; Poddar, P.V. Studies on activity of various extracts of Mentha arvensis Linn against drug induced gastric ulcer in mammals. World J. Gastrointest. Oncol. 2009, 1, 82. [Google Scholar] [CrossRef]
- Langella, C.; Naviglio, D.; Marino, M.; Calogero, A.; Gallo, M. New food approaches to reduce and/or eliminate increased gastric acidity related to gastroesophageal pathologies. Nutrition 2018, 54, 26–32. [Google Scholar] [CrossRef]
- Cengher, M.; Strohmeier, C.W. Behavioral Assessment and Treatment of Aerophagia. Clin. Case Stud. 2021, 21, 15346501211064584. [Google Scholar] [CrossRef]
- Gaddamwar, A.G.; Rajput, P.; Parsodkar, V. Extraction of basil, padina, ajwain and development of oxygen garden in the school yard as a preventive measure for covid-19. Int. J. Res. Appl. Sci. Eng. Technol. 2020, 8, 1408–1411. [Google Scholar] [CrossRef]
- Amzajerdi, A.; Keshavarz, M.; Montazeri, A.; Bekhradi, R. Effect of mint aroma on nausea, vomiting and anxiety in pregnant women. J. Fam. Med. Prim. Care 2019, 8, 2597. [Google Scholar]
- Agus, Y.; Hidayat, R.A. The Effect of Ginger Water and Mint Leaves for Reduce Nausea and Vomiting in the 1st Trimester of Pregnancy. In Proceedings of the 1st International Conference on Health Science, ICHS 2020, Jakarta, Indonesia, 26–27 October 2020. [Google Scholar]
- Haider, M.; Zhong, L. Ethno-medicinal uses of plants from district Bahawalpur, Pakistan. Curr. Res. J. Biol. Sci. 2014, 6, 183–190. [Google Scholar]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with a bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [PubMed]
- Mikaili, P.; Mojaverrostami, S.; Moloudizargari, M.; Aghajanshakeri, S. Pharmacological and therapeutic effects of Mentha longifolia L. and its main constituent, menthol. Anc. Sci. Life 2013, 33, 131–138. [Google Scholar] [CrossRef]
- Mthiyane, F.T.; Dludla, P.V.; Ziqubu, K.; Mthembu, S.X.H.; Muvhulawa, N.; Hlengwa, N.; Nkambule, B.B.; Mazibuko-Mbeje, S.E. A Review on the Antidiabetic Properties of Moringa oleifera Extracts: Focusing on Oxidative Stress and Inflammation as Main Therapeutic Targets. Front. Pharmacol. 2022, 13, 940572. [Google Scholar] [CrossRef]
- Pereira, A.S.P.; Banegas-Luna, A.J.; Peña-García, J.; Pérez-Sánchez, H.; Apostolides, Z. Evaluation of the Anti-Diabetic Activity of Some Common Herbs and Spices: Providing New Insights with Inverse Virtual Screening. Molecules 2019, 24, 4030. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Paramasivam, S.; Arulkumar, A. Evaluation of the lemongrass plant (Cymbopogon citratus) extracted in different solvents for antioxidant and antibacterial activity against human pathogens. Asian Pac. J. Trop. Dis. 2014, 4, S134–S139. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. LC-MS/MS Characterization of Phenolic Metabolites and Their Antioxidant Activities from Australian Native Plants. Metabolites 2022, 12, 1016. [Google Scholar] [CrossRef]
- Moore-Neibel, K.; Gerber, C.; Patel, J.; Friedman, M.; Ravishankar, S. Antimicrobial activity of lemongrass oil against Salmonella enterica on organic leafy greens. J. Appl. Microbiol. 2012, 112, 485–492. [Google Scholar] [CrossRef]
- Ferrare, K.; Bidel, L.P.R.; Awwad, A.; Poucheret, P.; Cazals, G.; Lazennec, F.; Azay-Milhau, J.; Tournier, M.; Lajoix, A.D.; Tousch, D. Increase in insulin sensitivity by the association of chicoric acid and chlorogenic acid contained in a natural chicoric acid extract (NCRAE) of chicory (Cichorium intybus L.) for an antidiabetic effect. J. Ethnopharmacol. 2018, 215, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.K.; Saggu, S.; Sakeran, M.I.; Zidan, N.; Rehman, H.; Ansari, A.A. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J. Biol. Sci. 2015, 22, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Shad, M.; Nawaz, H.; Rehman, T.; Ikram, N. Determination of some biochemicals, phytochemicals and antioxidant properties of different parts of Cichorium intybus L.: A comparative study. J. Anim. Plant Sci. 2013, 23, 1060–1066. [Google Scholar]
- Shad, M.A.; Nawaz, H.; Rehman, T.; Ahmad, H.B.; Hussain, M. Optimization of extraction efficiency of tannins from Cichorium intybus L.: Application of response surface methodology. J. Med. Plants Res. 2012, 6, 4467–4474. [Google Scholar]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Bahmani, M.; Shahinfard, N.; Rafieian-Kopaei, M.; Saki, K.; Shahsavari, S.; Taherikalani, M.; Ghafourian, S.; Baharvand-Ahmadi, B. Chicory: A review on ethnobotanical effects of Cichorium intybus L. J. Chem. Pharm. Sci. 2015, 8, 672–682. [Google Scholar]
- Pinseel, E.; Kulichová, J.; Scharfen, V.; Urbánková, P.; Van de Vijver, B.; Vyverman, W. Extensive cryptic diversity in the terrestrial diatom Pinnularia borealis (Bacillariophyceae). Protist 2019, 170, 121–140. [Google Scholar] [CrossRef]
- Siedliska, A.; Baranowski, P.; Pastuszka-Woźniak, J.; Zubik, M.; Krzyszczak, J. Identification of plant leaf phosphorus content at different growth stages based on hyperspectral reflectance. BMC Plant Biol. 2021, 21, 28. [Google Scholar] [CrossRef]
- Sinkovič, L.; Demšar, L.; Žnidarčič, D.; Vidrih, R.; Hribar, J.; Treutter, D. Phenolic profiles in leaves of chicory cultivars (Cichorium intybus L.) as influenced by organic and mineral fertilizers. Food Chem. 2015, 166, 507–513. [Google Scholar] [CrossRef]
- Afzal, S.; Shehzad, A.; Randhawa, M.A.; Asghar, A.; Shoaib, M.; Jahangir, M.A. Health benefits and importance of utilizing wheat and rye. Pak. J. Food Sci. 2013, 23, 212–222. [Google Scholar]
- Hefni, M.; Witthöft, C.M. Effect of germination and subsequent oven-drying on folate content in different wheat and rye cultivars. J. Cereal Sci. 2012, 56, 374–378. [Google Scholar] [CrossRef]
- Aziz, M.A.; Adnan, M.; Khan, A.H.; Shahat, A.A.; Al-Said, M.S.; Ullah, R. Traditional uses of medicinal plants practiced by the indigenous communities at Mohmand Agency, FATA, Pakistan. J. Ethnobiol. Ethnomed. 2018, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Fatiha, B.; Madani, K.; Chibane, M.; Duez, P. Chemical Composition and Biological Activities of Mentha Species. In Aromatic and Medicinal Plants; Hany, A.E.-S., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 47–79. [Google Scholar] [CrossRef]
- Iqbal, Y.; Ponnampalam, E.N.; Suleria, H.A.R.; Cottrell, J.J.; Dunshea, F.R. LC-ESI/QTOF-MS Profiling of Chicory and Lucerne Polyphenols and Their Antioxidant Activities. Antioxidants 2021, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Y.; Ponnampalam, E.N.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Extraction and characterization of polyphenols from non-conventional edible plants and their antioxidant activities. Food Res. Int. 2022, 157, 111205. [Google Scholar] [CrossRef] [PubMed]
- Moyo, B.; Oyedemi, S.; Masika, P.J.; Muchenje, V. Polyphenolic content and antioxidant properties of Moringa oleifera leaf extracts and enzymatic activity of liver from goats supplemented with Moringa oleifera leaves/sunflower seed cake. Meat Sci. 2012, 91, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Bayazid, A.B.; Park, S.H.; Kim, J.G.; Lim, B.O. Green chicory leaf extract exerts anti-inflammatory effects through suppressing LPS-induced MAPK/NF-κB activation and hepatoprotective activity in vitro. Food Agric. Immunol. 2020, 31, 513–532. [Google Scholar] [CrossRef]
- Baiano, A.; Romaniello, R.; Giametta, F.; Fiore, A. Optimization of Process Variables for the Sustainable Extraction of Phenolic Compounds from Chicory and Fennel By-Products. Appl. Sci. 2023, 13, 4191. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, Q.; Yang, Y. Comprehensive Investigation of Moringa oleifera from Different Regions by Simultaneous Determination of 11 Polyphenols Using UPLC-ESI-MS/MS. Molecules 2020, 25, 676. [Google Scholar] [CrossRef]
- Sankhalkar, S.; Vernekar, V. Quantitative and Qualitative Analysis of Phenolic and Flavonoid Content in Moringa oleifera Lam and Ocimum tenuiflorum L. Pharmacogn. Res. 2016, 8, 16–21. [Google Scholar] [CrossRef]
- Urías-Orona, V.; Gutiérrez-Soto, G.; Ruiz-Bautista, J.; Flores-Alonso, R.; Montiel-Ramos, I.; Martínez-Ávila, G.C.G.; Aranda-Ruiz, J.; Niño-Medina, G. Influence of extraction solvent on phenolic content and antioxidant capacity level of a commercial food supplement from Moringa oleifera leaves. Arch. Latinoam. Nutr. 2017, 67, 211–217. [Google Scholar]
- Povilaitis, D.; Šulniūtė, V.; Venskutonis, P.R.; Kraujalienė, V. Antioxidant properties of wheat and rye bran extracts obtained by pressurized liquid extraction with different solvents. J. Cereal Sci. 2015, 62, 117–123. [Google Scholar] [CrossRef]
- Ali, A.; Bashmil, Y.M.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. LC-MS/MS-QTOF Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential. Antioxidants 2021, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Ivanišová, E.; Kántor, A.; Kačániová, M. Antioxidant activity and total polyphenol content of different varieties of grape from the small Carpathians wine region of Slovakia. Anim. Sci. Biotechnol. 2019, 52, 78–82. [Google Scholar]
- Nouri, S.; Moslehishad, M.; Hosseini, S.M.; Etemadi, M. Comparison of Antioxidant and Alpha-Glucosidase Inhibitory Properties of Moringa peregrina and Ferulago carduchorum Leaf Extracts and Microencapsulation of Superior Plant. J. Food Qual. 2022, 2022, 5887180. [Google Scholar] [CrossRef]
- Salah Eddine, N.; Tlais, S.; Alkhatib, A.; Hamdan, R. Effect of Four Grape Varieties on the Physicochemical and Sensory Properties of Unripe Grape Verjuice. Int. J. Food Sci. 2020, 2020, 6457982. [Google Scholar] [CrossRef]
- Salih Mahdi, H.; Parveen, A. Biosynthesis, Characterization and Antibacterial Activity of Gold Nanoparticles (Au-NPs) using Black Lemon Extract. Mater. Today Proc. 2019, 18, 5164–5169. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Tang, Y.-L.; Chan, S.-W. A Review of the Pharmacological Effects of Piceatannol on Cardiovascular Diseases. Phytother. Res. 2014, 28, 1581–1588. [Google Scholar] [CrossRef]
- Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and Their Derivatives from Plants as Antivirals. Molecules 2020, 25, 183. [Google Scholar] [CrossRef]
- Jeya Shree, T.; Poompavai, S.; Mahaboob Begum, S.M.F.; Gowrisree, V.; Hemalatha, S.; Sieni, E.; Sundararajan, R. Cancer-Fighting Phytochemicals: Another Look. J. Nanomed. Biother. Discov. 2019, 9, 162. [Google Scholar]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Antioxidant, Alpha-Glucosidase Inhibition Activities, In Silico Molecular Docking and Pharmacokinetics Study of Phenolic Compounds from Native Australian Fruits and Spices. Antioxidants 2023, 12, 254. [Google Scholar] [CrossRef]
- Attique, S.A.; Hassan, M.; Usman, M.; Atif, R.M.; Mahboob, S.; Al-Ghanim, K.A.; Bilal, M.; Nawaz, M.Z. A Molecular Docking Approach to Evaluate the Pharmacological Properties of Natural and Synthetic Treatment Candidates for Use against Hypertension. Int. J. Environ. Res. Public. Health 2019, 16, 923. [Google Scholar] [CrossRef] [PubMed]
- Khalfaoui, A.; Noumi, E.; Belaabed, S.; Aouadi, K.; Lamjed, B.; Adnan, M.; Defant, A.; Kadri, A.; Snoussi, M.; Khan, M.A.; et al. LC-ESI/MS-Phytochemical Profiling with Antioxidant, Antibacterial, Antifungal, Antiviral and In Silico Pharmacological Properties of Algerian Asphodelus tenuifolius (Cav.) Organic Extracts. Antioxidants 2021, 10, 628. [Google Scholar] [CrossRef]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Characterization, Antioxidant Potential, and Pharmacokinetics Properties of Phenolic Compounds from Native Australian Herbs and Fruits. Plants 2023, 12, 993. [Google Scholar] [CrossRef]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds through LC-ESI-QTOF-MS2 and Their Antioxidant Potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Identification and characterization of anthocyanins and non-anthocyanin phenolics from Australian native fruits and their antioxidant, antidiabetic, and anti-Alzheimer potential. Food Res. Int. 2022, 162, 111951. [Google Scholar] [CrossRef] [PubMed]
- Severo, J.; Tiecher, A.; Chaves, F.C.; Silva, J.A.; Rombaldi, C.V. Gene transcript accumulation associated with physiological and chemical changes during developmental stages of strawberry cv. Camarosa. Food Chem. 2011, 126, 995–1000. [Google Scholar] [CrossRef]
- Fia, G.; Gori, C.; Bucalossi, G.; Borghini, F.; Zanoni, B. A Naturally Occurring Antioxidant Complex from Unripe Grapes: The Case of Sangiovese (v. Vitis vinifera). Antioxidants 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M. Ferrous Ion Chelating Activity (FICA)—A Comparative Antioxidant Activity Evaluation of Extracts of Eleven Naturally Growing Plants of Gujarat, India. Int. J. Sci. Res. 2012, 2, 426–428. [Google Scholar] [CrossRef]






| Variables | Lemongrass | Chicory | Ryegrass | Moringa |
|---|---|---|---|---|
| 80% methanol | 11.91 ± 0.56 b | 4.76 ± 0.11 a | 3.43 ± 0.09 c | 18.0 ± 1.51 a |
| 80% ethanol | 11.31 ± 0.19 b | 4.57 ± 0.07 ab | 3.12 ± 0.02 cd | 16.2 ± 1.33 b |
| 80% acidified methanol | 12.86 ± 0.12 a | 4.85 ± 0.08 a | 3.54 ± 0.03 cd | 18.5 ± 1.01 a |
| 80% acidified ethanol | 11.01 ± 0.41 b | 4.45 ± 0.14 ab | 3.17 ± 0.01 cd | 17.1 ± 1.32 ab |
| 80% acetone | 8.95 ± 0.13 c | 3.91 ± 0.06 b | 1.84 ± 0.01 c | 16.1 ± 1.09 b |
| 80% acidified acetone | 7.42 ± 0.35 cd | 3.76 ± 0.12 b | 1.63 ± 0.02 c | 17.2 ± 1.47 ab |
| Water | 2.91 ± 0.05 e | 0.45 ± 0.02 c | 0.24 ± 0.02 b | 4.01 ± 0.09 c |
| Samples | TPC (mg GAE/g) | TFC (mg QE/g) | TCT (mg CE/g) |
|---|---|---|---|
| Lemongrass | 12.9 ± 0.12 b | 6.19 ± 0.09 b | 0.90 ± 0.76 a |
| Chicory | 4.85 ± 0.06 c | 1.06 ± 0.01 c | 2.31 ± 0.15 c |
| Ryegrass | 3.54 ± 0.08 c | 1.52 ± 0.13 c | 0.58 ± 0.05 c |
| Moringa | 18.5 ± 1.01 a | 10.1 ± 0.83 a | 1.45 ± 0.01 b |
| Variables | DPPH (mg AAE/g) | ABTS (mg AAE/g) | FICA (μg EDTA/g) | •OH-RSA (mg AAE/g) | α-Glucosidase Inhibition Activity (μg/mL) |
|---|---|---|---|---|---|
| Lemongrass | 25.73 ± 0.18 a | 44.8 ± 0.93 a | 3.42 ± 0.11 a | 29.73 ± 0.48 a | 2.15 ± 0.13 a |
| Chicory | 16.61 ± 0.23 b | 35.4 ± 0.68 b | 2.52 ± 0.15 b | 17.69 ± 0.27 b | 16.41 ± 1.21 b |
| Ryegrass | 11.18 ± 0.21 c | 18.4 ± 0.56 c | 1.81 ± 0.05 c | 16.33 ± 0.39 b | 29.02 ± 2.17 c |
| Moringa | 34.16 ± 2.32 a | 54.9 ± 4.24 a | 7.49 ± 0.39 a | 41.6 ± 3.52 a | 1.89 ± 0.01 a |
| Variables | TPC | TFC | TCT | DPPH | ABTS | FICA | •OH-RSA |
|---|---|---|---|---|---|---|---|
| TFC | 0.99 | ||||||
| TCT | −0.03 | −0.12 | |||||
| DPPH | 0.99 | 0.97 | 0.18 | ||||
| ABTS | 0.93 | 0.87 | 0.32 | 0.97 | |||
| FICA | 0.93 | 0.93 | 0.15 | 0.93 | 0.86 | ||
| •OH-RSA | 0.99 | 0.99 | −0.02 | 0.98 | 0.91 | 0.96 | |
| α-glu | 0.94 | 0.89 | 0.11 | 0.96 | 0.97 | 0.79 | 0.91 |
| No. | Retention Time | ESI +/− | Theoretical m/z | Precursor m/z | Mass Error | Product Ions | Formula | Compound Name | Samples |
|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||||
| Hydroxybenzoic acids | |||||||||
| 1 | 6.216 | [M − H]− | 171.0288 | 171.0298 | 5.9 | 125 | C7H6O5 | Gallic acid | L, M, C, R |
| 2 | 10.718 | [M + H]+ | 155.0339 | 155.0341 | 1.3 | 109 | C7H6O4 | Protocatechuic acid | L, M, R, C |
| 3 | 13.549 | [M − H]− | 315.0721 | 315.0725 | 1.3 | 153 | C13H16O9 | Protocatechuic acid 4-O-glucoside | L, M |
| 4 | 15.982 | [M − H]− | 137.0244 | 137.0252 | 4.8 | 93 | C7H6O3 | p-Hydroxybenzoic acid | R, L, M, R |
| Hydroxycinnamic acids | |||||||||
| 5 | 6.302 | * [M + H]+ | 399.1286 | 399.1279 | −1.8 | 223, 191 | C18H22O10 | 3-Sinapoylquinic acid | L, C, M |
| 6 | 6.302 | [M + H]+ | 311.1125 | 311.1133 | 2.6 | 147, 131, 103 | C15H18O7 | Cinnamoyl glucose | L, M |
| 7 | 13.038 | * [M − H]− | 353.0878 | 353.0879 | 0.3 | 253, 190, 144 | C16H18O9 | 3-Caffeoylquinic acid | L, M, C, R |
| 8 | 13.170 | [M − H]− | 359.0772 | 359.0769 | −0.8 | 197, 179, 161, 135 | C18H16O8 | Rosmarinic acid | C, M |
| 9 | 13.720 | [M − H]− | 355.0671 | 355.0684 | 3.7 | 179, 161 | C15H16O10 | Caffeic acid 4-O-glucuronide | C, R, M |
| 10 | 14.041 | [M − H]− | 385.114 | 385.1140 | 0.0 | 223, 193 | C17H22O10 | 1-O-Sinapoyl-beta-D-glucose | R, L |
| 11 | 18.203 | * [M − H]− | 179.035 | 179.0349 | −0.6 | 143, 135, 133 | C9H8O4 | Caffeic acid | L, M, C, R |
| 12 | 18.341 | [M − H]− | 367.1034 | 367.1035 | 0.3 | 298, 288, 192, 191 | C17H20O9 | 3-Feruloylquinic acid | L, C, M, R |
| 13 | 18.697 | * [M − H]− | 337.0929 | 337.0926 | −0.9 | 265, 173, 162 | C16H18O8 | 3-p-Coumaroylquinic acid | L, M |
| 14 | 21.546 | * [M − H]− | 223.0612 | 223.0607 | −2.2 | 193, 179, 149, 134 | C11H12O5 | Sinapic acid | L, M, R |
| 15 | 22.719 | [M − H]− | 221.0455 | 221.0453 | −0.9 | 163 | C11H10O5 | p-Coumaroyl glycolic acid | C, M |
| 16 | 23.085 | [M − H]− | 193.0506 | 193.0502 | −2.1 | 178, 149, 134 | C10H10O4 | Ferulic acid | L, M, C, R |
| 17 | 24.020 | [M − H]− | 693.2036 | 693.2016 | −2.9 | 193, 134 | C32H38O17 | 1,2-Diferuloylgentiobiose | L, R |
| 18 | 24.095 | [M − H]− | 325.0929 | 325.0936 | 2.2 | 163, 119 | C15H18O8 | p-Coumaric acid 4-O-glucoside | L, C |
| 19 | 24.681 | [M − H]− | 295.0459 | 295.0471 | 4.1 | 115 | C13H12O8 | p-Coumaroyl tartaric acid | C, M |
| 20 | 26.759 | * [M − H]− | 147.0451 | 147.0450 | −0.7 | 129, 103 | C9H8O2 | Cinnamic acid | L, C, M, R |
| 21 | 29.106 | * [M − H]− | 163.04 | 163.0402 | 1.2 | 119 | C9H8O3 | p-Coumaric acid | L, C, M |
| 22 | 29.184 | [M − H]− | 529.1351 | 529.1354 | 0.6 | 193, 191, 179 | C26H26O12 | 1-Caffeoyl-5-feruloylquinic acid | C, L |
| 23 | 33.081 | * [M − H]− | 543.1508 | 543.1492 | −2.9 | 193, 191, 134 | C27H28O12 | 3,5-Diferuloylquinic acid | L, M |
| 24 | 36.425 | * [M + H]+ | 871.2655 | 871.2618 | −4.2 | 676, 195, 177 | C42H46O20 | 1,2,2′-Triferuloylgentiobiose | L, R, M |
| Flavonoids | |||||||||
| Flavanols | |||||||||
| 25 | 4.873 | [M − H]− | 715.1304 | 715.1310 | 0.8 | 565, 139 | C36H28O16 | Theaflavin 3-O-gallate | L, R |
| 26 | 17.002 | [M − H]− | 305.0667 | 305.0673 | 2.0 | 269, 219 | C15H14O7 | (+)-Gallocatechin | C, M |
| 27 | 21.174 | * [M − H]− | 577.1351 | 577.1354 | 2.3 | 451, 425, 289 | C30H26O12 | Procyanidin B2 | L, M, C, R |
| 28 | 21.246 | * [M + H]+ | 291.0863 | 291.0850 | −4.5 | 291 | C15H14O6 | (-)-Epicatechin | L, M, C, R |
| 29 | 22.814 | [M + H]+ | 483.1133 | 483.1133 | 0.0 | 483 | C21H22O13 | (−)-Epigallocatechin 7-O-glucuronide | L, C |
| 30 | 23.438 | [M − H]− | 865.1985 | 865.2009 | 4.6 | 739, 695, 577, 451 | C45H38O18 | Procyanidin trimer C1 | C, M |
| 31 | 23.590 | [M − H]− | 563.1195 | 563.1206 | 2.0 | C29H24O12 | Theaflavin | C, R | |
| 32 | 24.681 | [M − H]− | 457.0776 | 457.0780 | 0.9 | 305, 169 | C22H18O11 | (−)-Epigallocatechin 3-O-gallate | C, M |
| Flavanones | |||||||||
| 33 | 4.136 | * [M − H]− | 609.1825 | 609.1855 | 4.9 | 301 | C28H34O15 | Hesperidin | L, C, M |
| 34 | 14.151 | [M − H]− | 595.1668 | 595.1659 | −1.5 | 459, 287, 151 | C27H32O15 | Neoeriocitrin | C, R, M |
| 35 | 20.214 | * [M − H]− | 579.1719 | 579.1739 | 3.5 | 271 | C27H32O14 | Naringin | L, M |
| 36 | 26.216 | [M + H]+ | 435.1286 | 435.1290 | 0.9 | 273 | C21H22O10 | Naringenin 7-O-glucoside | L, C, M |
| Flavones | |||||||||
| 37 | 11.827 | * [M + H]+ | 403.1388 | 403.1394 | 1.5 | 237, 188, 145, 59 | C21H22O8 | Nobiletin | L, R |
| 38 | 15.587 | [M − H]− | 577.1563 | 577.1583 | 4.2 | 431, 269 | C27H30O14 | Rhoifolin | C, M |
| 39 | 19.524 | [M − H]− | 621.1097 | 621.1073 | −3.9 | 271 | C27H26O17 | Apigenin 7-O-diglucuronide | R |
| 40 | 20.550 | [M − H]− | 343.0823 | 343.0819 | −1.2 | 327, 255, 241 | C18H16O7 | Cirsilineol | L, C |
| 41 | 21.076 | * [M − H]− | 593.1512 | 593.1504 | −1.3 | 449, 283 | C27H30O15 | Apigenin 6,8-di-C-glucoside | L, C |
| 42 | 21.174 | * [M − H]− | 431.0983 | 431.1002 | 4.4 | 269 | C21H20O10 | Apigenin 6-C-glucoside | M |
| 43 | 24.378 | * [M − H]− | 607.1668 | 607.1651 | −2.8 | 301, 300 | C28H32O15 | Diosmin | L, M |
| 44 | 24.681 | [M − H]− | 637.1046 | 637.1075 | 4.6 | 285 | C27H26O18 | Luteolin 7-O-diglucuronide | C, L |
| 45 | 26.169 | [M − H]− | 461.1089 | 461.1094 | 1.1 | 299 | C22H22O11 | Chrysoeriol 7-O-glucoside | C |
| 46 | 28.604 | * [M + H]+ | 287.055 | 287.0555 | 1.7 | 287 | C15H10O6 | 3,4′,7-Tetrahydroxyflavone | L, M |
| Flavonols | |||||||||
| 47 | 4.706 | [M − H]− | 609.1097 | 609.1100 | 0.5 | 301 | C26H26O17 | Quercetin 3-O-xylosyl-glucuronide | R |
| 48 | 13.680 | [M − H]− | 627.1567 | 627.1570 | 0.5 | 303 | C27H32O17 | Taxifolin 4′,7-diglucoside | C, R |
| 49 | 17.320 | * [M − H]− | 579.1355 | 579.1350 | −0.9 | 285 | C26H28O15 | Kaempferol 3-O-xylosyl-glucoside | L |
| 50 | 21.683 | [M − H]− | 463.0882 | 463.0872 | −2.2 | 317 | C21H20O12 | Myricetin 3-O-rhamnoside | L, M |
| 51 | 21.694 | * [M − H]− | 461.0725 | 461.0746 | 4.6 | 285, 113, 85 | C21H18O12 | Kaempferol 3-O-glucuronide | R, L |
| 52 | 22.899 | [M − H]− | 298.0483 | 298.0475 | −2.7 | 283, 151 | C16H11O6 | Kaempferide | L |
| 53 | 23.391 | [M − H]− | 535.1093 | 535.1117 | 4.5 | 359 | C24H24O14 | Jaceidin 4′-O-glucuronide | R, C |
| 54 | 23.701 | [M − H]− | 461.1089 | 461.1076 | −2.8 | 315 | C22H22O11 | Isorhamnetin 3-O-rutinoside | L |
| 55 | 30.894 | * [M − H]− | 329.0667 | 329.0680 | 4.0 | 314, 299, 271 | C17H14O7 | 3,7-Dimethylquercetin | L, M |
| Isoflavonoids | |||||||||
| 56 | 4.501 | [M + H]+ | 533.129 | 533.1297 | 1.3 | 533 | C25H24O13 | 6″-O-Malonylglycitin | L, M |
| 57 | 22.837 | * [M − H]− | 429.0827 | 429.0828 | 0.2 | 253 | C21H18O10 | Daidzein 7-O-glucuronide | L, C |
| 58 | 23.010 | [M − H]− | 517.0987 | 517.1013 | 5.0 | 271 | C24H22O13 | 6″-O-Malonylgenistin | M, C |
| 59 | 26.707 | [M + H]+ | 271.0965 | 271.0968 | 1.1 | 253, 137 | C16H14O4 | Dihydroformononetin | L, M |
| 60 | 54.991 | * [M − H]− | 459.0933 | 459.0916 | −3.7 | 441, 283, 267 | C22H20O11 | Glycitein 7-O-glucuronide | M, L |
| 61 | 55.134 | [M − H]− | 417.1191 | 417.1184 | −1.7 | 241 | C21H22O9 | Equol 7-O-glucuronide | M |
| Stilbenes | |||||||||
| 62 | 6.905 | [M − H]− | 243.0663 | 243.0671 | 3.3 | 225, 201, 174, 159 | C14H12O4 | Piceatannol | M, C |
| 63 | 9.737 | [M + H]+ | 303.1227 | 303.1225 | −0.7 | 285 | C17H18O5 | 3′-Hydroxy-3,4,5,4′-tetramethoxystilbene | L, M |
| Lignans | |||||||||
| 64 | 14.203 | [M + H]+ | 389.1595 | 389.1595 | 0.0 | 389 | C21H24O7 | Medioresinol | L, M |
| 65 | 18.156 | * [M − H]− | 555.2235 | 555.2220 | −2.7 | 359 | C30H36O10 | Lariciresinol-sesquilignan | L, C, M |
| 66 | 19.885 | [M − H]− | 557.2392 | 557.2390 | −0.4 | 539, 521, 509, 361 | C30H38O10 | Secoisolariciresinol-sesquilignan | M |
| 67 | 43.624 | [M − H]− | 359.15 | 359.1504 | 1.1 | 329, 192, 178, 175, 160 | C20H24O6 | Lariciresinol | M |
| 68 | 45.726 | [M − H]− | 357.1343 | 357.1345 | 0.6 | C20H22O6 | Matairesinol | M | |
| Other compounds | |||||||||
| 69 | 4.551 | [M − H]− | 345.1707 | 345.1693 | −3.9 | 301 | C20H26O5 | Rosmanol | M |
| 70 | 38.744 | * [M + H]+ | 151.1118 | 151.1121 | 2.0 | 107 | C10H14O | Carvacrol | L, M |
| 71 | 45.817 | [M − H]− | 331.1915 | 331.1920 | 1.5 | 287 | C20H28O4 | Carnosic acid | L, R, M |
| 72 | 50.044 | [M − H]− | 329.1758 | 329.1766 | 2.4 | 285 | C20H26O4 | Carnosol | L, M |
| 73 | 62.904 | [M − H]− | 343.1551 | 343.1556 | 1.5 | 299 | C20H24O5 | Rosmadial | M |
| 74 | 49.606 | [M − H]− | 315.1085 | 315.1097 | 3.8 | 153, 123 | C14H20O8 | Hydroxytyrosol 4-O-glucoside | M |
| 75 | 4.009 | * [M + H]+ | 127.039 | 127.0387 | −2.4 | 127 | C6H6O3 | Pyrogallol | L, C, M |
| 76 | 16.729 | [M − H]− | 717.1461 | 717.1479 | 2.5 | 520, 357, 179, 161 | C36H30O16 | Salvianolic acid B | R, C |
| 77 | 17.201 | [M − H]− | 491.0983 | 491.0978 | −1.0 | 311, 267, 249 | C26H20O10 | Salvianolic acid C | C, L |
| 78 | 4.331 | * [M − H]− | 245.0455 | 245.0444 | −4.5 | 215, 201 | C13H10O5 | Isopimpinellin | R, C, L |
| 79 | 18.203 | * [M − H]− | 135.0451 | 135.0450 | −0.7 | 107, 93, 79 | C8H8O2 | p-Anisaldehyde | L, M |
| 80 | 14.188 | [M − H]− | 191.035 | 191.0343 | −3.7 | 175, 147 | C10H8O4 | Scopoletin | M, C |
| 81 | 20.611 | * [M − H]− | 145.0295 | 145.0293 | −1.4 | 101 | C9H6O2 | Coumarin | M, L |
| 82 | 26.806 | [M − H]− | 339.0721 | 339.0705 | −4.7 | 177 | C15H16O9 | Esculin | M, L |
| 83 | 26.949 | * [M − H]− | 161.0244 | 161.0247 | 1.9 | 133 | C9H6O3 | Umbelliferone | L, M |
| 84 | 42.621 | [M − H]− | 177.0557 | 177.0553 | −2.3 | 133 | C10H10O3 | Mellein | M, L |
| 85 | 30.115 | [M − H]− | 473.0725 | 473.0736 | 2.3 | 293, 311 | C22H18O12 | Chicoric acid | R, C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiani, H.S.; Ahmad, W.; Nawaz, S.; Farah, M.A.; Ali, A. Optimized Extraction of Polyphenols from Unconventional Edible Plants: LC-MS/MS Profiling of Polyphenols, Biological Functions, Molecular Docking, and Pharmacokinetics Study. Molecules 2023, 28, 6703. https://doi.org/10.3390/molecules28186703
Kiani HS, Ahmad W, Nawaz S, Farah MA, Ali A. Optimized Extraction of Polyphenols from Unconventional Edible Plants: LC-MS/MS Profiling of Polyphenols, Biological Functions, Molecular Docking, and Pharmacokinetics Study. Molecules. 2023; 28(18):6703. https://doi.org/10.3390/molecules28186703
Chicago/Turabian StyleKiani, Hafiza Sehrish, Waheed Ahmad, Sana Nawaz, Mohammad Abul Farah, and Akhtar Ali. 2023. "Optimized Extraction of Polyphenols from Unconventional Edible Plants: LC-MS/MS Profiling of Polyphenols, Biological Functions, Molecular Docking, and Pharmacokinetics Study" Molecules 28, no. 18: 6703. https://doi.org/10.3390/molecules28186703