Hispidulin Inhibits the Vascular Inflammation Triggered by Porphyromonas gingivalis Lipopolysaccharide
Abstract
:1. Introduction
2. Results
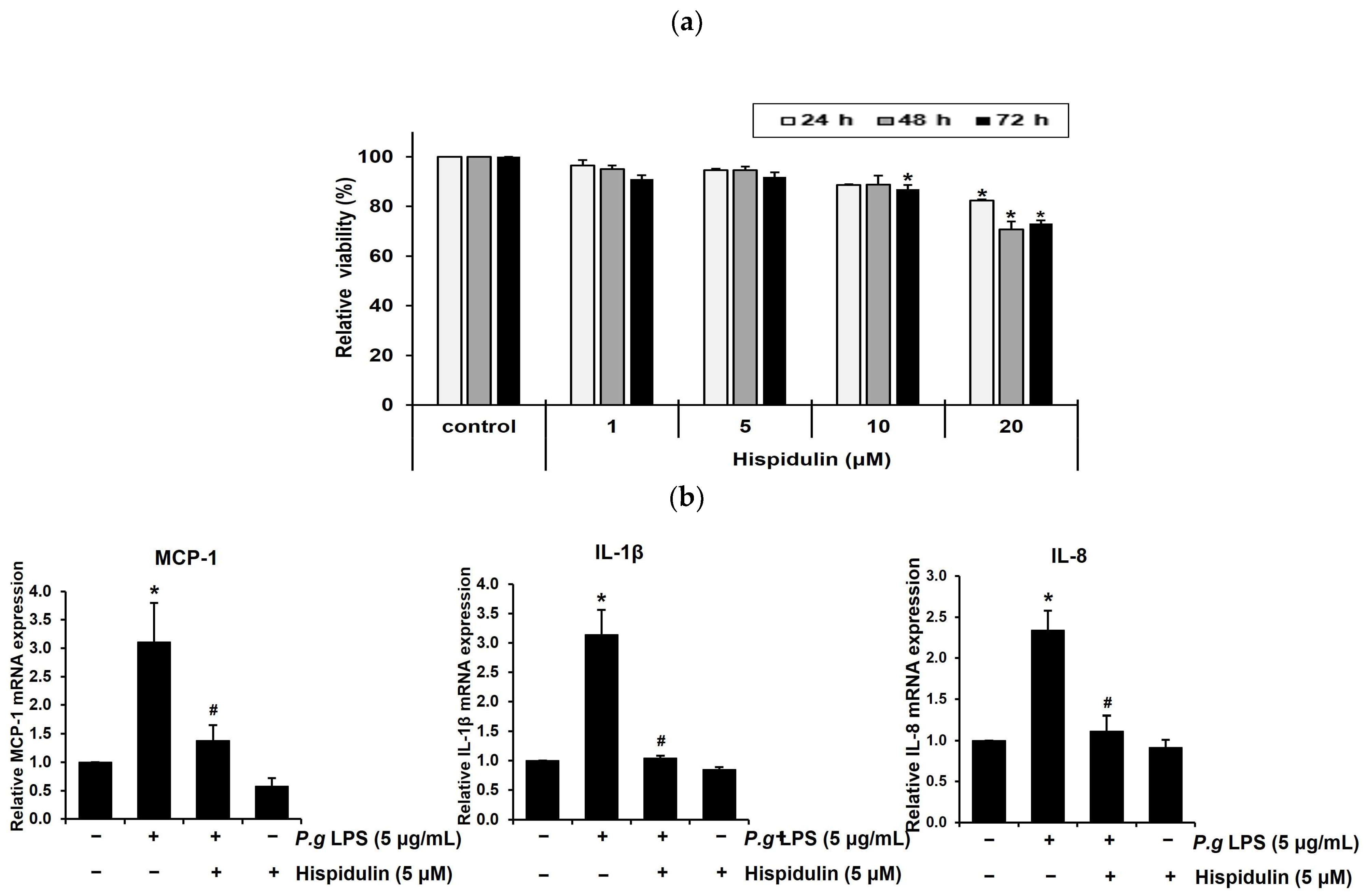
2.1. Hispidulin Decreases the P. gingivalis LPS-Induced Expression of Inflammatory Cytokines in Vascular Endothelial Cells
2.2. Hispidulin Decreases the P. gingivalis LPS-Induced Expression of ICAM-1 in Endothelial Cells
2.3. Hispidulin Inhibits Monocyte Adhesion to P. gingivalis LPS-Stimulated Vascular Endothelial Cells
2.4. Hispidulin Decreases the P. gingivalis LPS-Induced Transendothelial Migration of Monocytes
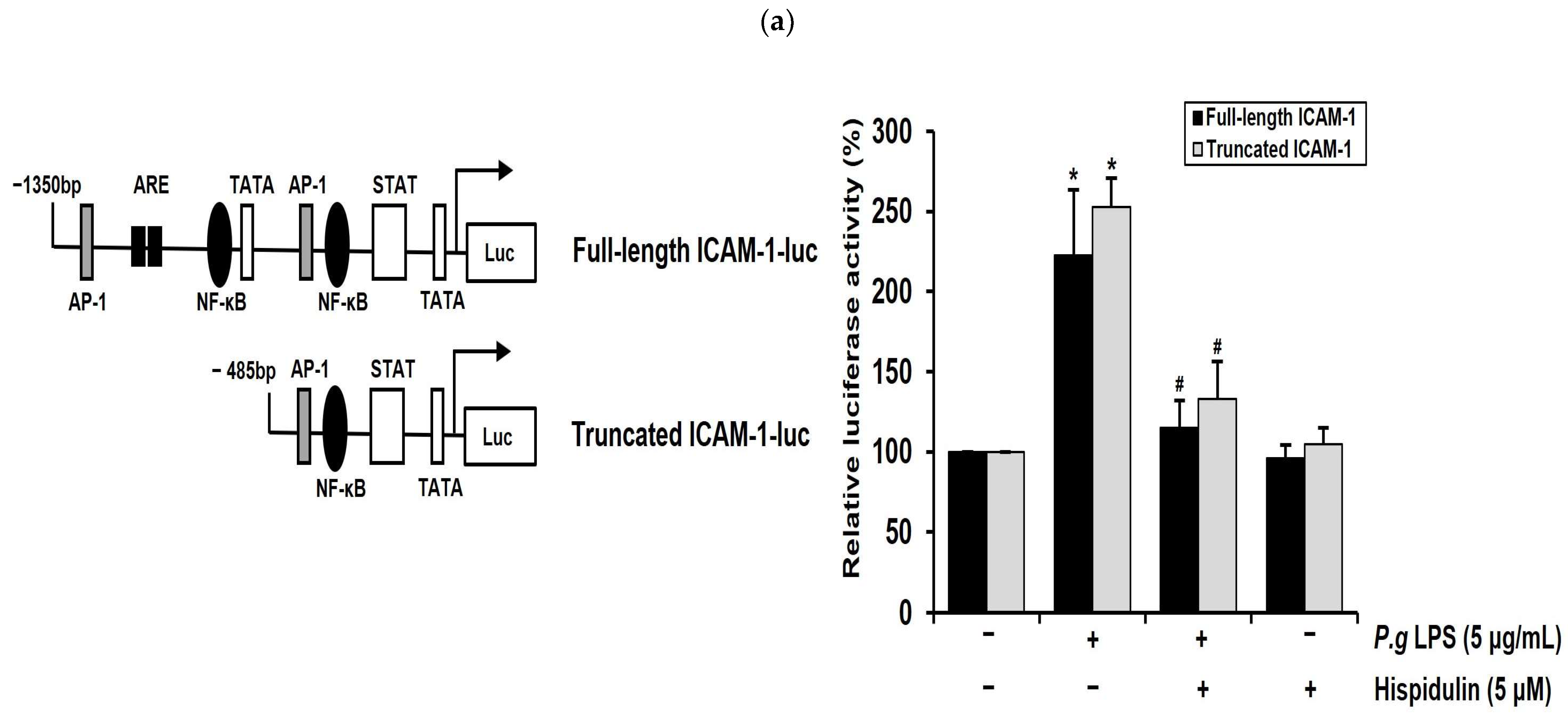
2.5. Hispidulin Downregulates the P. gingivalis LPS-Induced Transcriptional Activation of ICAM-1 through NF-κB Activation
2.6. Hispidulin Downregulates P. gingivalis LPS-Induced MAPKs and AKT in Vascular Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cell Proliferation Assay
4.4. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
4.5. Western Immunoblot Analysis
4.6. Flow Cytometry Analysis
4.7. In Vitro Monocyte Adhesion Assay
4.8. Ex Vivo Monocyte Adhesion Assay
4.9. Transmigration Assay
4.10. Transient Transfection and Reporter Gene Analysis
4.11. Immunocytochemistry
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sharaf, S.; Hijazi, K. Modulatory Mechanisms of Pathogenicity in Porphyromonas gingivalis and Other Periodontal Pathobionts. Microorganisms 2022, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Mysak, J.; Podzimek, S.; Sommerova, P.; Lyuya-Mi, Y.; Bartova, J.; Janatova, T.; Prochazkova, J.; Duskova, J. Porphyromonas gingivalis: Major Periodontopathic Pathogen Overview. J. Immunol. Res. 2014, 2014, 476068. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Zhi, A.; Lai, P.F.H.; Wang, G.; Xia, Y.; Xiong, Z.; Zhang, H.; Che, N.; Ai, L. The Oral Microbiota—A Mechanistic Role for Systemic Diseases. Br. Dent. J. 2018, 224, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Kholy, K.E.; Genco, R.J.; Van Dyke, T.E. Oral Infections and Cardiovascular Disease. Trends Endocrinol. Metab. 2015, 26, 315–321. [Google Scholar] [CrossRef]
- de Jongh, C.A.; de Vries, T.J.; Bikker, F.J.; Gibbs, S.; Krom, B.P. Mechanisms of Porphyromonas gingivalis to Translocate Over the Oral Mucosa and Other Tissue Barriers. J. Oral Microbiol. 2023, 15, 2205291. [Google Scholar] [CrossRef]
- Gerhardt, T.; Ley, K. Monocyte Trafficking Across the Vessel Wall. Cardiovasc. Res. 2015, 107, 321–330. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Gutiérrez-Venegas, G. Flavonoids Exert Multiple Periodontic Benefits Including Anti-Inflammatory, Periodontal Ligament-Supporting, and Alveolar Bone-Preserving Effects. Life Sci. 2018, 209, 435–454. [Google Scholar] [CrossRef]
- Cai, Y.; Kurita-Ochiai, T.; Hashizume, T.; Yamamoto, M. Green Tea Epigallocatechin-3-Gallate Attenuates Porphyromonas gingivalis-Induced Atherosclerosis. Pathog. Dis. 2013, 67, 76–83. [Google Scholar] [CrossRef]
- Liu, K.; Zhao, F.; Yan, J.; Xia, Z.; Jiang, D.; Ma, P. Hispidulin: A Promising Flavonoid with Diverse Anti-Cancer Properties. Life Sci. 2020, 259, 118395. [Google Scholar] [CrossRef]
- Ashaq, A.; Maqbool, M.F.; Maryam, A.; Khan, M.; Shakir, H.A.; Irfan, M.; Qazi, J.I.; Li, Y.; Ma, T. Hispidulin: A Novel Natural Compound with Therapeutic Potential Against Human Cancers. Phytother. Res. 2021, 35, 771–789. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Medicinal Importance, Pharmacological Activities, and Analytical Aspects of Hispidulin: A Concise Report. J. Tradit. Complement. Med. 2016, 7, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wang, Z.; Ma, C. Hispidulin Exerts Anti-Osteoporotic Activity in Ovariectomized Mice Via Activating AMPK Signaling Pathway. Cell Biochem. Biophys. 2014, 69, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Min, K.J.; Kim, M.J.; Kim, S.H.; Kwon, T.K. Hispidulin Inhibits Mast Cell-Mediated Allergic Inflammation through Down-Regulation of Histamine Release and Inflammatory Cytokines. Molecules 2019, 24, 2131. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Lee, S.; Kang, J.; Choi, Y.A.; Lee, B.; Kwon, T.K.; Jang, Y.H.; Kim, S.H. Hispidulin Alleviates Imiquimod-Induced Psoriasis-Like Skin Inflammation by Inhibiting Splenic Th1/Th17 Cell Population and Keratinocyte Activation. Int. Immunopharmacol. 2020, 87, 106767. [Google Scholar] [CrossRef]
- Saba, E.; Lee, Y.; Yang, W.; Lee, Y.Y.; Kim, M.; Woo, S.; Kim, K.; Kwon, Y.; Kim, T.; Kwak, D.; et al. Effects of a Herbal Formulation, KGC3P, and its Individual Component, Nepetin, on Coal Fly Dust-Induced Airway Inflammation. Sci. Rep. 2020, 10, 14036. [Google Scholar] [CrossRef]
- Yu, C.; Cheng, C.; Kang, Y.F.; Chang, P.C.; Lin, I.P.; Kuo, Y.H.; Jhou, A.J.; Lin, M.Y.; Chen, C.Y.; Lee, C.H. Hispidulin Inhibits Neuroinflammation in Lipopolysaccharide-Activated BV2 Microglia and Attenuates the Activation of Akt, NF-κB, and STAT3 Pathway. Neurotox Res. 2020, 38, 163–174. [Google Scholar] [CrossRef]
- He, L.; Wu, Y.; Lin, L.; Wang, J.; Wu, Y.; Chen, Y.; Yi, Z.; Liu, M.; Pang, X. Hispidulin, a Small Flavonoid Molecule, Suppresses the Angiogenesis and Growth of Human Pancreatic Cancer by Targeting Vascular Endothelial Growth Factor Receptor 2-Mediated PI3K/Akt/mTOR Signaling Pathway. Cancer Sci. 2011, 102, 219–225. [Google Scholar] [CrossRef]
- Taleb, S. Inflammation in Atherosclerosis. Arch. Cardiovasc. Dis. 2016, 109, 708–715. [Google Scholar] [CrossRef]
- Davies, M.J.; Gordon, J.L.; Gearing, A.J.; Pigott, R.; Woolf, N.; Katz, D.; Kyriakopoulos, A. The Expression of the Adhesion Molecules ICAM-1, VCAM-1, PECAM, and E-Selectin in Human Atherosclerosis. J. Pathol. 1993, 171, 223–229. [Google Scholar] [CrossRef]
- Park, H.J.; Jeong, S.K.; Kim, S.R.; Bae, S.K.; Kim, W.S.; Jin, S.D.; Koo, T.H.; Jang, H.Y.; Yun, I.; Kim, K.W.; et al. Resveratrol Inhibits Porphyromonas gingivalis Lipopolysaccharide-Induced Endothelial Adhesion Molecule Expression by Suppressing NF-kappaB Activation. Arch. Pharm. Res. 2009, 32, 583–591. [Google Scholar] [CrossRef]
- Toriuchi, K.; Kihara, T.; Aoki, H.; Kakita, H.; Takeshita, S.; Ueda, H.; Inoue, Y.; Hayashi, H.; Shimono, Y.; Yamada, Y.; et al. Monocyte-Derived miRNA-1914-5p Attenuates IL-1β-Induced Monocyte Adhesion and Transmigration. Int. J. Mol. Sci. 2023, 24, 2829. [Google Scholar] [CrossRef] [PubMed]
- Fenyo, I.M.; Gafencu, A.V. The Involvement of the Monocytes/Macrophages in Chronic Inflammation Associated with Atherosclerosis. Immunobiology 2013, 218, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Verma, I. Nuclear Factor (NF)-kappaB Proteins: Therapeutic Targets. Ann. Rheum. Dis. 2004, 63 (Suppl. S2), ii57–ii61. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Bae, Y.H.; Bae, S.K.; Choi, K.S.; Yoon, K.H.; Koo, T.H.; Jang, H.O.; Yun, I.; Kim, K.W.; Kwon, Y.G.; et al. Visfatin Enhances ICAM-1 and VCAM-1 Expression through ROS-Dependent NF-kappaB Activation in Endothelial Cells. Biochim. Biophys. Acta 2008, 1783, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Cynader, M.; Jia, W. TDP-43 Inhibits NF-κB Activity by Blocking p65 Nuclear Translocation. PLoS ONE 2015, 10, e0142296. [Google Scholar] [CrossRef]
- Jian, C.X.; Li, M.Z.; Zheng, W.Y.; He, Y.; Ren, Y.; Wu, Z.M.; Fan, Q.S.; Hu, Y.H.; Li, C.J. Tormentic Acid Inhibits LPS-Induced Inflammatory Response in Human Gingival Fibroblasts Via Inhibition of TLR4-Mediated NF-κB and MAPK Signalling Pathway. Arch. Oral Biol. 2015, 60, 1327–1332. [Google Scholar] [CrossRef]
- Yilmaz, O.; Jungas, T.; Verbeke, P.; Ojcius, D.M. Activation of the Phosphatidylinositol 3-Kinase/Akt Pathway Contributes to Survival of Primary Epithelial Cells Infected with the Periodontal Pathogen Porphyromonas gingivalis. Infect. Immun. 2004, 72, 3743–3751. [Google Scholar] [CrossRef]
- An, P.; Xie, J.; Qiu, S.; Liu, Y.; Wang, J.; Xiu, X.; Li, L.; Tang, M. Hispidulin Exhibits Neuroprotective Activities Against Cerebral Ischemia Reperfusion Injury through Suppressing NLRP3-Mediated Pyroptosis. Life Sci. 2019, 232, 116599. [Google Scholar] [CrossRef]
- Dabaghi-Barbosa, P.; Mariante Rocha, A.; Franco da Cruz Lima, A.; Heleno de Oliveira, B.; Benigna Martinelli de Oliveira, M.; Gunilla Skare Carnieri, E.; Cadena, S.M.S.C.; Eliane Merlin Rocha, M. Hispidulin: Antioxidant Properties and Effect on Mitochondrial Energy Metabolism. Free Radic. Res. 2005, 39, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, S.; Kim, N.; Dhakal, H.; Choi, Y.A.; Kwon, T.K.; Khang, D.; Kim, S.H. Hispidulin Alleviates 2,4-Dinitrochlorobenzene and House Dust Mite Extract-Induced Atopic Dermatitis-Like Skin Inflammation. Biomed. Pharmacother. 2021, 137, 111359. [Google Scholar] [CrossRef]
- Kim, K.; Leem, J. Hispidulin Ameliorates Endotoxin-Induced Acute Kidney Injury in Mice. Molecules 2022, 27, 2019. [Google Scholar] [CrossRef] [PubMed]
- Heine, H.; Zamyatina, A. Therapeutic Targeting of TLR4 for Inflammation, Infection, and Cancer: A Perspective for Disaccharide Lipid A Mimetics. Pharmaceuticals 2022, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Nativel, B.; Couret, D.; Giraud, P.; Meilhac, O.; d’Hellencourt, C.L.; Viranaïcken, W.; Da Silva, C.R. Porphyromonas gingivalis Lipopolysaccharides Act Exclusively through TLR4 with a Resilience between Mouse and Human. Sci. Rep. 2017, 7, 15789. [Google Scholar] [CrossRef]
- Kocgozlu, L.; Elkaim, R.; Tenenbaum, H.; Werner, S. Variable Cell Responses to P. gingivalis Lipopolysaccharide. J. Dent. Res. 2009, 88, 741–745. [Google Scholar] [CrossRef]
- Gutiérrez-Venegas, G.; Alonso Luna, O.; Ventura-Arroyo, J.A.; Hernández-Bermúdez, C. Myricetin Suppresses Lipoteichoic Acid-Induced Interleukin-1β and Cyclooxygenase-2 Expression in Human Gingival Fibroblasts. Microbiol. Immunol. 2013, 57, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Jeong, G.S.; Lee, S.H.; Jeong, S.N.; Kim, Y.C.; Kim, E.C. Anti-Inflammatory Effects of Apigenin on Nicotine- and Lipopolysaccharide-Stimulated Human Periodontal Ligament Cells Via Heme Oxygenase-1. Int. Immunopharmacol. 2009, 9, 1374–1380. [Google Scholar] [CrossRef]
- Yetkin Ay, Z.; Bakır, B.; Bozkurt, S.B.; Kayis, S.A.; Hakki, S.S. Positive Effect of Curcumin on Experimental Peridontitis Via Suppression of IL-1-Beta and IL-6 Expression Level. Int. J. Vitam. Nutr. Res. 2022, 92, 231–239. [Google Scholar] [CrossRef]
- Cheng, W.C.; Huang, R.Y.; Chiang, C.Y.; Chen, J.K.; Liu, C.H.; Chu, C.L.; Fu, E. Ameliorative Effect of Quercetin on the Destruction Caused by Experimental Periodontitis in Rats. J. Periodontal. Res. 2010, 45, 788–795. [Google Scholar] [CrossRef]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 615486. [Google Scholar] [CrossRef]
- Fernández, A.; Herrera, D.; Hoare, A.; Hernández, M.; Torres, V.A. Lipopolysaccharides from Porphyromonas Endodontalis and Porphyromonas gingivalis Promote Angiogenesis Via Toll-Like-Receptors 2 and 4 Pathways in Vitro. Int. Endod. J. 2023, 56, 1270–1293. [Google Scholar] [CrossRef]
- Nepal, M.; Choi, H.J.; Choi, B.; Yang, M.; Chae, J.; Li, L.; Soh, Y. Hispidulin Attenuates Bone Resorption and Osteoclastogenesis Via the RANKL-Induced NF-κB and NFATc1 Pathways. Eur. J. Pharmacol. 2013, 715, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Tabas, I.; García-Cardeña, G.; Owens, G.K. Recent Insights into the Cellular Biology of Atherosclerosis. J. Cell Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Dri, E.; Lampas, E.; Lazaros, G.; Lazarou, E.; Theofilis, P.; Tsioufis, C.; Tousoulis, D. Inflammatory Mediators of Endothelial Dysfunction. Life 2023, 13, 1420. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K.; Leducq Transatlantic Network on Atherothrombosis. Inflammation in Atherosclerosis: From Pathophysiology to Practice. J. Am. Coll. Cardiol. 2009, 54, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bian, T.; Lyu, J.; Cui, D.; Lei, L.; Yan, F. Human Β-Defensin-3 Alleviates the Progression of Atherosclerosis Accelerated by Porphyromonas gingivalis Lipopolysaccharide. Int. Immunopharmacol. 2016, 38, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Links between Atherosclerotic and Periodontal Disease. Exp. Mol. Pathol. 2016, 100, 220–235. [Google Scholar] [CrossRef]
- Schmitten, T.; Livak, K. Analyzing real-time PCR data by the comparative Ct method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Lee, H.; Park, H.-J.; Kim, M.-K.; Kim, Y.-I.; Kim, H.J.; Bae, S.-K.; Kim, Y.-J.; Bae, M.-K. Hispidulin Inhibits the Vascular Inflammation Triggered by Porphyromonas gingivalis Lipopolysaccharide. Molecules 2023, 28, 6717. https://doi.org/10.3390/molecules28186717
Kim Y, Lee H, Park H-J, Kim M-K, Kim Y-I, Kim HJ, Bae S-K, Kim Y-J, Bae M-K. Hispidulin Inhibits the Vascular Inflammation Triggered by Porphyromonas gingivalis Lipopolysaccharide. Molecules. 2023; 28(18):6717. https://doi.org/10.3390/molecules28186717
Chicago/Turabian StyleKim, Yeon, Hoyong Lee, Hyun-Joo Park, Mi-Kyoung Kim, Yong-Il Kim, Hyung Joon Kim, Soo-Kyung Bae, Yung-Jin Kim, and Moon-Kyoung Bae. 2023. "Hispidulin Inhibits the Vascular Inflammation Triggered by Porphyromonas gingivalis Lipopolysaccharide" Molecules 28, no. 18: 6717. https://doi.org/10.3390/molecules28186717





