Boosted Photocatalytic Performance for Antibiotics Removal with Ag/PW12/TiO2 Composite: Degradation Pathways and Toxicity Assessment
Abstract
:1. Introduction
2. Results and Discussion
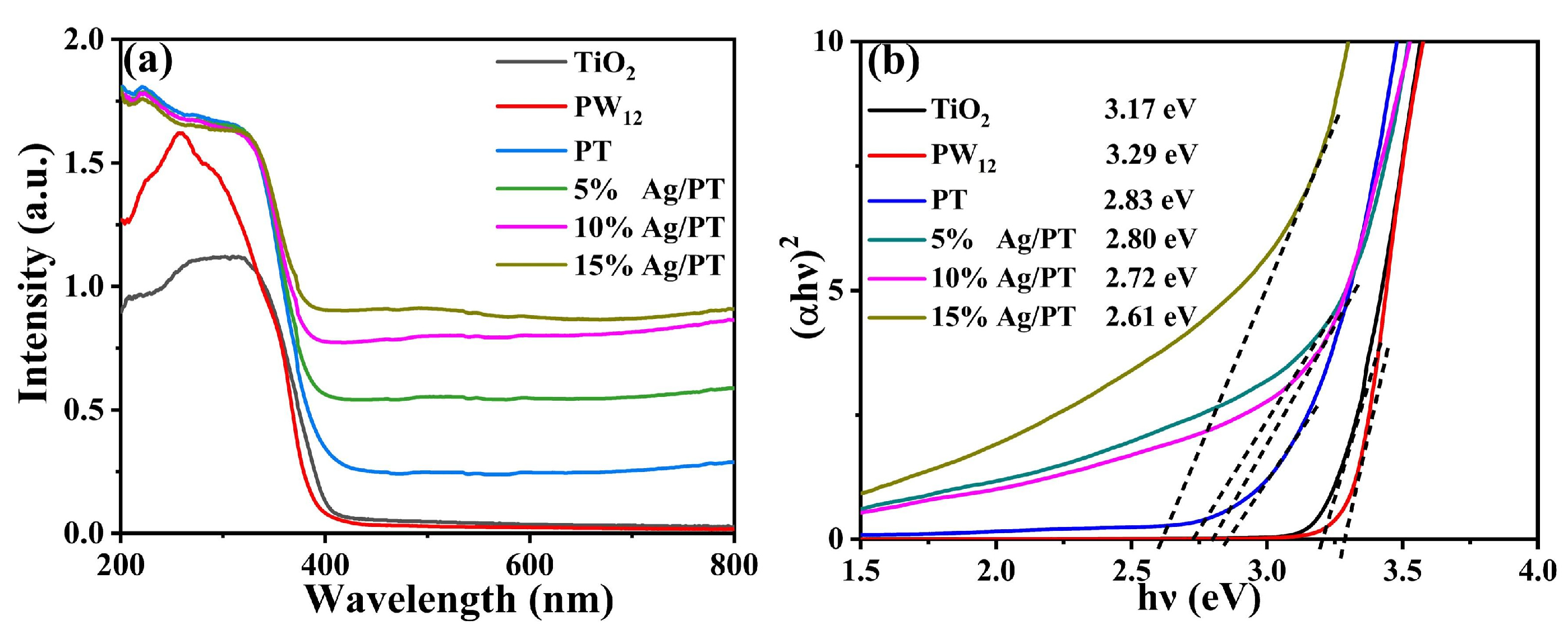
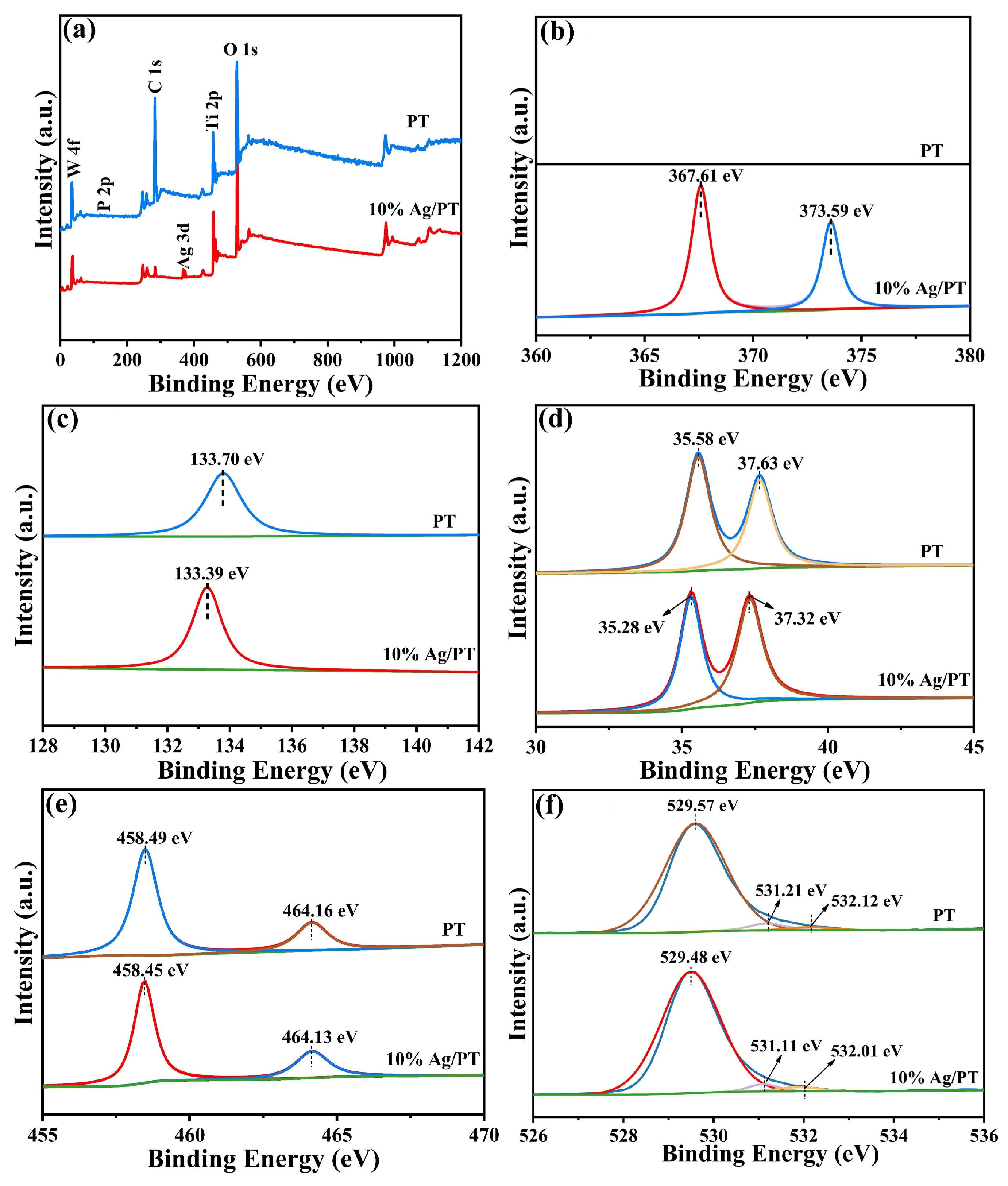
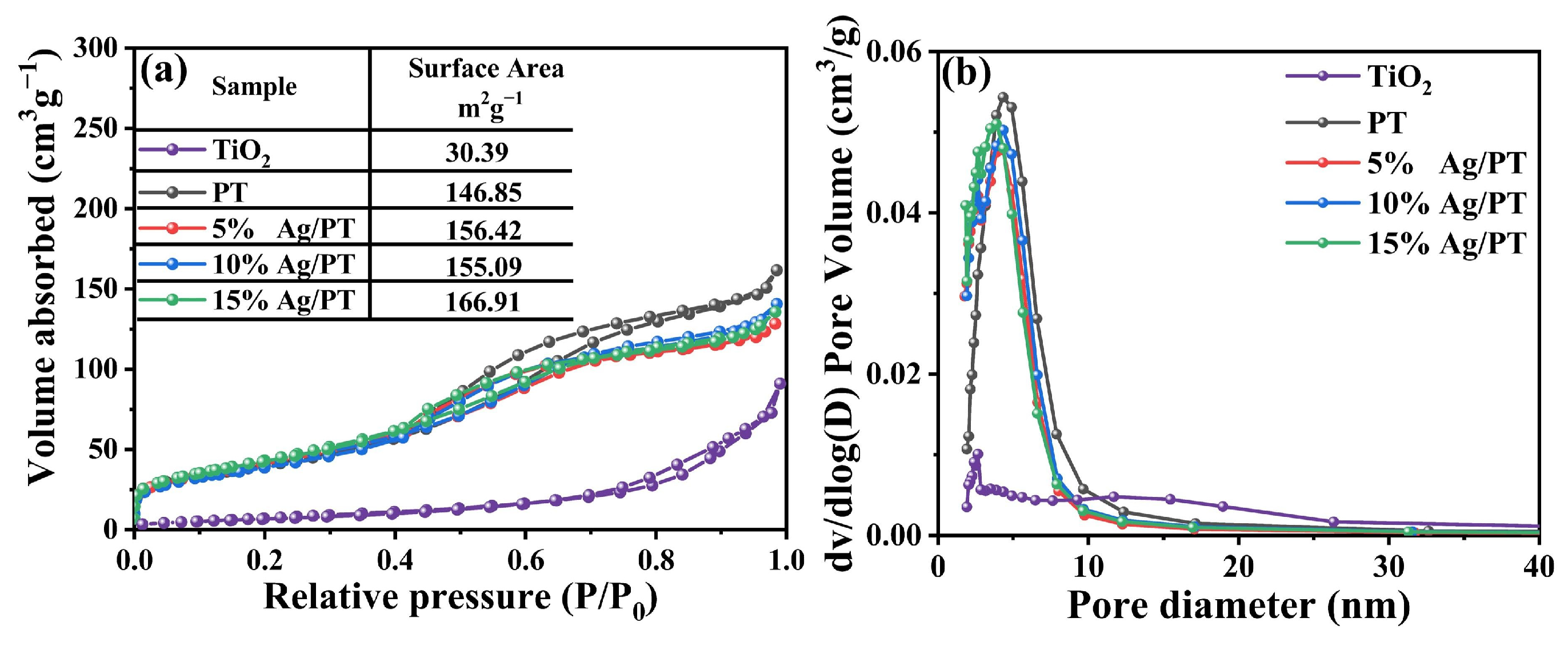
2.1. Characterization of Ag/PT Composites
2.2. Catalytic Activity Assessment of Ag/PT Composites
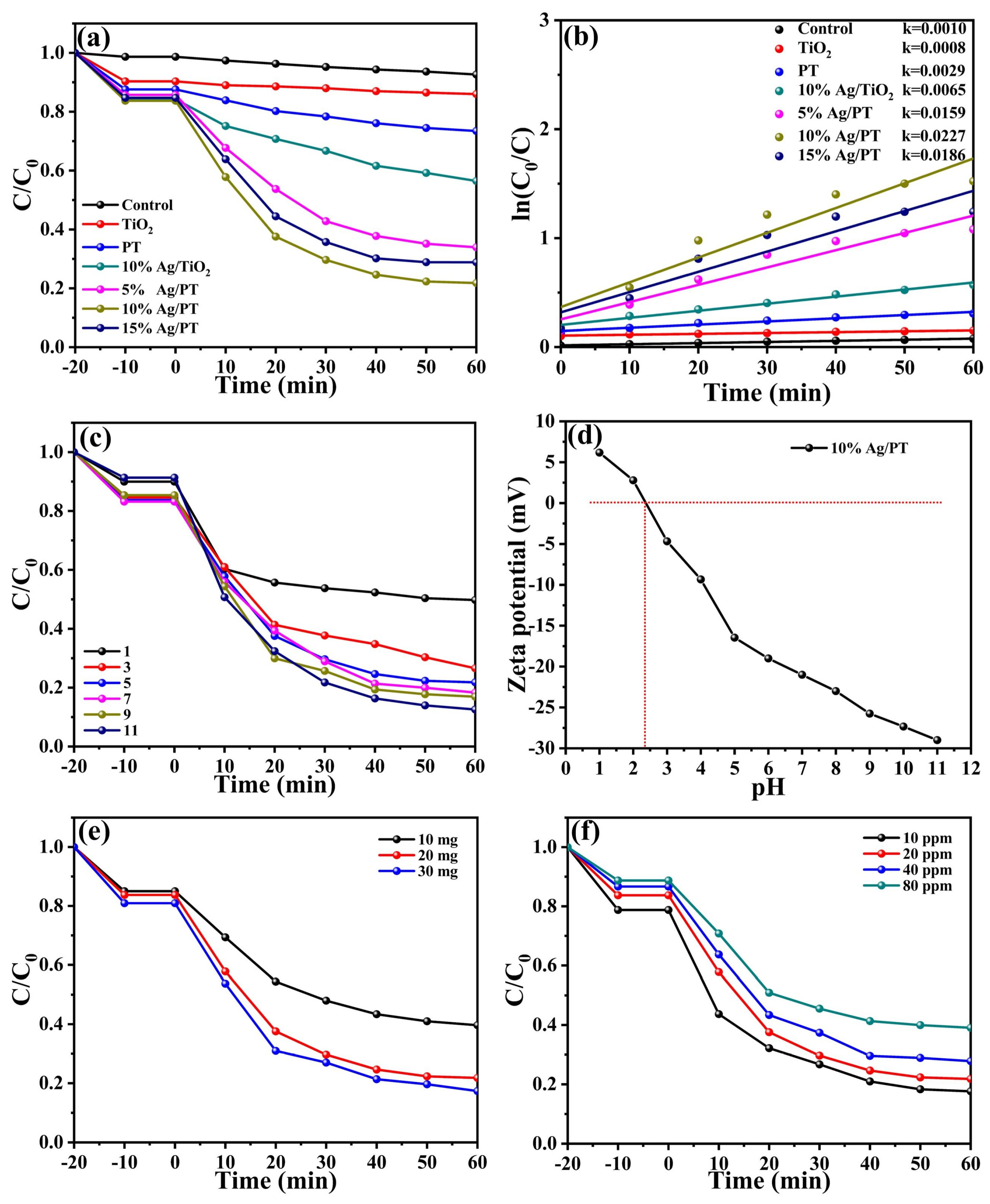
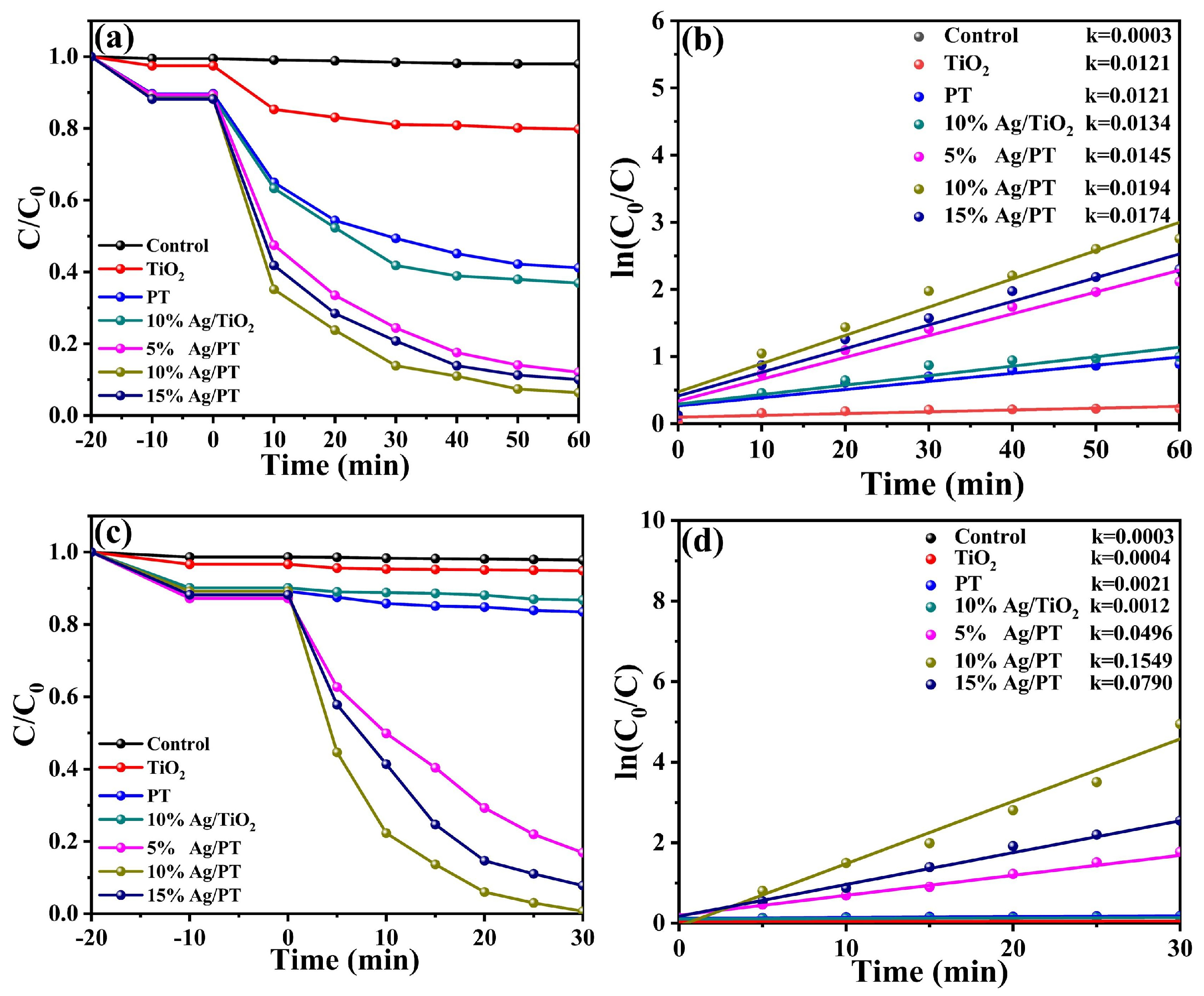
2.2.1. Photocatalytic Removal of TC
2.2.2. Photocatalytic Degradation of ENR and MO
2.3. Stability Test of Photocatalyst
2.4. Photocatalytic Mechanism Investigation
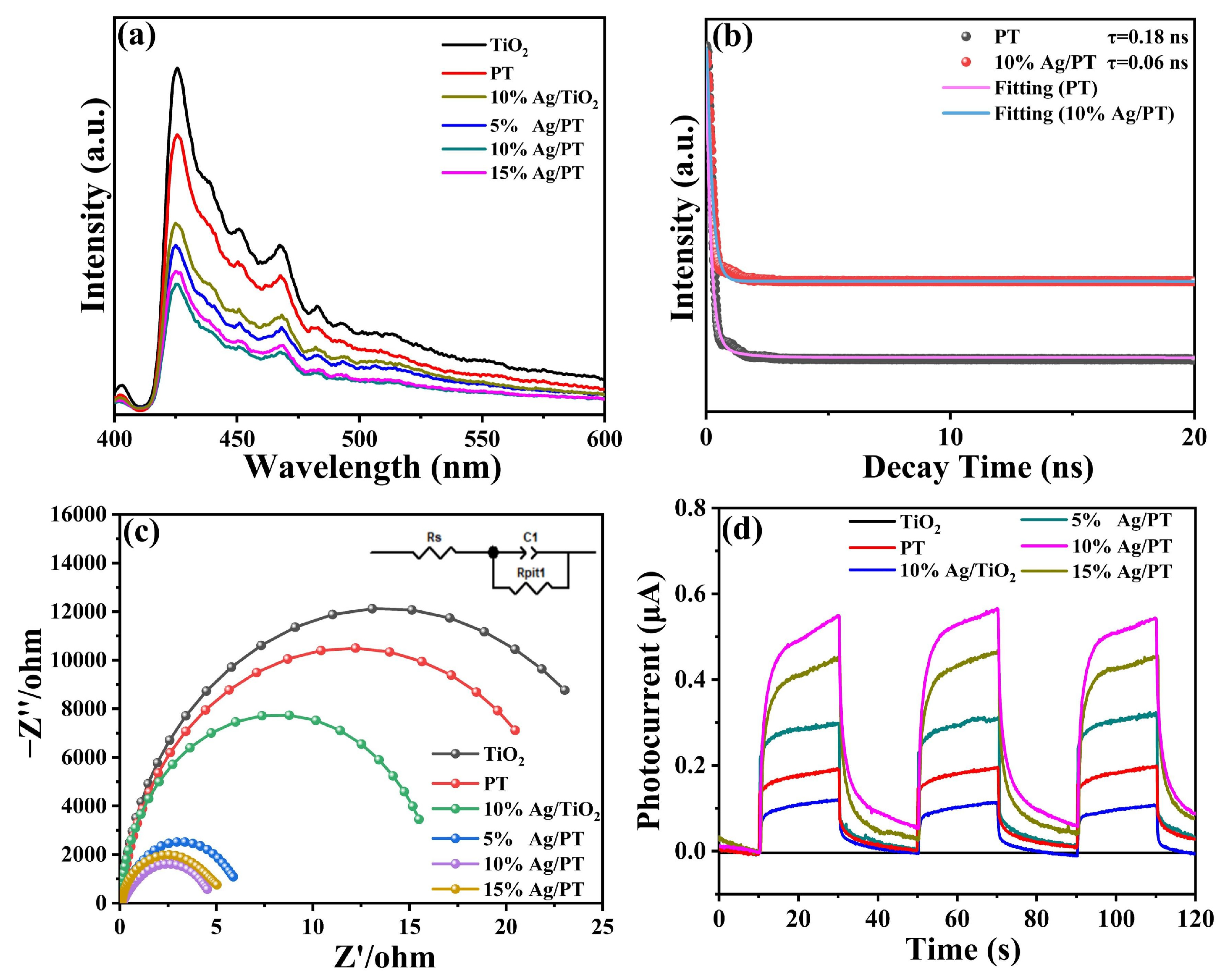
2.4.1. Photogenerated Carriers Behavior Analysis
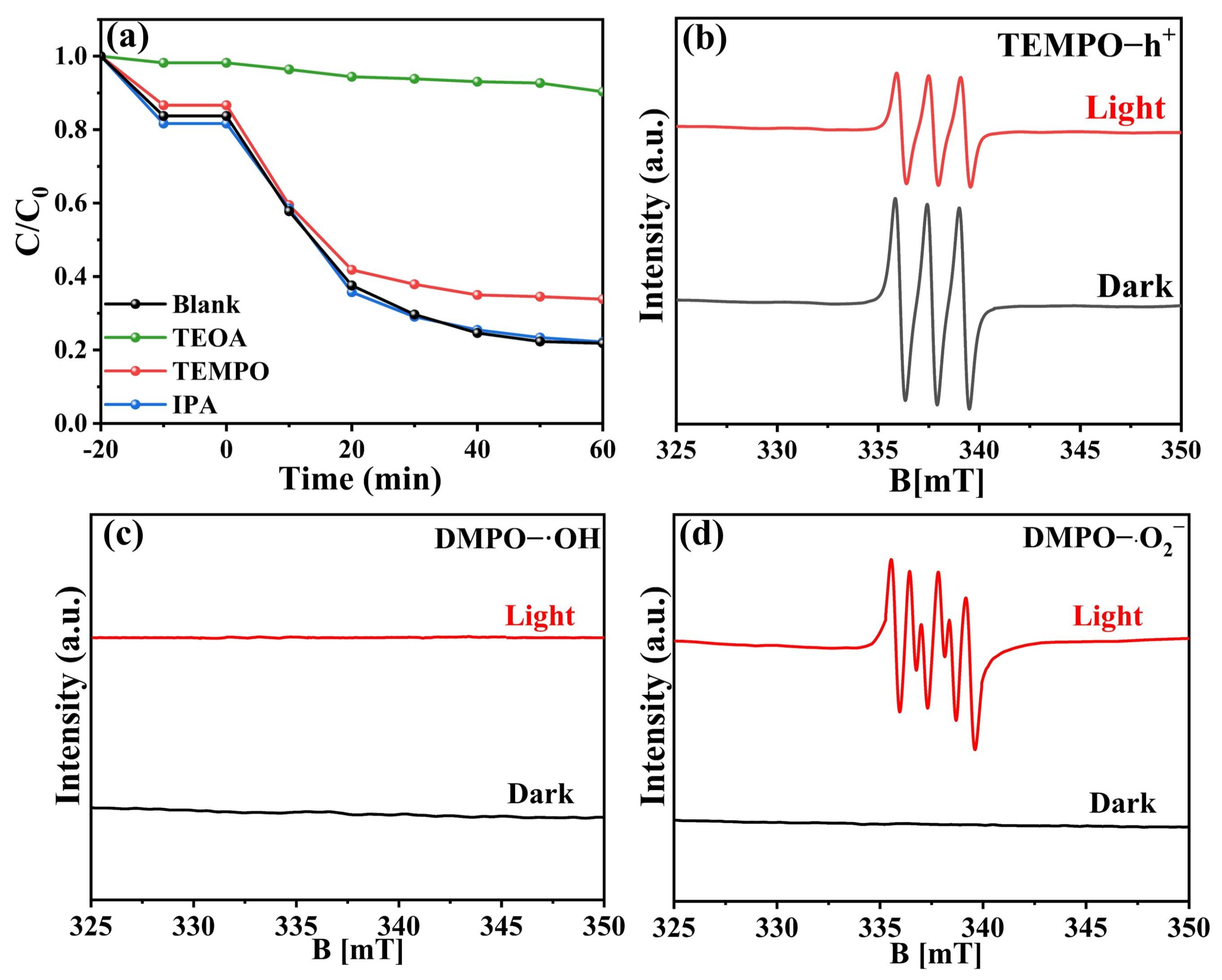
2.4.2. Active Species in Photocatalytic Reactions
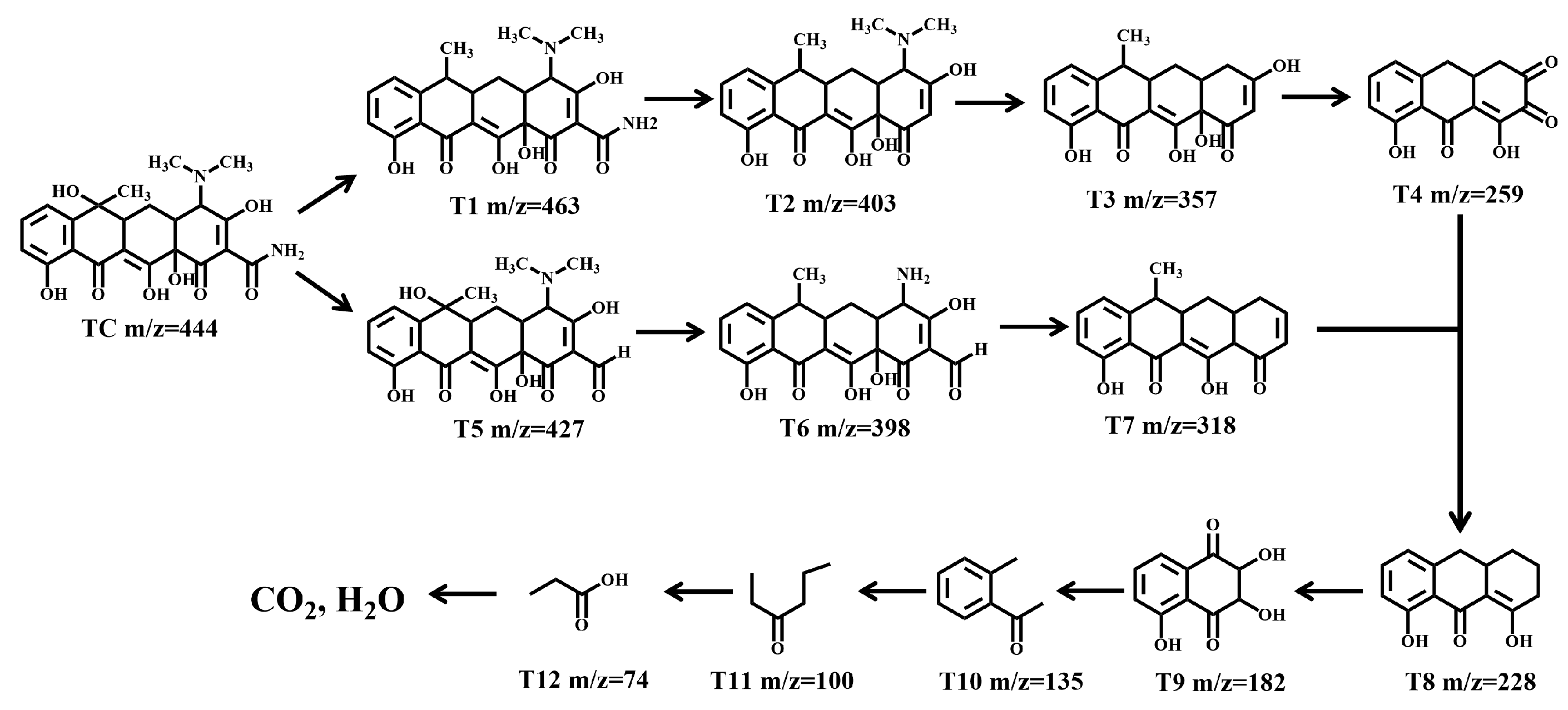
2.4.3. Degradation Pathways of TC and Toxicity Assessment
2.4.4. Possible Photocatalytic Mechanism
3. Experiments and Characterizations
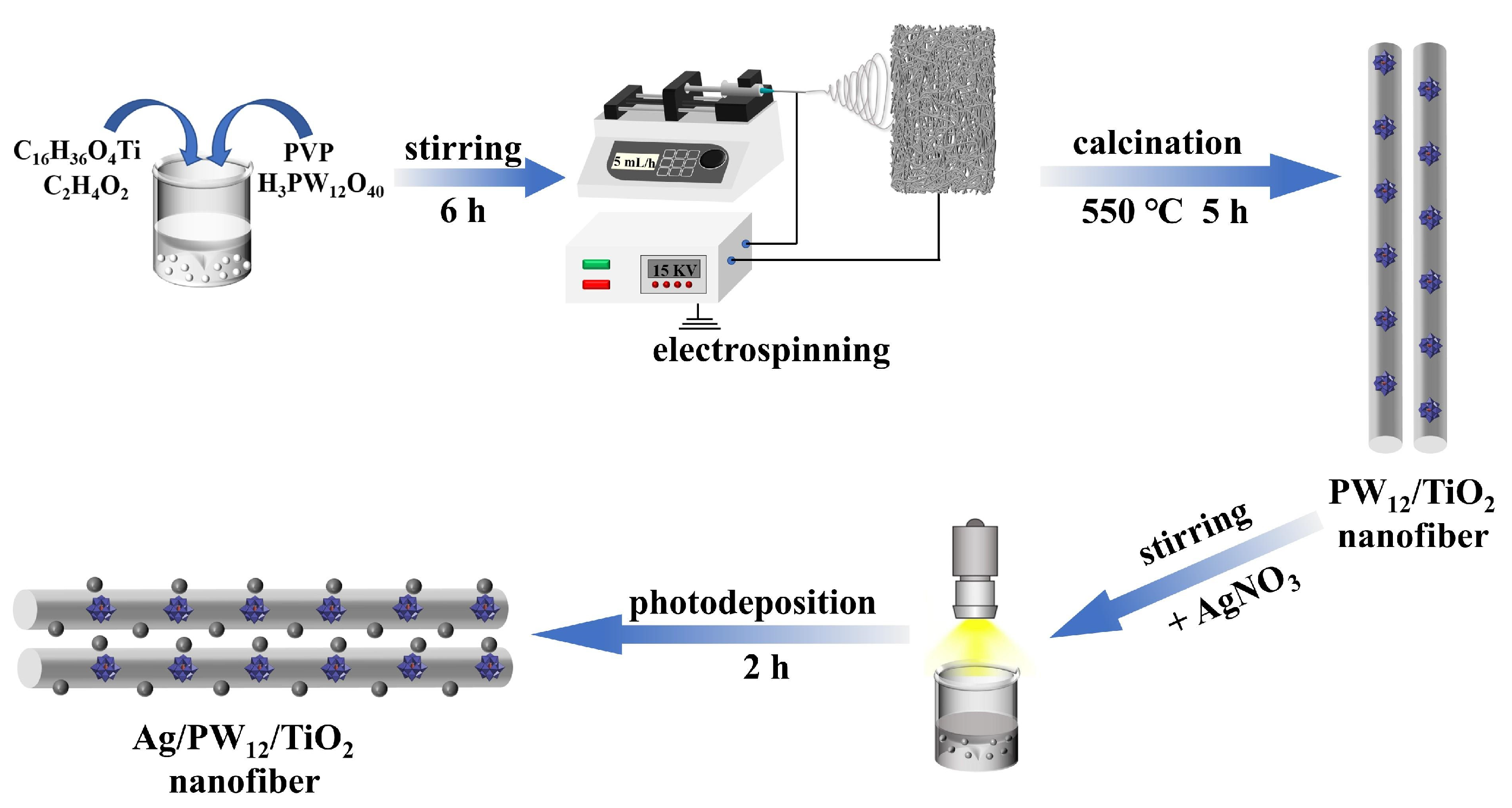
Construction of Ag/PT Photocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wu, Z.; Wang, M.Y.; Bai, Y.; Song, H.; Lv, J.X.; Mo, X.F.; Li, X.Q.; Lin, Z. Upcycling of nickel iron slags to hierarchical self-assembled flower-like photocatalysts for highly efficient degradation of high-concentration tetracycline. Chem. Eng. J. 2023, 464, 142532. [Google Scholar] [CrossRef]
- Miao, Y.X.; Zhao, Y.X.; Zhang, S.; Shi, R.; Zhang, T.R. Strain engineering: A boosting strategy for photocatalysis. Adv. Mater. 2022, 34, 2200868. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tong, F.X.; Lv, M.; Wang, Z.Y.; Liu, Y.Y.; Wang, P.; Cheng, H.F.; Dai, Y.; Zheng, Z.K.; Huang, B.B. In situ monitoring charge transfer on topotactic epitaxial heterointerface for tetracycline degradation at the single-particle level. ACS Catal. 2022, 12, 9114–9124. [Google Scholar] [CrossRef]
- Liccardo, L.; Bordin, M.; Sheverdyaeva, P.M.; Belli, M.; Moras, P.; Vomiero, A.; Moretti, E. Surface defect engineering in colored TiO2 hollow spheres toward efficient photocatalysis. Adv. Funct. Mater. 2023, 33, 2212486. [Google Scholar] [CrossRef]
- Cao, H.; Liu, F.Y.; Tai, Y.T.; Wang, W.; Li, X.Y.; Li, P.Y.; Zhao, H.Z.; Xia, Y.Q.; Wang, S.J. Promoting photocatalytic performance of TiO2 nanomaterials by structural and electronic modulation. Chem. Eng. J. 2023, 466, 143219. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Huang, Q.; Du, J.; Sun, L.; Li, F.; Meyer, T.J. Stabilization of a molecular water oxidation catalyst on a dye-sensitized photoanode by apyridyl anchor. Nat. Commun. 2020, 11, 4610. [Google Scholar] [CrossRef]
- Xing, F.Y.; Wang, C.Z.; Liu, S.Q.; Jin, S.H.; Jin, H.B.; Li, J.B. Interfacial chemical bond engineering in a direct Z-Scheme g-C3N4/MoS2 heterojunction. ACS Appl. Mater. Interface 2023, 15, 11731–11740. [Google Scholar] [CrossRef]
- Sun, G.T.; Tai, Z.G.; Li, F.; Ye, Q.; Wang, T.; Fang, Z.Y.; Jia, L.C.; Liu, W.; Wang, H.Q. Construction of ZnIn2S4/CdS/PdS S-Scheme heterostructure for efficient photocatalytic H2 production. Small 2023, 19, 2207758. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhang, Y.; Zhao, Y.N.; Tan, H.Q.; Zhao, Z.; Shi, H.F.; Wang, E.B.; Li, Y.G. AgxH3-xPMo12O40/Ag nanorods/g-C3N4 1D/2D Z-scheme heterojunction for highly efficient visible-light photocatalysis. Dalton Trans. 2019, 48, 6484–6491. [Google Scholar] [CrossRef]
- Horn, M.R.; Singh, A.; Alomari, S.; Goberna-Ferrón, S.; Benages-Vilau, R.; Chodankar, N.; Motta, N.; Ostrikov, K.; MacLeod, J.; Sonar, P.; et al. Polyoxometalates (POMs): From electroactive clusters to energy materials. Energy Environ. Sci. 2021, 14, 1652–1700. [Google Scholar] [CrossRef]
- Shi, H.F.; Yu, Y.C.; Zhang, Y.; Feng, X.J.; Zhao, X.Y.; Tan, H.Q.; Khan, S.U.; Li, Y.G.; Wang, E.B. Polyoxometalate/TiO2/Ag composite nanofibers with enhanced photocatalytic performance under visible light. Appl. Catal. B-Environ. 2018, 221, 280–289. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.L.; Wang, X.L.; Li, Y.G.; Su, Z.M.; Wang, E.B. Polyoxometalates in dye-sensitized solar cells. Chem. Soc. Rev. 2019, 48, 260–284. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Eshtiagh-Hosseini, H.; Alipour, M.; Frontera, A. Recent developments in the crystal engineering of diverse coordination modes (0–12) for Keggin-type polyoxometalates in hybrid inorganic-organic architectures. Coord. Chem. Rev. 2014, 275, 1–18. [Google Scholar] [CrossRef]
- Xing, F.S.; Zeng, R.Y.; Cheng, C.C.; Liu, Q.W.; Huang, C.J. POM-incorporated ZnIn2S4 Z-scheme dual-functional photocatalysts for cooperative benzyl alcohol oxidation and H2 evolution in aqueous solution. Appl. Catal. B Environ. 2022, 306, 121087. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, S.M.; Wang, X. Helical microporous nanorods assembled by polyoxometalate clusters for the photocatalytic oxidation of toluene. Angew. Chem. Int. Ed. 2021, 60, 17404–17409. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Liu, M.X.; Chen, L. Polyoxometalate built-in conjugated microporous polymers for visible-light heterogeneous photocatalysis. J. Mater. Chem. A. 2017, 5, 13757–13762. [Google Scholar] [CrossRef]
- He, B.W.; Luo, C.; Wang, Z.L.; Zhang, L.Y.; Yu, J.G. Synergistic enhancement of solar H2O2 and HCOOH production over TiO2 by dual co-catalyst loading in a tri-phase system. Appl. Catal. B-Environ. 2023, 323, 122200. [Google Scholar] [CrossRef]
- Pellejero, I.; Clemente, A.; Reinoso, S.; Cornejo, A.; Navajas, A.; Vesperinas, J.J.; Urbiztondo, M.A.; Gandía, L.M. Innovative catalyst integration on transparent silicone microreactors for photocatalytic applications. Catal. Today 2022, 383, 164–172. [Google Scholar] [CrossRef]
- Shi, H.F.; Zhao, T.T.; Zhang, Y.; Tan, H.Q.; Shen, W.H.; Wang, W.D.; Li, Y.G.; Wang, E.B. Pt/POMs/TiO2 composite nanofibers with enhanced visible-light photocatalytic performance for environmental remediation. Dalton Trans. 2019, 48, 13353–13359. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.P.; Shen, X.C.; Zhang, Y.; Lou, Y.; Pan, C.S.; Zhu, Y.F.; Xu, J. A plasmonic Z-scheme Ag@AgCl/PDI photocatalyst for the efficient elimination of organic pollutants, antibiotic resistant bacteria and antibiotic resistance genes. Appl. Catal. B-Environ. 2023, 324, 122220. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Guo, C.Y.; Liu, X.; Li, L.; Wang, L.; Xu, H.L.; Zhang, D.K.; Li, C.H.; Li, Q.; Wang, W.T. Ag nanoparticles anchored organic/inorganic Z-scheme 3DOMM-TiO2−x-based heterojunction for efficient photocatalytic and photoelectrochemical water splitting. Chin. J. Catal. 2022, 43, 1360–1370. [Google Scholar] [CrossRef]
- Zeng, Q.L.; Xie, X.F.; Wang, X.; Wang, Y.; Lu, G.H.; Pui, D.Y.H.; Sun, J. Enhanced photocatalytic performance of Ag@TiO2 for the gaseous acetaldehyde photodegradation under fluorescent lamp. Chem. Eng. J. 2018, 341, 83–92. [Google Scholar] [CrossRef]
- Kumar, P.S.; Sundaramurthy, J.; Sundarrajan, S.; Babu, V.J.; Singh, G.; Allakhverdiev, S.I.; Ramakrishna, S. Hierarchical electrospun nanofibers for energy harvesting, production and environmental remediation. Energy Environ. Sci. 2014, 7, 3192–3222. [Google Scholar] [CrossRef]
- Li, C.X.; Zhao, Y.X.; Song, Y.X.; Qiu, X.J.; Wang, S.Z.; Sun, P.Z. Optimization of electron transport pathway: A novel strategy to solve the photocorrosion of Ag-based photocatalysts. Environ. Sci. Technol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Jiang, X.Y.; Huang, J.D.; Lu, W.; Zhang, Z.Y. Plasmon-enhanced photocatalytic overall water-splitting over Au nanoparticle-decorated CaNb2O6 electrospun nanofibers. J. Mater. Chem. A 2022, 10, 20048–20058. [Google Scholar] [CrossRef]
- Le, T.T.; Lee, M.; Chae, K.H.; Moon, G.H.; Kim, S.H. Control of copper element in mesoporous iron oxide photocatalysts towards UV light-assisted superfast mineralization of isopropyl alcohol with peroxydisulfate. Chem. Eng. J. 2023, 451, 139048. [Google Scholar] [CrossRef]
- Shi, H.F.; Zhao, T.T.; Wang, J.B.; Wang, Y.T.; Chen, Z.; Liu, B.L.; Ji, H.F.; Wang, W.D.; Zhang, G.L.; Li, Y.G. Fabrication of g-C3N4/PW12/TiO2 composite with significantly enhanced photocatalytic performance under visible light. J. Alloys Compd. 2021, 860, 157924. [Google Scholar] [CrossRef]
- Mahadadalkar, M.A.; Park, N.; Yusuf, M.; Nagappan, S.; Nallal, M.; Park, K.H. Electrospun Fe doped TiO2 fiber photocatalyst for efficient wastewater treatment. Chemosphere 2023, 330, 138599. [Google Scholar] [CrossRef]
- Ni, J.X.; Liu, D.M.; Wang, W.; Wang, A.W.; Jia, J.L.; Tian, J.Y.; Xing, Z.P. Hierarchical defect-rich flower-like BiOBr/Ag nanoparticles/ultrathin g-C3N4 with transfer channels plasmonic Z-scheme heterojunction photocatalyst for accelerated visible-light-driven photothermal-photocatalytic oxytetracycline degradation. Chem. Eng. J. 2021, 419, 129969. [Google Scholar] [CrossRef]
- Huang, X.Y.; Liu, X. Highly polymerized linear polyimide/H3PW12O40 photocatalyst with full visible light region absorption. Chemosphere 2021, 283, 131230. [Google Scholar] [CrossRef]
- You, Y.L.J.; Gao, S.Y.; Yang, Z.; Cao, M.N.; Cao, R. Facile synthesis of polyoxometalate-thionine composite via direct precipitation method and its photocatalytic activity for degradation of rhodamine B under visible light. J. Colloid Interface Sci. 2012, 365, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.B.; Guo, Y.M.; Qi, S.P.; Zhang, K.; Yang, J.F.; Li, B.N.; Chen, J.X.; Zhao, Y.X.; Lou, Y.B. Cu7S4/MnIn2S4 heterojunction for efficient photocatalytic hydrogen generation. J. Alloys Compd. 2021, 884, 161035. [Google Scholar] [CrossRef]
- Gao, B.Q.; Tao, K.K.; Xi, Z.H.; El-Sayed, M.M.H.; Shoeib, T.; Yang, H. Fabrication of 3D lignosulfonate composited sponges impregnated by BiVO4/polyaniline/Ag ternary photocatalyst for synergistic adsorption-photodegradation of fluoroquinolones in water. Chem. Eng. J. 2022, 446, 137282. [Google Scholar] [CrossRef]
- Kong, W.H.; Wang, S.L.; Wu, D.; Chen, C.R.; Luo, Y.S.; Pei, Y.T.; Tian, B.Z.; Zhang, J.L. Fabrication of 3D sponge@AgBr-AgCl/Ag and tubular photoreactor for continuous wastewater purification under sunlight irradiation. ACS Sustain. Chem. Eng. 2019, 7, 14051–14063. [Google Scholar] [CrossRef]
- Tamilselvan, S.; Soniya, R.M.; Vasantharaja, R.; Kannan, M.; Supriya, S. Silver nanoparticles based spectroscopic sensing of eight metal ions in aqueous solutions. Environ. Res. 2022, 212, 113585. [Google Scholar] [CrossRef]
- Shi, H.F.; Jin, T.; Li, J.P.; Li, Y.L.; Chang, Y.Q.; Jin, Z.H.; Jiang, W.; Qu, X.S.; Chen, Z. Construction of Z-scheme Cs3PMo12O40/g-C3N4 composite photocatalyst with highly efficient photocatalytic performance under visible light irradiation. J. Solid State Chem. 2022, 311, 123069. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A review on plasmonic metal–TiO2 composite for generation, trapping, storing and dynamic vectorial transfer of photogenerated electrons across the Schottky junction in a photocatalytic system. Appl. Surf. Sci. 2016, 360, 601–622. [Google Scholar] [CrossRef]
- Mu, F.H.; Liu, C.X.; Xie, Y.; Zhou, S.J.; Dai, B.L.; Xia, D.H.; Huang, H.B.; Zhao, W.; Sun, C.; Kong, Y.; et al. Metal-organic framework-derived rodlike AgCl/Ag/In2O3: A plasmonic Z-scheme visible light photocatalyst. Chem. Eng. J. 2021, 415, 129010. [Google Scholar] [CrossRef]
- Basumatary, B.; Basumatary, R.; Ramchiary, A.; Konwar, D. Evaluation of Ag@TiO2/WO3 heterojunction photocatalyst for enhanced photocatalytic activity towards methylene blue degradation. Chemosphere 2022, 286, 131848. [Google Scholar] [CrossRef]
- Yang, R.X.; Zhong, S.; Zhang, L.S.; Liu, B.J. PW12/CN@Bi2WO6 composite photocatalyst prepared based on organic-inorganic hybrid system for removing pollutants in water. Sep. Purif. Technol. 2020, 235, 116270. [Google Scholar] [CrossRef]
- Lu, N.; Wang, Y.Q.; Ning, S.Q.; Zhao, W.J.; Qian, M.; Ma, Y.; Wang, J.; Fan, L.Y.; Guan, J.N.; Yuan, X. Design of plasmonic Ag-TiO2/H3PW12O40 composite film with enhanced sunlight photocatalytic activity towards o-chlorophenol degradation. Sci. Rep. 2017, 7, 17298. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, M.H.; Xu, L.; Li, F.Y. Limitation of WO3 in Zn-Co3O4 nanopolyhedra by the pyrolysis of H3PW12O40@BMZIF: Synergistic effect of heterostructure and oxygen vacancies for enhanced nitrogen fixation. Inorg. Chem. 2023, 62, 8710–8718. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, H.X.; Wang, K.; Chen, J.; Li, M.; Wang, Y.; Tsiakaras, P.; Song, S.Q. Enhanced TiO2 photocatalytic 2 e– oxygen reduction reaction via interfacial microenvironment regulation and mechanism analysis. ACS Catal. 2023, 13, 6497–6508. [Google Scholar] [CrossRef]
- Gao, Y.T.; Chen, F.; Chen, Z.; Shi, H.F. NixCo1−xS as an effective noble metal-free cocatalyst for enhanced photocatalytic activity of g-C3N4. J. Mater. Sci. Technol. 2020, 56, 227–235. [Google Scholar] [CrossRef]
- Guo, J.; Gan, W.; Ding, C.S.; Lu, Y.Q.; Li, J.R.; Qi, S.H.; Zhang, M.; Sun, Z.Q. Black phosphorus quantum dots and Ag nanoparticles co-modified TiO2 nanorod arrays as powerful photocatalyst for tetracycline hydrochloride degradation: Pathways, toxicity assessment, and mechanism insight. Sep. Purif. Technol. 2022, 297, 121454. [Google Scholar] [CrossRef]
- Li, J.H.; Kang, W.L.; Yang, X.; Yu, X.D.; Xu, L.L.; Guo, Y.H.; Fang, H.B.; Zhang, S.D. Mesoporous titania-based H3PW12O40 composite by a block copolymer surfactant-assisted templating route: Preparation, characterization, and heterogeneous photocatalytic properties. Desalination 2010, 255, 107–116. [Google Scholar] [CrossRef]
- Yang, C.D.; Feng, S.; Ma, C.C.; Zhou, Y.; Dai, X.J.; Ye, Z.W.; Wang, Y. Bi2Sn2O7/UiO-66-NH2 heterojunction photocatalyst simultaneously adsorbed and photodegraded tetracycline. J. Environ. Chem. Eng. 2023, 11, 109664. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, L.; Zhao, X.L.; Zhang, J.Q.; Ao, Z.M.; Liu, W.R.; Wu, H.; Shi, L.; Yin, Y.; Xu, X.Y.; et al. Efficient photocatalytic overall water splitting on metal-free 1D SWCNT/2D ultrathin C3N4 heterojunctions via novel non-resonant plasmonic effect. Appl. Catal. B-Environ. 2020, 278, 119312. [Google Scholar] [CrossRef]
- Li, S.Y.; Niu, Z.W.; Pan, D.Q.; Cui, Z.P.; Shang, H.W.; Lian, J.; Wu, W.S. Efficient photoreduction strategy for uranium immobilization based on graphite carbon nitride/activated carbon nanocomposites. Chin. Chem. Lett. 2022, 33, 3581–3584. [Google Scholar] [CrossRef]
- Wu, C.; Dai, J.N.; Ma, J.; Zhang, T.Y.; Qiang, L.S.; Xue, J.Q. Mechanistic study of B-TiO2/BiVO4 S-scheme heterojunction photocatalyst for tetracycline hydrochloride removal and H2 production. Sep. Purif. Technol. 2023, 312, 123398. [Google Scholar] [CrossRef]
- Zhu, L.D.; Zhou, Y.X.; Fei, L.Y.; Cheng, X.L.; Zhu, X.X.; Deng, L.Q.; Ma, X. Z-scheme CuO/Fe3O4/GO heterojunction photocatalyst: Enhanced photocatalytic performance for elimination of tetracycline. Chemosphere 2022, 309, 136721. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, H.J.; Liu, J.J.; Liu, X.; Song, X.H.; Zhou, W.Q.; Zhang, J.S.; Huo, P.W. Enhanced degradation of polyethylene terephthalate plastics by CdS/CeO2 heterojunction photocatalyst activated peroxymonosulfate. J. Hazard. Mater. 2023, 452, 131375. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.F.D.; Xavier, É.S.; Zerner, M.C.; Almeida, W.B.D. Spectroscopic investigation of the Al(III)-anhydrotetracycline complexation process. J. Mol. Struct. 2000, 527, 193–202. [Google Scholar] [CrossRef]
- Yang, J.H.; Sun, J.L.; Chen, S.; Lan, D.Q.; Li, Z.H.; Li, Z.J.; Wei, J.W.; Yu, Z.B.; Zhu, H.X.; Wang, S.F.; et al. S-scheme 1 T phase MoSe2/AgBr heterojunction toward antibiotic degradation: Photocatalytic mechanism, degradation pathways, and intermediates toxicity evaluation. Sep. Purif. Technol. 2022, 290, 120881. [Google Scholar] [CrossRef]
- Gao, P.; Li, Z.X.; Feng, L.; Liu, Y.Z.; Du, Z.W.; Zhang, L.Q. Construction of novel MWCNTs/Bi4O5I2 nanosheets with enhanced adsorption and photocatalytic performance for the degradation of tetracycline: Efficiency, mechanism and regeneration. Chem. Eng. J. 2022, 429, 132398. [Google Scholar] [CrossRef]
- Abilarasu, A.; Kumar, P.S.; Vo, D.V.N.; Krithika, D.; Ngueagni, P.T.; Joshiba, G.J.; Carolin, C.F.; Prasannamedha, G. Enhanced photocatalytic degradation of diclofenac by Sn0.15Mn0.85Fe2O4 catalyst under solar light. J. Environ. Chem. Eng. 2021, 9, 104875. [Google Scholar] [CrossRef]
- Shi, H.F.; Zhu, H.W.; Jin, T.; Chen, L.; Zhang, J.Y.; Qiao, K.Y.; Chen, Z. Construction of Bi/Polyoxometalate doped TiO2 composite with efficient visible-light photocatalytic performance: Mechanism insight, degradation pathway and toxicity evaluation. Appl. Surf. Sci. 2023, 615, 156310. [Google Scholar] [CrossRef]
- Chen, D.D.; Yi, X.H.; Zhao, C.; Fu, H.F.; Wang, P.; Wang, C.C. Polyaniline modified MIL-100(Fe) for enhanced photocatalytic Cr(VI) reduction and tetracycline degradation under white light. Chemosphere 2020, 245, 125659. [Google Scholar] [CrossRef]
- Wang, H.X.; Liao, B.; Lu, T.; Ai, Y.L.; Liu, G. Enhanced visible-light photocatalytic degradation of tetracycline by a novel hollow BiOCl@CeO2 heterostructured microspheres: Structural characterization and reaction mechanism. J. Hazard. Mater. 2020, 385, 12155. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, Y.T.; Chen, F.; Shi, H.F. Metallic NiSe cocatalyst decorated g-C3N4 with enhanced photocatalytic activity. Chem. Eng. J. 2023, 413, 127474. [Google Scholar] [CrossRef]
- Liu, C.; He, X.X.; Xu, Q.X.; Chen, M. A general way to realize the bi-directional promotion effects on the photocatalytic removal of heavy metals and organic pollutants in real water by a novel S-scheme heterojunction: Experimental investigations, QSAR and DFT calculations. J. Hazard. Mater. 2023, 445, 130551. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, Z.T.; Feng, Y.; Dai, H.L.; Zhao, Y.F.; Han, N.; Zhang, Q.F.; Zou, Z.G. Ultrathin Z-scheme 2D/2D N-doped HTiNbO5 nanosheets/g-C3N4 porous composites for efficient photocatalytic degradation and H2 generation under visible light. J. Colloid Interface Sci. 2021, 583, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.F.; Yan, G.; Zhang, Y.; Tan, H.Q.; Zhou, W.Z.; Ma, Y.Y.; Li, Y.G.; Chen, W.L.; Wang, E.B. Ag/AgxH3-xPMo12O40 nanowires with enhanced visible light-driven photocatalytic performance. ACS Appl. Mater. Interface 2017, 9, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gao, Y.T.; Mu, D.Z.; Shi, H.F.; Lou, D.W.; Liu, S.Y. Recyclable magnetic NiFe2O4/C yolk–shell nanospheres with excellent visible-light-Fenton degradation performance of tetracycline hydrochloride. Dalton Trans. 2019, 48, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.F.; Tan, H.Q.; Zhu, W.B.; Sun, Z.C.; Ma, Y.J.; Wang, E.B. Electrospun Cr-doped Bi4Ti3O12/Bi2Ti2O7 heterostructure fibers with enhanced visible-light photocatalytic properties. J. Mater. Chem. A 2015, 3, 6586–6591. [Google Scholar] [CrossRef]
- Wang, Y.H.; Han, D.M.; Wang, Z.H.; Gu, F.B. Efficient photocatalytic degradation of tetracycline under visible light by an all-solid-state Z-Scheme Ag3PO4/MIL-101(Cr) heterostructure with metallic Ag as a charge transmission bridge. ACS Appl. Mater. Interface 2023, 15, 22085–22100. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Xie, T.P.; Wang, J.K.; Yang, J.W.; Kong, D.S.; Liu, C.W.; Chen, S.L.; Yang, F.L.; Pan, M.J.; Yang, J.; et al. Photocatalytic activation of PMS over magnetic heterojunction photocatalyst SrTiO3/BaFe12O19 for tetracycline ultrafast degradation. Chem. Eng. J. 2023, 470, 143900. [Google Scholar] [CrossRef]
- Li, S.J.; Yan, R.Y.; Cai, M.J.; Jiang, W.; Zhang, M.Y.; Li, X. Enhanced antibiotic degradation performance of Cd0.5Zn0.5S/Bi2MoO6 S-scheme photocatalyst by carbon dot modification. J. Mater. Sci. Technol. 2023, 164, 59–67. [Google Scholar] [CrossRef]
- Li, X.L.; Yang, G.Q.; Li, S.S.; Xiao, N.; Li, N.; Gao, Y.Q.; Lv, D.; Ge, L. Novel dual co-catalysts decorated Au@HCS@PdS hybrids with spatially separated charge carriers and enhanced photocatalytic hydrogen evolution activity. Chem. Eng. J. 2020, 379, 122350. [Google Scholar] [CrossRef]
- Li, S.J.; Cai, M.J.; Wang, C.C.; Liu, Y.P. Ta3N5/CdS core–shell S-scheme heterojunction nanofibers for efficient photocatalytic removal of antibiotic tetracycline and Cr(VI): Performance and mechanism insights. Adv. Fiber Mater. 2023, 5, 994–1007. [Google Scholar] [CrossRef]
- Li, S.J.; Wang, C.C.; Dong, K.X.; Zhang, P.; Chen, X.B.; Li, X. MIL-101(Fe)/BiOBr S-scheme photocatalyst for promoting photocatalytic abatement of Cr(VI) and enrofloxacin antibiotic: Performance and mechanism. Chin. J. Catal. 2023, 51, 101–112. [Google Scholar] [CrossRef]
- Cai, Z.Q.; Song, Y.G.; Jin, X.B.; Wang, C.C.; Ji, H.D.; Liu, W.; Sun, X.B. Highly efficient AgBr/h-MoO3 with charge separation tuning for photocatalytic degradation of trimethoprim: Mechanism insight and toxicity assessment. Sci. Total. Environ. 2021, 781, 146754. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.L.; Chen, J.; Tian, L.; Fan, C.; Xu, W.T.; Zhang, Y.J.; Gan, T.; Hu, H.Y.; Huang, Z.Q.; Qin, Y.B. Construction of a recyclable chitosan-based aerogel-supported TiO2 catalyst for treating high-concentration surfactants. Compos. Part. B-Eng. 2023, 251, 110475. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.H.; Zhang, L.F.; Jiang, B.; Sun, Y.L. In-situ constructed 2D/2D ZnIn2S4/Bi4Ti3O12 S-scheme heterojunction for degradation of tetracycline: Performance and mechanism insights. J. Hazard. Mater. 2022, 438, 129438. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Li, W.J.; Xie, H.X.; Li, X.; Ge, B.; Yang, L.Q.; Chang, M.J.; Du, H.L.; Song, S.J. Controllable fabrication of Bi4Ti3O12/C/Bi2S3/MoS2 heterojunction with effective suppression of Bi2S3 assisted by amorphous carbon interlayer for significantly enhanced photocatalysis. J. Taiwan Inst. Chem. E 2023, 146, 104882. [Google Scholar] [CrossRef]
- Shi, H.F.; Fu, J.C.; Jiang, W.; Wang, Y.T.; Liu, B.L.; Liu, J.X.; Ji, H.F.; Wang, W.D.; Chen, Z. Construction of g-C3N4/Bi4Ti3O12 hollow nanofibers with highly efficient visible-light-driven photocatalytic performance. Colloid Surf. A 2021, 615, 126063. [Google Scholar] [CrossRef]
- Sayed, M.; Yu, J.G.; Liu, G.; Jaroniec, M. Non-noble plasmonic metal-based photocatalysts. Chem. Rev. 2022, 122, 10484–10537. [Google Scholar] [CrossRef]
- Jin, Z.Z.; Li, J.R.; Zhang, Y.M.; Liu, D.; Ding, H.; Mamba, B.B.; Kuvarega, A.T.; Gui, J.Z. Rational design of efficient visible-light photocatalysts (1D@2D/0D) ZnO@Ni-doped BiOBr/Bi heterojunction: Considerations on hierarchical structures, doping and SPR effect. J. Mater. Sci. Technol. 2022, 125, 38–50. [Google Scholar] [CrossRef]
- He, D.; Chen, Y.; Situ, Y.; Zhong, L.; Huang, H. Synthesis of ternary g-C3N4/Ag/-FeOOH photocatalyst: An integrated heterogeneous fenton-like system for effectively degradation of azo dye methyl orange under visible light. Appl. Surf. Sci. 2017, 425, 862–872. [Google Scholar] [CrossRef]
- Liang, J.X.; Hou, Y.P.; Zhu, H.X.; Xiong, J.H.; Huang, W.Y.; Yu, Z.B.; Wang, S.F. Levofloxacin degradation performance and mechanism in the novel electro-Fenton system constructed with vanadium oxide electrodes under neutral pH. Chem. Eng. J. 2021, 433, 133574. [Google Scholar] [CrossRef]
- Gong, Y.N.; Wang, Y.; Tang, M.M.; Zhang, H.; Wu, P.; Liu, C.J.; He, J.; Jiang, W. A two-step process coupling photocatalysis with adsorption to treat tetracycline-Copper(II) hybrid wastewaters, degradation mechanism, pathways and biotoxicity evaluation. J. Water. Process. Eng. 2022, 47, 102710. [Google Scholar] [CrossRef]
- Chen, L.J.; Li, Y.H.; Zhang, J.W.; Li, M.X.; Yin, W.Y.; Chen, X. Oxidative degradation of tetracycline hydrochloride by Mn2O3/Bi2O3 photocatalysis activated peroxymonosulfate. Inorg. Chem. Commun. 2022, 140, 109414. [Google Scholar] [CrossRef]
- Zhang, X.M.; Wang, H.; Gao, M.M.; Zhao, P.F.; Xia, W.L.; Yang, R.L.; Huang, Y.C.; Wang, L.; Liu, M.X.; Wei, T.; et al. Template-directed synthesis of pomegranate-shaped zinc oxide@zeolitic imidazolate framework for visible light photocatalytic degradation of tetracycline. Chemosphere 2022, 294, 133782. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.Q.; Cao, X.J.; Wang, B.; Jiang, Q.; Chen, Z.G.; Xia, J.X. In-situ synthesis of MoS2/BiOBr material via mechanical ball milling for boosted photocatalytic degradation pollutants performance. ChemistrySelect 2021, 6, 928–936. [Google Scholar] [CrossRef]
- Wu, S.Q.; Li, X.Y.; Tian, Y.Q.; Lin, Y.; Hu, Y.H. Excellent photocatalytic degradation of tetracycline over black anatase-TiO2 under visible light. Chem. Eng. J. 2021, 406, 126747. [Google Scholar] [CrossRef]
- Li, S.Y.; Tang, Y.W.; Wang, M.; Kang, J.; Jin, C.Y.; Liu, J.Y.; Li, Z.L.; Zhu, J.W. NiO/g-C3N4 2D/2D heterojunction catalyst as efficient peroxymonosulfate activators toward tetracycline degradation: Characterization, performance and mechanism. J. Alloys Compd. 2021, 880, 160547. [Google Scholar] [CrossRef]
- Shen, X.F.; Zhang, Y.; Shi, Z.; Shan, S.D.; Liu, J.S.; Zhang, L.S. Construction of C3N4/CdS nanojunctions on carbon fiber cloth as a filter-membrane-shaped photocatalyst for degrading flowing wastewater. J. Alloys Compd. 2021, 851, 156743. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Ranjith, K.S.; Lee, H.; Park, B.; Norouzi, M.; Nikoo, S.Z.; Kim, W.S.; Han, Y.K.; Huh, Y.S. Tuning the phase composition of 1D TiO2 by Fe/Sn co-doping strategy for enhanced visible-light-driven photocatalytic and photoelectrochemical performances. J. Alloys Compd. 2021, 851, 156826. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Q.; Chen, P.; Zheng, H.; Shi, J.; Shu, H.; Liu, Y. Photocatalytic degradation of tetracycline by using a regenerable (Bi)BiOBr/rGO composite. J. Clean. Prod. 2022, 339, 130771. [Google Scholar] [CrossRef]
- Chen, Z.J.; Guo, H.; Liu, H.Y.; Niu, C.G.; Huang, D.W.; Yang, Y.Y.; Liang, C.; Li, L.; Li, J.C. Construction of dual S-scheme Ag2CO3/Bi4O5I2/g-C3N4 heterostructure photocatalyst with enhanced visible-light photocatalytic degradation for tetracycline. Chem. Eng. J. 2022, 438, 135471. [Google Scholar] [CrossRef]
- Cestaro, R.; Philippe, L.; Serrà, A.; Gómez, E.; Schmutz, P. Electrodeposited manganese oxides as efficient photocatalyst for the degradation of tetracycline antibiotics pollutant. Chem. Eng. J. 2023, 462, 142202. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Rafiee, E.; Eavani, S. Photocatalytic removal of toxic dyes, liquorice and tetracycline wastewaters by a mesoporous photocatalyst under irradiation of different lamps and sunlight. J. Environ. Manag. 2022, 313, 115023. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G.; Chen, X.L.; Di, J.; Liu, Y.L.; Yin, S.; Xia, J.X.; Li, H.M. Graphene-like boron nitride modified bismuth phosphate materials for boosting photocatalytic degradation of enrofloxacin. J. Colloid Interface Sci. 2017, 492, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Li, G.B.; Wang, D.; Zhong, Z.C.; Hu, K.B.; Zhang, C.Q.; Hu, G.P.; Li, X.W.; Wan, Y.H. Lanthanide-doped upconversion glass-ceramic photocatalyst fabricated from fluorine-containing waste for the degradation of organic pollutants. J. Colloid Interface Sci. 2023, 638, 461–473. [Google Scholar] [CrossRef]
- Huang, J.X.; Li, D.G.; Li, R.B.; Chen, P.; Zhang, Q.X.; Liu, H.J.; Lv, W.Y.; Liu, G.G.; Feng, Y.P. One-step synthesis of phosphorus/oxygen co-doped g-C3N4/anatase TiO2 Z-scheme photocatalyst for significantly enhanced visible-light photocatalysis degradation of enrofloxacin. J. Hazard. Mater. 2020, 386, 12. [Google Scholar] [CrossRef]
- Wen, X.J.; Niu, C.G.; Zhang, L.; Liang, C.; Zeng, G.M. A novel Ag2O/CeO2 heterojunction photocatalysts for photocatalytic degradation of enrofloxacin: Possible degradation pathways; mineralization activity and an in depth mechanism insight. Appl. Catal. B-Environ. 2018, 221, 701–714. [Google Scholar] [CrossRef]
- Cai, M.J.; Liu, Y.P.; Wang, C.C.; Lin, W.; Li, S.J. Novel Cd0.5Zn0.5S/Bi2MoO6 S-scheme heterojunction for boosting the photodegradation of antibiotic enrofloxacin: Degradation pathway; mechanism and toxicity assessment. Sep. Purif. Technol. 2023, 304, 11. [Google Scholar] [CrossRef]
- Li, T.C.; Liu, J.X.; Shi, F.; Zhang, H.Y.; Zhang, H.J.; Ma, C.C.; Wasim, M. A novel S-type CsxWO3/BiOI heterojunction photocatalyst constructed in graphene aerogel with high degradation efficiency for enrofloxacin: Degradation mechanism and DFT calculation. J. Environ. Chem. Eng. 2023, 11, 109301. [Google Scholar] [CrossRef]
- Xiao, L.Q.; Zhang, S.Y.; Chen, B.Q.; Wu, P.P.; Feng, N.D.; Deng, F.; Wang, Z. Visible-light photocatalysis degradation of enrofloxacin by crawfish shell biochar combined with g-C3N4: Effects and mechanisms. J. Environ. Chem. Eng. 2023, 11, 109693. [Google Scholar] [CrossRef]
- Huang, P.Q.; Luan, J.F. Synthesis of a GaOOH/ZnBiTaO5 heterojunction photocatalyst with enhanced photocatalytic performance toward enrofloxacin. RSC Adv. 2020, 10, 4286–4292. [Google Scholar] [CrossRef]
- Sciscenko, I.; Mestre, S.; Climent, J.; Valero, F.; Escudero-Onate, C.; Oller, I.; Arques, A. Magnetic Photocatalyst for Wastewater Tertiary Treatment at Pilot Plant Scale: Disinfection and Enrofloxacin Abatement. Water 2021, 13, 12. [Google Scholar] [CrossRef]
- Su, Y.H.; Chen, P.; Wang, F.L.; Zhang, Q.X.; Chen, T.S.; Wang, Y.F.; Yao, K.; Lv, W.Y.; Liu, G.G. Decoration of TiO2/g-C3N4 Z-scheme by carbon dots as a novel photocatalyst with improved visible-light photocatalytic performance for the degradation of enrofloxacin. RSC Adv. 2017, 7, 34096–34103. [Google Scholar] [CrossRef]
- Huang, P.Q.; Luan, J.F. Dispersed GaOOH rods loaded on the surface of ZnBiNbO5 particles with enhanced photocatalytic activity toward enrofloxacin. RSC Adv. 2019, 9, 32027–32033. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Yan, L.; Cheng, J.M.; Jing, C.Y. Mechanistic insights into TiO2 thickness in Fe3O4@TiO2-GO composites for enrofloxacin photodegradation. Chem. Eng. J. 2017, 325, 647–654. [Google Scholar] [CrossRef]
- Mahjoub, A.R.; Rahmani, H.; Khazaee, Z. Bimetallic CuAg alloyed nanoparticles anchored on CdS nanorods for the photocatalytic degradation of enrofloxacin. ACS Appl. Nano. Mater. 2023, 6, 4554–4566. [Google Scholar]
- Luan, J.F.; Liu, W.L.; Yao, Y.; Ma, B.B.; Niu, B.W.; Yang, G.M.; Wei, Z.J. Synthesis and Property Examination of Er2FeSbO7/BiTiSbO6 Heterojunction Composite Catalyst and Light-Catalyzed Retrogradation of Enrofloxacin in Pharmaceutical Waste Water under Visible Light Irradiation. Materials 2022, 15, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Liu, X.T.; Gu, S.N.; Liu, J.M. Enhanced photocatalytic performance of rhodamine B and enrofloxacin by Pt loaded Bi4V2O11: Boosted separation of charge carriers; additional superoxide radical production; and the photocatalytic mechanism. RSC Adv. 2021, 11, 9746–9755. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.L.; Wu, S.; Liu, S.X.; Zhang, W. Photothermal enhancement of highly efficient photocatalysis with bioinspired thermal radiation balance characteristics. Appl. Surf. Sci. 2022, 592, 153304. [Google Scholar] [CrossRef]
- Sane, P.K.; Rakte, D.; Tambat, S.; Bhalinge, R.; Sontakke, S.M.; Nemade, P. Enhancing solar photocatalytic activity of Bi5O7I photocatalyst with activated carbon heterojunction. Adv. Powder. Technol. 2022, 33, 103357. [Google Scholar] [CrossRef]
- Harikumar, B.; Okla, M.K.; Alaraidh, I.A.; Mohebaldi, A.; Soufa, W.; Abdel-Maksoud, M.A.; Aufy, M.; Thomas, A.M.; Raju, L.L.; Khan, S. Robust visible light active CoNiO2–BiFeO3–NiS ternary nanocomposite for photo-fenton degradation of rhodamine B and methyl orange: Kinetics; degradation pathway and toxicity assessment. J. Environ. Manag. 2022, 317, 115321. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.F.; Liu, J.S.; Wu, Z.Y.; Zhu, K.J.; Suo, H.; Lv, X.L.; Fu, Y.L.; Jian, R.; Sun, Z.B. Construction of Bi2WO6/ZnIn2S4 with Z-scheme structure for efficient photocatalytic performance. Chem. Phys. Lett. 2021, 769, 138449. [Google Scholar] [CrossRef]
- Van, N.U.; Thuy, N.P.; Hanh, V.N.; Loan, D.T.; Vuong, D.B.; Thao, T.T. Low-temperature designing of BiVO4 nanocubes with coexposed {010}/{110} facets for solar light photocatalytic degradation of methyl orange and diazinon. Inorg. Chem. Commun. 2022, 136, 109136. [Google Scholar] [CrossRef]
- Hieu, V.Q.; Phung, T.K.; Nguyen, T.; Khan, A.; Doan, V.D.; Tran, V.A.; Le, V.T. Photocatalytic degradation of methyl orange dye by Ti3C2-eTiO2 heterojunction under solar light. Chemosphere 2021, 276, 130154. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, T.; Tao, L.L.; Lei, H.W.; Ma, P.Y.; Liu, J. A novel copper-doped porous carbon nanospheres film prepared by onestep ultrasonic spray pyrolytic of sugar for photocatalytic degradation of methyl orange. Process. Saf. Environ. 2022, 158, 79–86. [Google Scholar] [CrossRef]
- Menon, S.G.; Bedyala, A.K.; Pathakc, T.; Kumara, V.; Swart, H.C. Sr4Al14O25: Eu2+, Dy3+@ZnO nanocomposites as highly efficient visible light photocatalysts for the degradation of aqueous methyl orange. J. Alloys Compd. 2021, 860, 158370. [Google Scholar] [CrossRef]
- Li, J.W.; He, M.Z.; Yan, J.K.; Liu, J.H.; Zhang, J.X.; Ma, J.J. Room temperature engineering crystal facet of Cu2O for photocatalytic degradation of methyl orange. Nanomaterials 2022, 10, 1697. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Jasni, M.A.A.; Lim, Y.C. A highly photoresponsive and efficient molybdenum modifiedtitanium dioxide photocatalyst for the degradation of methyl orange. Int. J. Environ. Sci. Technol. 2022, 19, 5579–5594. [Google Scholar] [CrossRef]
- Lu, G.F.; Liu, X.D.; Zhang, P.; Xu, S.T.; Gao, Y.J.; Yu, S.Y. Preparation and Photocatalytic Studies on Nanocomposites of 4-Hydroxylphenyl-Substituted Corrole/TiO2 towards Methyl Orange Photodegradation. ChemistrySelect 2021, 6, 6841–6846. [Google Scholar] [CrossRef]
- Kourab, P.; Mukherjee, S.P. CsPbBr3/Cs4PbBr6 perovskite@COF nanocomposites for visible-light-driven photocatalytic applications in water. J. Mater. Chem. A. 2021, 9, 6819–6826. [Google Scholar]
- Arumugam, M.; Seralathan, K.; Praserthdam, S.; Tahir, M.P. Synthesis of novel graphene aerogel encapsulated bismuth oxyiodide composite towards effective removal of methyl orange azo-dye under visible light. Chemosphere 2022, 303, 135121. [Google Scholar] [CrossRef]
- Tang, H.D.; Zhang, W.J.; Meng, Y.; Xie, B.; Ni, Z.M.; Xia, S.J. Investigation onto the performance and mechanism of visible light photodegradation of methyl orange catalyzed by M/CeO2 (M = Pt; Ag; Au). Mater. Res. Bull. 2021, 144, 111497. [Google Scholar] [CrossRef]
- Aadnan, I.; Zegaoui, O.; Mragui, A.E.; Silva, J.C.G.E. Physicochemical and photocatalytic properties under visible light of ZnO-Bentonite/Chitosan hybrid-biocompositefor water remediation. Nanomaterials 2022, 12, 102. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, H.; Wang, H.; Zhang, E.; Qu, X.; Li, J.; Zhao, S.; Gao, H.; Chen, Z. Boosted Photocatalytic Performance for Antibiotics Removal with Ag/PW12/TiO2 Composite: Degradation Pathways and Toxicity Assessment. Molecules 2023, 28, 6831. https://doi.org/10.3390/molecules28196831
Shi H, Wang H, Zhang E, Qu X, Li J, Zhao S, Gao H, Chen Z. Boosted Photocatalytic Performance for Antibiotics Removal with Ag/PW12/TiO2 Composite: Degradation Pathways and Toxicity Assessment. Molecules. 2023; 28(19):6831. https://doi.org/10.3390/molecules28196831
Chicago/Turabian StyleShi, Hongfei, Haoshen Wang, Enji Zhang, Xiaoshu Qu, Jianping Li, Sisi Zhao, Huajing Gao, and Zhe Chen. 2023. "Boosted Photocatalytic Performance for Antibiotics Removal with Ag/PW12/TiO2 Composite: Degradation Pathways and Toxicity Assessment" Molecules 28, no. 19: 6831. https://doi.org/10.3390/molecules28196831




