Electrochemical and Spectroscopic (FTIR) Evidence of Conducting Polymer-Cu Ions Interaction
Abstract
:1. Introduction
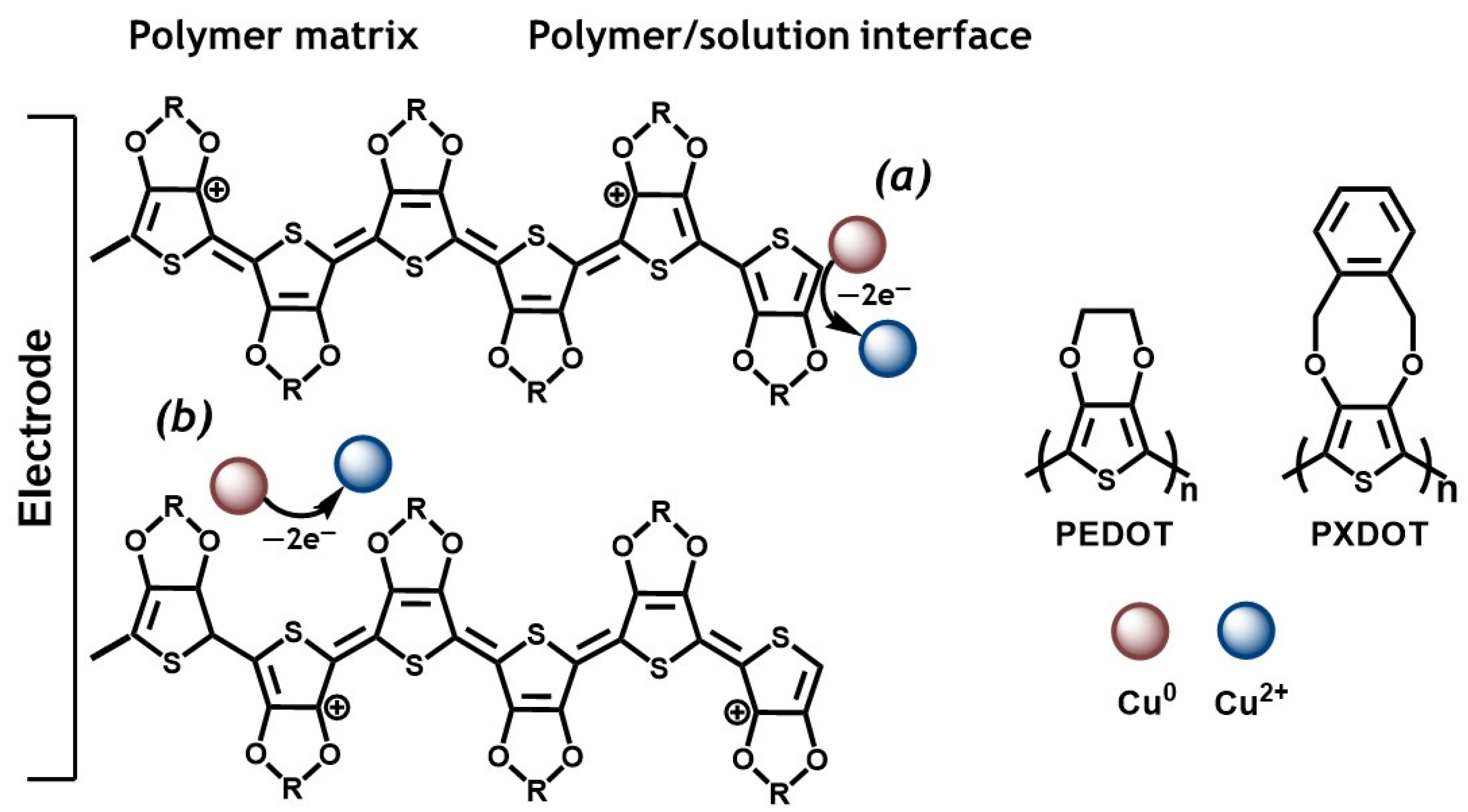
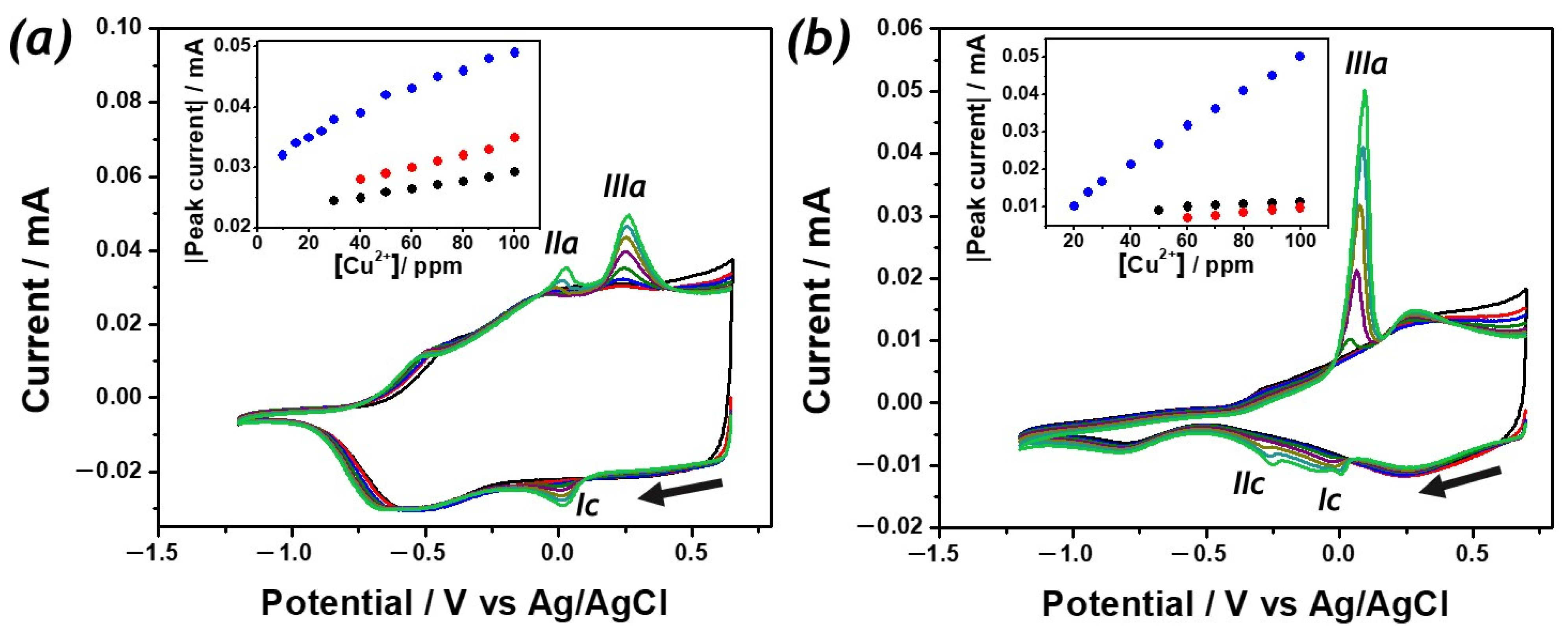
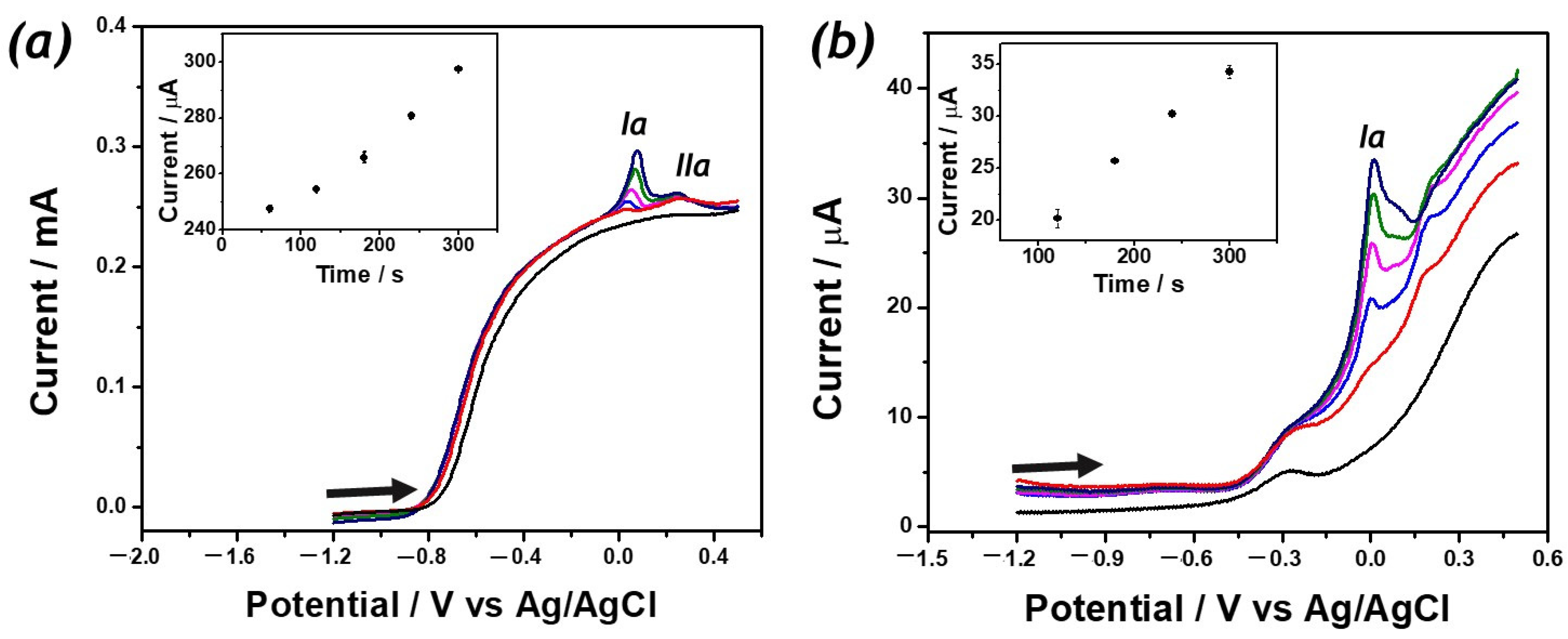
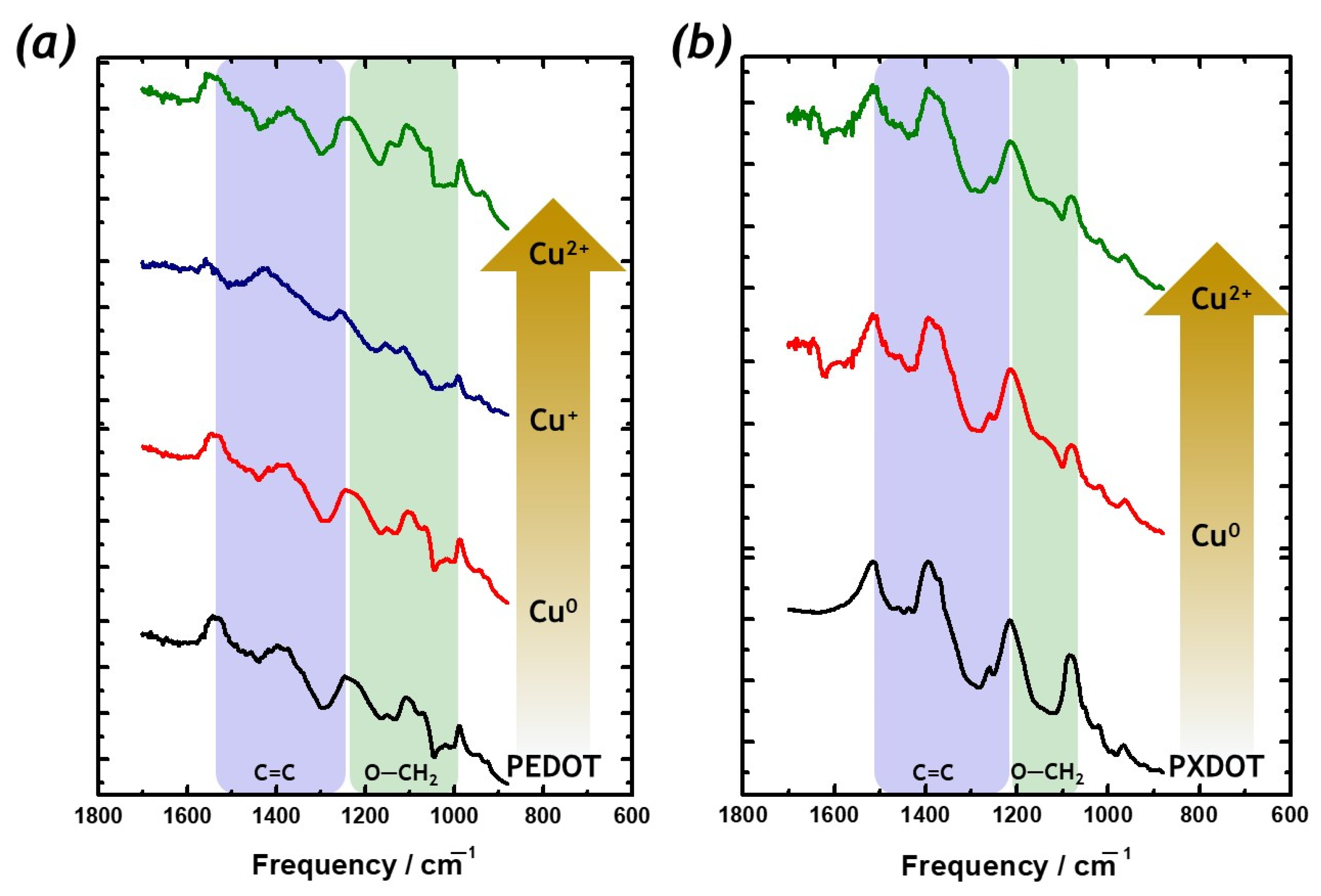
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Arrigan, D.W.M. Tutorial review. Voltammetric determination of trace metals and organics after accumulation at modified electrodes. Analyst 1994, 19, 1953–1966. [Google Scholar] [CrossRef]
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrodes: Mini review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterization and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef]
- Lange, U.; Roznyatovskaya, N.V.; Mirsky, V.M. Conducting polymers in chemical sensors and arrays. Anal. Chim. Acta 2008, 614, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Salinas, G.; Frontana-Uribe, B.A. Electrochemical Analysis of Heavy Metal Ions Using Conducting Polymer Interfaces. Electrochem 2022, 3, 492–506. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Shirsat, M.D.; Ramanaviciene, A.; Ramanavicius, A. Composites based on conducting polymers and carbon nanomaterials for heavy metal ion sensing. Crit. Rev. Anal. Chem. 2018, 48, 293–304. [Google Scholar] [CrossRef] [PubMed]
- El-Said, W.A.; Abdelshakour, M.; Choi, J.H.; Choi, J.W. Application of Conducting Polymer Nanostructures to Electrochemical Biosensors. Molecules 2020, 25, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trojanowicz, M. Application of Conducting Polymers in Chemical Analysis. Microchim. Acta 2003, 143, 75–91. [Google Scholar] [CrossRef]
- Tsakova, V.; Winkels, S.; Schultze, J.W. Crystallization kinetics of Pd in composite films of PEDT. J. Electroanal. Chem. 2001, 500, 574–583. [Google Scholar] [CrossRef]
- Tsakova, V. How to affect number, size, and location of metal particles deposited in conducting polymer layers. J. Solid State Electrochem. 2008, 12, 1421–1434. [Google Scholar] [CrossRef]
- Ma, Z.H.; Tan, K.L.; Kang, E.T. Electroless plating of palladium and copper on polyaniline films. Synth. Met. 2000, 114, 17–25. [Google Scholar] [CrossRef]
- Lim, V.W.L.; Kang, E.T.; Neoh, K.G. Electroless plating of palladium and copper on polypyrrole films. Synth. Met. 2001, 123, 107–115. [Google Scholar] [CrossRef]
- Liu, Y.; Hwang, B. Mechanism of conductivity decay of polypyrrole exposed to water and enhancement of conductivity stability of copper(I)-modified polypyrrole. J. Electroanal. Chem. 2001, 501, 100–106. [Google Scholar] [CrossRef]
- Liu, Y.C.; Hwang, B.J. Interaction of copper(I)-polypyrrole complexes prepared by depositing–dissolving copper onto and from polypyrroles. Thin Solid Film. 1999, 339, 233–239. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Ger, M. Mechanism of underpotential deposition of metal on conducting polymers. Synth. Met. 2002, 126, 337–345. [Google Scholar] [CrossRef]
- Song, F.; Shiu, K. Preconcentration and electroanalysis of silver species at polypyrrole film modified glassy carbon electrodes. J. Electroanal. Chem. 2001, 498, 161–170. [Google Scholar] [CrossRef]
- Rau, J.; Lee, J.; Chen, S. Study of Au(I)-polypyrrole interaction. Synth. Met. 1996, 79, 69–74. [Google Scholar] [CrossRef]
- Lanchkar, A.; Selmani, A.; Sacher, E.; Leclerc, M.; Mokhliss, R. Metallization of polythiophenes I. Interaction of vapor-deposited Cu, Ag and Au with poly(3-hexylthiophene) (P3HT). Synth. Met. 1994, 66, 209–215. [Google Scholar] [CrossRef]
- Ilieva, M.; Tsakova, V. Copper modified poly(3,4-ethylenedioxythiophene): Part I. Galvanostatic experiments. Synth. Met. 2004, 141, 281–285. [Google Scholar] [CrossRef]
- Ilieva, M.; Tsakova, V. Copper modified poly(3,4-ethylenedioxythiophene): Part II: Potentiostatic experiments. Synth. Met. 2004, 141, 287–292. [Google Scholar] [CrossRef]
- Nakova, A.; Ilieva, M.; Czibula, C.; Teichert, C.; Tsakova, V. PEDOT-supported Pd nanocatalysts—Oxidation of formic acid. Electrochim. Acta 2021, 374, 137931. [Google Scholar] [CrossRef]
- Karabozhikova, V.; Tsakova, V. Electroless deposition of silver on poly(3, 4-ethylenedioxythiophene): Role of the organic ions used in the course of electrochemical synthesis. Chem. Pap. 2017, 71, 339–346. [Google Scholar] [CrossRef]
- Nakova, A.; Ilieva, M.; Boiadjieva-Scherzer, T.; Tsakova, V. Electroless deposition of palladium nanoparticles on poly (3,4-ethylene-dioxythiophene)—Role of the electrode substrate. J Solid State Electrochem. 2018, 22, 1901–1908. [Google Scholar] [CrossRef]
- Karabozhikova, V.; Tsakova, V. Role of the doping ions for the electrocrystallization of silver on poly (3,4-ethylenedyoxythiophene)-modified electrodes. Electrochim. Acta 2016, 217, 218–225. [Google Scholar] [CrossRef]
- Castillo-Lara, D.A.; Vasquez-Medrano, R.; Ibanez, J.G.; Frontana-Uribe, B.A.; Salinas, G. Electrochemical behavior of the Cu (II)/Cu (0) system on vitreous carbon electrodes modified with PEDOT electropolymerized in aqueous media. ECS Trans. 2018, 84, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Salinas, G.; Ibanez, J.G.; Vásquez-Medrano, R.; Frontana-Uribe, B.A. Electrochemical behavior of poly-bithiophene, poly-3,4-ethylendioxythiophene and poly-3,4-orthoxylendioxythiophene in EtOH/H2O (1:1) mixture. Synth. Met. 2018, 237, 65–72. [Google Scholar] [CrossRef]
- Salinas, G.; Del-Oso, J.A.; Espinoza-Montero, P.J.; Heinze, J.; Frontana-Uribe, B.A. Electrochemical polymerization, characterization and in-situ conductivity studies of poly-3,4-ortho-xylendioxythiophene (PXDOT). Synth. Met. 2018, 245, 135–143. [Google Scholar] [CrossRef]
- Nicolini, T.; Villarroel Marquez, A.; Goudeau, B.; Kuhn, A.; Salinas, G. In situ spectroelectrochemical-conductance measurements as an efficient tool for the evaluation of charge trapping in conducting polymers. J. Phys. Chem. Lett. 2021, 12, 10422–11042. [Google Scholar] [CrossRef]
- Salinas, G.; Frontana-Uribe, B.A.; Reculusa, S.; Garrigue, P.; Kuhn, A. Highly ordered macroporous poly-3,4-orthoxylendioxythiophene electrodes as a sensitive analytical tool for heavy metal quantification. Anal. Chem. 2018, 90, 11770–11774. [Google Scholar] [CrossRef] [Green Version]
- Salinas, G.; Ibanez, J.G.; Vasquez-Medrano, R.; Frontana-Uribe, B.A. Analysis of Cu in Mezcal Commercial Samples using Square Wave Anodic Stripping Voltammetry. J. Electrochem. Sci. Technol. 2018, 9, 276–281. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas D’équilibres Electrochimiques; Gauthier-Villars & Ci.: Paris, France, 1963; pp. 384–387. [Google Scholar]
- Mirceski, V.; Sebez, B.; Jancovska, M.; Ogorevc, B.; Hocevar, S.B. Mechanisms and kinetics of electrode processes at bismuth and antimony film and bare glassy carbon surfaces under square-wave anodic stripping voltammetry conditions. Electrochim. Acta 2013, 105, 254–260. [Google Scholar] [CrossRef]
- Mirceski, V.; Laborda, E.; Guziejewski, D.; Compton, R.G. New Approach to electrode kinetic measurements in square-wave voltammetry: Amplitude-based quasireversible maximum. Anal. Chem. 2013, 85, 5586–5594. [Google Scholar] [CrossRef] [PubMed]
- Mirceski, V.; Gulaboski, R.; Lovric, M.; Bogeski, I.; Kappl, R.; Hoth, M. Square-wave voltammetry: A review on the recent progress. Electroanalysis 2013, 25, 2411–2422. [Google Scholar] [CrossRef] [Green Version]
- Brewer, G. The risks of free copper in the body and the development of useful anticopper drugs. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 727–732. [Google Scholar] [CrossRef]
- Langner, C.; Denk, H. Wilson disease. Virchows Arch. 2004, 445, 111–118. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincon, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting polymers in the fields of energy, environmental remediation, and chemical-chiral sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef]
- Cisneros-Pérez, P.A.; Martínez-Otero, D.; Cuevas-Yañez, E.; Frontana-Uribe, B.A. Diprotodecarboxylation reactions of 3,4-dialkoxythiophene-2,5-dicarboxylic acids mediated by Ag2CO3 and microwaves. Synth. Commun. 2014, 44, 222–230. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas, G.; Frontana-Uribe, B.A. Electrochemical and Spectroscopic (FTIR) Evidence of Conducting Polymer-Cu Ions Interaction. Molecules 2023, 28, 569. https://doi.org/10.3390/molecules28020569
Salinas G, Frontana-Uribe BA. Electrochemical and Spectroscopic (FTIR) Evidence of Conducting Polymer-Cu Ions Interaction. Molecules. 2023; 28(2):569. https://doi.org/10.3390/molecules28020569
Chicago/Turabian StyleSalinas, Gerardo, and Bernardo A. Frontana-Uribe. 2023. "Electrochemical and Spectroscopic (FTIR) Evidence of Conducting Polymer-Cu Ions Interaction" Molecules 28, no. 2: 569. https://doi.org/10.3390/molecules28020569






