Validation of a Modified QuEChERS Method for the Determination of Selected Organochlorine Compounds in Honey
Abstract
:1. Introduction
2. Results and Discussion
2.1. Linearity
2.2. Recovery and Repeatability
2.3. Reproducibility
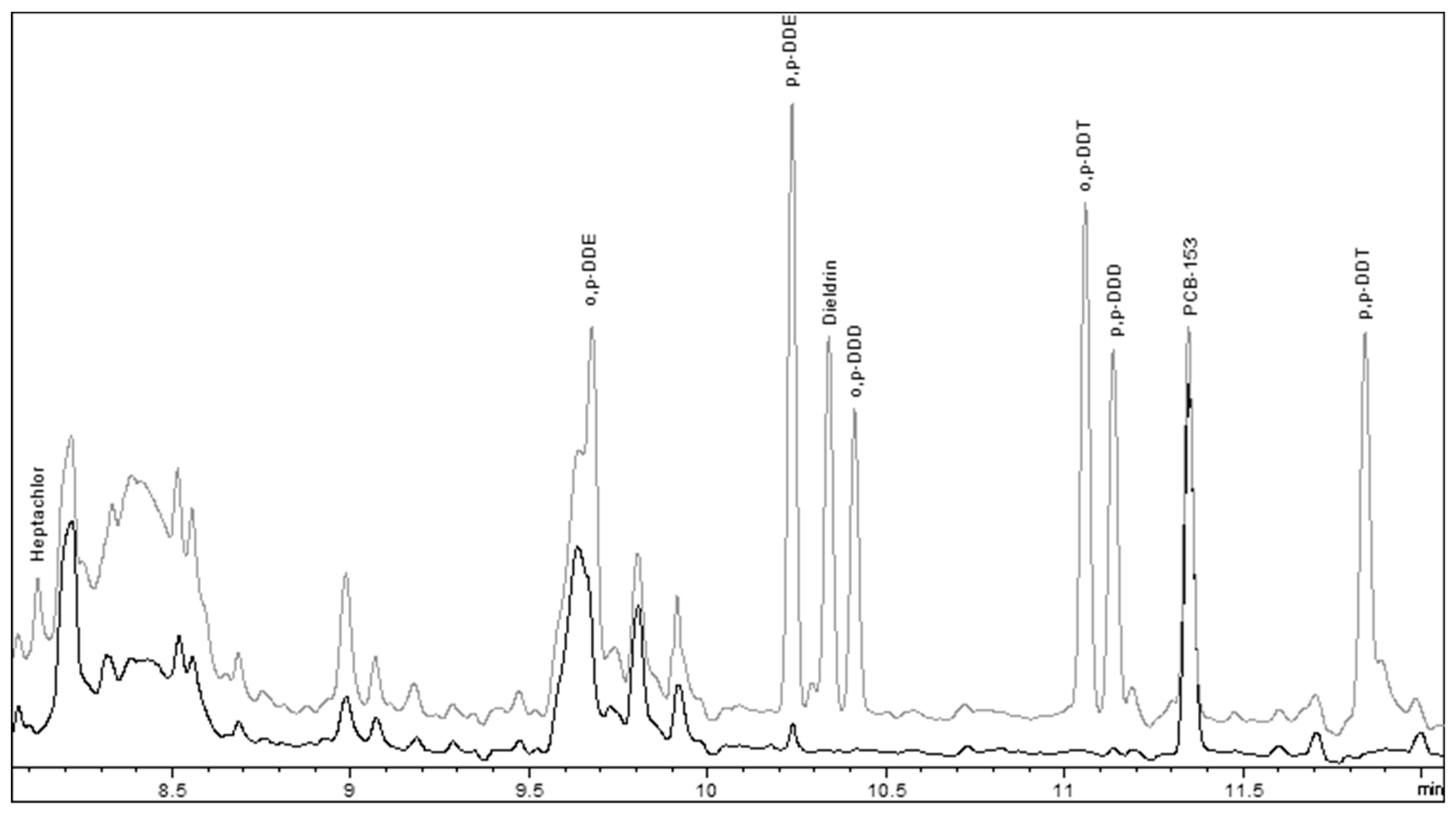
2.4. Role of the Keeper
2.5. Limit of Quantification
3. Materials and Methods
3.1. Material
3.2. Reagents
3.3. Standard Solutions
3.4. Sample Preparation
3.5. Chromatography
3.6. Validation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; González-Paramás, A.M. Chemical Composition of Honey. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer: Cham, Switzerland, 2017; pp. 43–82. [Google Scholar] [CrossRef]
- Saranraj, P.; Sivasakthi, S. Comprehensive Review on Honey: Biochemical and Medicinal Properties. J. Acad. Ind. Res. 2018, 6, 165–181. [Google Scholar]
- Burlando, B.; Cornara, L. Honey in dermatology and skin care: A review. J. Cosmet. Dermatol. 2013, 12, 306–313. [Google Scholar] [CrossRef]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the Antimicrobial Composition of Honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gałczyńska, M.; Gamrat, R.; Bosiacki, M.; Sotek, Z.; Stasińska, M.; Ochmian, I. Micro and Macroelements in Honey and Atmospheric Pollution (NW and Central Poland). Resources 2021, 10, 86. [Google Scholar] [CrossRef]
- Fakhri, Y.; Abtahi, M.; Atamaleki, A.; Raoofi, A.; Atabati, H.; Asadi, A.; Miri, A.; Shamloo, E.; Alinejad, A.; Keramati, H.; et al. The concentration of potentially toxic elements (PTEs) in honey: A global systematic review and meta-analysis and risk assessment. Trends Food Sci. Technol. 2019, 91, 498–506. [Google Scholar] [CrossRef]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A.; Ansari, M.J. Antibiotic, Pesticide, and Microbial Contaminants of Honey: Human Health Hazards. Sci. World J. 2012, 2012, 930849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maliszewska-Kordybach, B.; Smreczak, B.; Klimkowicz-Pawlas, A. Evaluation of the Status of Contamination of Arable Soils in Poland with DDT and HCH Residues; National and Regional Scales. Pol. J. Environ. Stud. 2014, 23, 139–148. [Google Scholar]
- Malusá, E.; Tartanus, M.; Danelski, W.; Miszczak, A.; Szustakowska, E.; Kicińska, J.; Furmanczyk, E.M. Monitoring of DDT in Agricultural Soils under Organic Farming in Poland and the Risk of Crop Contamination. Environ. Manag. 2020, 66, 916–929. [Google Scholar] [CrossRef]
- Piotrowski, S. Presence of polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) in bottom sediments of the Odra RiverEstuary (NW Poland). Prz. Geol. 2003, 51, 835–840. [Google Scholar]
- Ukalska-Jaruga, A.; Smreczak, B.; Siebielec, G. Assessment of Pesticide Residue Content in Polish Agricultural Soils. Molecules 2020, 25, 587. [Google Scholar] [CrossRef] [Green Version]
- Herrera, A.; Perez-Arquillue, C.; Conchello, P.; Bayarri, S.; Lazaro, R.; Yague, C.; Arino, A. Determination of pesticides and PCBs in honey by solid-phase extraction cleanup followed by gas chromatography with electron-capture and nitrogen–phosphorus detection. Anal. Bioanal. Chem. 2005, 381, 695–701. [Google Scholar] [CrossRef]
- Wilczynska, A.; Przybylowski, P. Residues of Organochlorine Pesticides in Polish Honeys. APIACTA 2007, 42, 16–24. [Google Scholar]
- Rissato, S.R.; Galhiane, M.S.; Almeida, M.V.; Gerenutti, M.; Apon, B.M. Multiresidue determination of pesticides in honey samples by gas chromatography–mass spectrometry and application in environmental contamination. Food Chem. 2007, 101, 1719–1726. [Google Scholar] [CrossRef]
- Yavuz, H.; Guler, G.O.; Aktumsek, A.; Cakmak, Y.S.; Ozparlak, H. Determination of some organochlorine pesticide residues in honeys from Konya, Turkey. Environ. Monit. Assess. 2010, 168, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Steffan-Dewenter, I.; Kuhn, A. Honeybee foraging in differentially structured landscapes. Proc. R. Soc. Lond B 2003, 270, 569–575. [Google Scholar] [CrossRef] [Green Version]
- Struciński, P.; Ludwicki, J.K.; Góralczyk, K.; Czaja, K.; Olszewski, W.; Jethon, J.; Barańska, J.; Hernik, A. Levels of organochlorine insecticides in breast adipose tissue of polish women. Rocz. Panstw. Zakl. Hig. 2002, 53, 221–230. (In Polish) [Google Scholar]
- Struciński, P.; Góralczyk, K.; Ludwicki, J.K.; Czaja, K.; Hernik, A.; Korcz, W. Levels of selected organochlorine insecticides, polychlorinated biphenyls, phthalates and perfluorinated aliphatic substances in blood—Polish WWF study. Rocz. Panstw. Zakl. Hig. 2006, 57, 99–112. (In Polish) [Google Scholar]
- Hernik, A.; Góralczyk, K.; Struciński, P.; Czaja, K.; Kucharska, A.; Korcz, W.; Snopczyński, T.; Ludwicki, J.K. Polybrominated diphenyl ethers, polychlorinated biphenyls and organochlorine pesticides in human milk as markers of environmental exposure to these compounds. Ann. Agric. Environ. Med. 2011, 18, 113–118. [Google Scholar]
- Richardson, J.R.; Roy, A.; Shalat, S.L.; Von Stein, R.T.; Hossain, M.M.; Buckley, B.; Gearing, M.; Levey, A.I. and German, D.C. Elevated serum pesticide levels and risk for Alzheimer disease. JAMA Neurol. 2014, 71, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Agency for Toxic Substances and Disease Registry (ATSDR). CHAPTER 1. RELEVANCE TO PUBLIC HEALTH. In Toxicological Profile for DDT, DDE, DDD; U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2022; pp. 1–14. [Google Scholar]
- Singh, Z.; Kaur, J.; Kaur, R.; Hundal, S.S. Toxic Effects of Organochlorine Pesticides: A Review. Am. J. BioSci. 2016, 4, 11–18. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain. Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to DDT as an undesirable substance in animal feed. EFSA J. 2006, 4, 15–20. [Google Scholar] [CrossRef]
- Maqbool, F.; Mostafalou, S.; Bahadar, H.; Abdollah, M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanism. Life Sci. 2016, 145, 265–273. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. DDT. In DDT, Lindane and 2,4-D; IARC Working Group on the Evaluation of Carcinogenic Risks to Humans: Lyon, France, 2018; Volume 113, pp. 37–233. [Google Scholar]
- European Food Safety Authority (EFSA). Pesticide Residue Intake Model (PRIMo) rev. 3.1. Available online: https://www.efsa.europa.eu/en/applications/pesticides/tools (accessed on 12 September 2022).
- European Commission (EC). Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. Off. J. Eur. Comm. 2005, L70, 1–16. [Google Scholar]
- European Commission (EC). EU Pesticides Database. Available online: https://food.ec.europa.eu/plants/pesticides/eu-pesticides-database_en (accessed on 4 January 2023).
- Rahman, M.; Abd El-Aty, A.M.; Shim, J.H. Matrix enhancement effect: A blessing or a curse for gas chromatography?—A review. Anal. Chim. Acta 2013, 801, 14–21. [Google Scholar] [CrossRef]
- Tette, P.A.S.; Guidi, L.R.; Glória, M.B.A.; Fernandes, C. Pesticides in honey: A review on chromatographic analytical methods. Talanta 2016, 149, 124–141. [Google Scholar] [CrossRef]
- Blasco, C.; Lino, C.M.; Pico, Y.; Pena, A.; Font, G.; Silveira, M.I.N. Determination of organochlorine pesticide residues in honey from the central zone of Portugal and the Valencian community of Spain. J. Chromatogr. A 2004, 1049, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kujawski, M.W.; Pinteaux, E.; Namieśnik, J. Application of dispersive liquid–liquid microextraction for the determination of selected organochlorine pesticides in honey by gas chromatography–mass spectrometry. Eur. Food Res. Technol. 2012, 234, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Al-Alam, J.; Fajloun, Z.; Chbani, A.; Millet, M. A multiresidue method for the analysis of 90 pesticides, 16 PAHs, and 22 PCBs in honey using QuEChERS–SPME. Anal. Bioanal. Chem. 2017, 409, 5157–5169. [Google Scholar] [CrossRef] [PubMed]
- Wiest, L.; Buleté, A.; Giroud, B.; Fratta, C.; Amic, S.; Lambert, O.; Pouliquen, H.; Arnaudguilhem, C. Multi-residue analysis of 80 environmental contaminants in honeys, honeybees and pollens by one extraction procedure followed by liquid and gas chromatography coupled with mass spectrometric detection. J. Chromatogr. A 2011, 1218, 5743–5756. [Google Scholar] [CrossRef]
- Calatayud-Vernicha, P.; Simó, F.C.E.; Picó, Y. Efficiency of QuEChERS approach for determining 52 pesticide residues in honey and honey bees. MethodsX 2016, 3, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukiibi, S.B.; Nyanzi, S.A.; Kwetegyeka, J.; Olisah, C.; Taiwo, A.M.; Tebandeke, E.M.E.; Matovu, H.; Odongo, S.; Abayi, J.J.M.; Ngeno, E.C.; et al. Organochlorine pesticide residues in Uganda’s honey as a bioindicator of environmental contamination and reproductive health implications to consumers. Ecotoxicol. Environ. Saf. 2021, 214, 112094. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Abd El-Aty, A.M.; Kim, S.W.; Shin, S.C.; Shin, H.C.; Shim, J.H. Quick, easy, cheap, effective, rugged, and safe sample preparation approach for pesticide residue analysis using traditional detectors in chromatography: A review. J. Sep. Sci. 2017, 40, 203–212. [Google Scholar] [CrossRef]
- SANTE/11312/2021: Guidance Document on Pesticide Analytical Methods for Risk Assessment and Post-Approval Control and Monitoring Purposes. DG SANTE. 2022. Available online: https://food.ec.europa.eu/system/files/2021-02/pesticides_mrl_guidelines_2020-12830.pdf (accessed on 12 October 2022).
- Orso, D.; Martins, M.L.; Donato, F.F.; Rizzetti, T.M.; Kemmerich, M.; Adaime, M.B.; Zanella, R. Multiresidue Determination of Pesticide Residues in Honey by Modified QuEChERS Method and Gas Chromatography with Electron Capture Detection. J. Braz. Chem. Soc. 2014, 25, 1355–1364. [Google Scholar] [CrossRef]
- Vilca, F.Z.; Correia-Oliveira, M.E.; Leal, R.M.P.; Monteiro, S.H.; Zanardi, O.Z.; Marchini, L.C.; Tornisielo, V.L. Quechers Approach for the Determination of Seven Pesticide Residues in Brazilian Honey Samples Using GC-µECD. J. Food Sci. Eng. 2012, 2, 163–169. [Google Scholar]
- Abdallah, O.I.; Hanafi, A.; Ghani, S.B.A.; Ghisoni, S.; Lucini, L. Pesticides contamination in Egyptian honey samples. J. Consum. Prot. Food Saf. 2017, 12, 317–327. [Google Scholar] [CrossRef]

| Compound | RT (min) | R2 | Fortification I (2.9 ng/g) | Fortification II (20.0 ng/g) | ||
|---|---|---|---|---|---|---|
| Mean Recovery (%) n = 6 | RSD (%) | Mean Recovery (%) n = 6 | RSD (%) | |||
| Heptachlor | 8.126 | 0.9990 | 49.4 | 1.9 | 69.6 | 5.6 |
| o,p’-DDE | 9.663 | 0.9939 | 82.9 | 15.0 | 73.7 | 3.2 |
| p,p’-DDE | 10.236 | 0.9994 | 81.7 | 4.0 | 67.0 | 3.2 |
| Dieldrin | 10.336 | 0.9967 | 108.3 | 9.3 | 79.2 | 4.7 |
| o,p’-DDD | 10.410 | 0.9986 | 81.7 | 2.5 | 72.9 | 2.6 |
| o,p’-DDT | 11.057 | 0.9988 | 78.0 | 3.2 | 71.2 | 2.7 |
| p,p’-DDD | 11.135 | 0.9990 | 71.2 | 3.8 | 68.0 | 4.2 |
| p,p’-DDT | 11.841 | 0.9968 | 92.1 | 8.2 | 66.4 | 6.8 |
| Compound | RT (min) | R2 | Fortification I (2.9 ng/g) | Fortification II (20.0 ng/g) | ||
|---|---|---|---|---|---|---|
| Mean Recovery (%) n = 6 | RSD (%) | Mean Recovery (%) n = 6 | RSD (%) | |||
| Heptachlor | 8.124 | 0.9992 | 38.8 | 14.6 | 88.3 | 10.0 |
| o,p’-DDE | 9.654 | 0.9956 | 129.3 | 11.0 | 104.3 | 5.4 |
| p,p’-DDE | 10.224 | 0.9996 | 64.7 | 4.8 | 82.8 | 4.4 |
| Dieldrin | 10.326 | 0.9997 | 92.3 | 18.0 | 107.1 | 6.0 |
| o,p’-DDD | 10.399 | 0.9996 | 75.0 | 6.0 | 95.2 | 2.2 |
| o,p’-DDT | 11.043 | 0.9999 | 70.6 | 4.3 | 92.1 | 2.6 |
| p,p’-DDD | 11.122 | 0.9999 | 73.7 | 5.7 | 89.8 | 3.1 |
| p,p’-DDT | 11.827 | 0.9999 | 95.5 | 9.8 | 97.4 | 8.6 |
| Compound | Analyst I | Analyst II | ||||||
|---|---|---|---|---|---|---|---|---|
| RT (min) | R2 | Mean Recovery (%) n = 6 | RSD (%) | RT (min) | R2 | Mean Recovery (%) n = 6 | RSD (%) | |
| Heptachlor | 8.105 | 0.9994 | 77.4 | 7.0 | 8.105 | 0.9985 | 68.0% | 3.8 |
| Compound | Heptachlor | o,p’-DDE | p,p’-DDE | Dieldrin | o,p’-DDD | o,p’-DDT | p,p’-DDD | p,p’-DDT | |
|---|---|---|---|---|---|---|---|---|---|
| Fortification I (2.9 ng/g) | Mean recovery (%), n = 12 | 44.1 | 106.1 | 73.2 | 100.3 | 78.3 | 74.3 | 72.5 | 93.8 |
| RSD (%) | 15.1 | 25.2 | 12.4 | 15.8 | 6.2 | 6.2 | 5.2 | 9.3 | |
| Fortification II (20 ng/g) | Mean recovery (%), n = 12 | 79.0 | 89.0 | 74.9 | 93.2 | 84.0 | 81.7 | 78.9 | 81.9 |
| RSD (%) | 14.7 | 17.8 | 11.3 | 16 | 13.4 | 13.1 | 14.3 | 20.7 | |
| Method | Compound | LOQ (ng/g) | Recovery (%) 1 | Detection | Solvent | References |
|---|---|---|---|---|---|---|
| Method 1 | o,p’-DDD | 3.7 | 89.0 (10) | GC-MS | Acetonitrile | [36] |
| p,p’-DDT | 65.9 | 91.0 (60) | ||||
| Dieldrin | 29.5 | 90.0 (30) | ||||
| Method 2 | p,p’-DDE | 10 | 76.0 (20) | GC-ECD | Acetonitrile | [40] |
| Dieldrin | 10 | 76.0 (20) | ||||
| Heptachlor | 10 | 71.0 (20) | ||||
| Method 3 | Heptachlor | 33 | 128.0 (70) | GC-ECD | Ethyl acetate | [41] |
| Dieldrin | 8 | 91.6 (70) | ||||
| Method presented | p,p’-DDE | 2.9 | 81.7 (2.9) | GC-ECD | Acetonitrile | [–] |
| o,p’-DDD | 2.9 | 81.7 (2.9) | ||||
| p,p’-DDT | 2.9 | 92.1 (2.9) | ||||
| Dieldrin | 2.9 | 108.3 (2.9) | ||||
| Heptachlor | 5.6 | 77.4 (5.6) |
| Compound | Concentration (ng/mL) | Determination without n-Dodecane (n = 5) | Determination with n-Dodecane (n = 5) | ||||
|---|---|---|---|---|---|---|---|
| Concentration Measured(ng/mL) | Recovery (%) | RSD (%) | Concentration Measured(ng/mL) | Recovery (%) | RSD (%) | ||
| Heptachlor | 30.0 | 19.8 | 65.9 | 22.7 | 35.9 | 119.9 | 4.6 |
| o,p’-DDE | 30.4 | 14.5 | 47.7 | 24.3 | 31.5 | 103.7 | 3.7 |
| p,p’-DDE | 30.0 | 20.5 | 68.2 | 11.6 | 32.7 | 109.1 | 2.0 |
| Dieldrin | 30.5 | 13.5 | 44.4 | 27.8 | 34.2 | 112.3 | 3.9 |
| o,p’-DDD | 30.3 | 15.9 | 52.7 | 18.9 | 31.9 | 105.3 | 2.5 |
| o,p’-DDT | 30.4 | 15.7 | 51.6 | 17.2 | 30.6 | 100.7 | 1.4 |
| p,p’-DDD | 30.2 | 18.0 | 59.6 | 12.8 | 31.5 | 104.2 | 1.9 |
| p,p’-DDT | 30.1 | 15.9 | 52.7 | 12.3 | 27.8 | 92.2 | 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewiński, R.; Hernik, A.; Liszewska, M.; Buckley, B.; Czaja, K.; Korcz, W.; Słomczyńska, A.; Struciński, P. Validation of a Modified QuEChERS Method for the Determination of Selected Organochlorine Compounds in Honey. Molecules 2023, 28, 842. https://doi.org/10.3390/molecules28020842
Lewiński R, Hernik A, Liszewska M, Buckley B, Czaja K, Korcz W, Słomczyńska A, Struciński P. Validation of a Modified QuEChERS Method for the Determination of Selected Organochlorine Compounds in Honey. Molecules. 2023; 28(2):842. https://doi.org/10.3390/molecules28020842
Chicago/Turabian StyleLewiński, Radosław, Agnieszka Hernik, Monika Liszewska, Brian Buckley, Katarzyna Czaja, Wojciech Korcz, Anna Słomczyńska, and Paweł Struciński. 2023. "Validation of a Modified QuEChERS Method for the Determination of Selected Organochlorine Compounds in Honey" Molecules 28, no. 2: 842. https://doi.org/10.3390/molecules28020842






