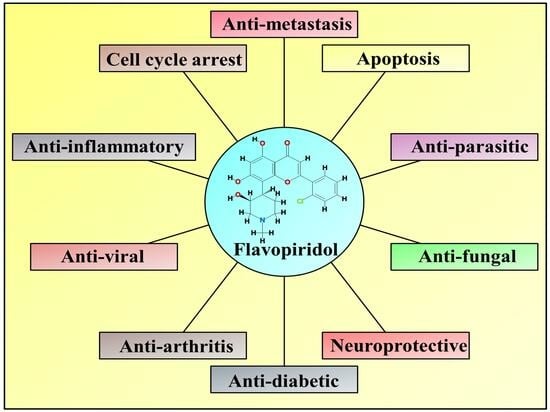
The Pharmacological Implications of Flavopiridol: An Updated Overview
Abstract
:1. Introduction
2. Chemistry of Flavopiridol
3. Molecular Insights into the Chemopreventive Actions of Flavopiridol
3.1. Apoptosis Activation and Cell Cycle Arrest
3.2. Anti-Metastatic Effect
3.3. Anti-Angiogenesis
3.4. Anti-Inflammatory Effects
4. Efficacy of Flavopiridol as a Therapeutic Agent
5. Derivatives of Flavopiridol
6. Adverse Effects of Flavopiridol
7. Clinical Trials: Results and Experiences
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuli, H.S.; Sharma, A.K.; Sandhu, S.S.; Kashyap, D. Cordycepin: A bioactive metabolite with therapeutic potential. Life Sci. 2013, 93, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Marwaha, R.K.; Marwaha, M.G.; Nandal, R.; Sharma, A.K. Flavopiridol as cyclin dependent kinase (CDK) inhibitor: A review. New J. Chem. 2018, 42, 18500–18507. [Google Scholar] [CrossRef]
- Raju, U.; Nakata, E.; Mason, K.A.; Ang, K.K.; Milas, L. Flavopiridol, a cyclin-dependent kinase inhibitor, enhances radiosensitivity of ovarian carcinoma cells. Cancer Res. 2003, 63, 3263–3267. [Google Scholar] [PubMed]
- Blachly, J.S.; Byrd, J.C. Emerging drug profile: Cyclin-dependent kinase inhibitors. Leuk. Lymphoma 2013, 54, 2133–2143. [Google Scholar] [CrossRef]
- Carlson, B.A.; Dubay, M.M.; Sausville, E.A.; Brizuela, L.; Worland, P.J. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 1996, 56, 2973–2978. [Google Scholar]
- Drees, M.; A Dengler, W.; Roth, T.; LaBonte, H.; Mayo, J.; Malspeis, L.; Grever, M.; A Sausville, E.; Fiebig, H.H. Flavopiridol (L86-8275): Selective antitumor activity in vitro and activity in vivo for prostate carcinoma cells. Clin. Cancer Res. 1997, 3, 273–279. [Google Scholar]
- Wirger, A.; E Perabo, F.G.; Burgemeister, S.; Haase, L.; Schmidt, D.H.; Doehn, C.; Mueller, S.C.; Jocham, D. Flavopiridol, an inhibitor of cyclin-dependent kinases, induces growth inhibition and apoptosis in bladder cancer cells in vitro and in vivo. Anticancer. Res. 2005, 25, 4341–4347. [Google Scholar]
- Jackman, K.M.; Frye, C.B.; Hunger, S.P. Flavopiridol displays preclinical activity in acute lymphoblastic leukemia. Pediatr. Blood Cancer 2008, 50, 772–778. [Google Scholar] [CrossRef]
- Garcia-Cuellar, M.-P.; Füller, E.; Mäthner, E.; Breitinger, C.; Hetzner, K.; Zeitlmann, L.; Borkhardt, A.; Slany, R.K. Efficacy of cyclin-dependent-kinase 9 inhibitors in a murine model of mixed-lineage leukemia. Leukemia 2014, 28, 1427–1435. [Google Scholar] [CrossRef]
- Stewart, Z.A.; Westfall, M.D.; Pietenpol, J.A. Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol. Sci. 2003, 24, 139–145. [Google Scholar] [CrossRef]
- Desai, D.; Gu, Y.; O Morgan, D. Activation of human cyclin-dependent kinases in vitro. Mol. Biol. Cell 1992, 3, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Senderowicz, A.M.; Sausville, E.A.; Figg, W.D. Flavopiridol, a novel cyclin-dependent kinase inhibitor, in clinical development. Ann. Pharmacother. 2002, 36, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Zaharevitz, D.W.; Gussio, R.; Leost, M.; Senderowicz, A.M.; Lahusen, T.; Kunick, C.; Meijer, L.; A Sausville, E. Discovery and initial characterization of the paullones, a novel class of small-molecule inhibitors of cyclin-dependent kinases. Cancer Res. 1999, 59, 2566–2569. [Google Scholar] [PubMed]
- Arguello, F.; Alexander, M.; Sterry, J.A.; Tudor, G.; Smith, E.M.; Kalavar, N.T.; Greene, J.F.; Koss, W.; Morgan, C.D.; Stinson, S.F.; et al. Flavopiridol induces apoptosis of normal lymphoid cells, causes immunosuppression, and has potent antitumor activity in vivo against human leukemia and lymphoma xenografts. Blood J. Am. Soc. Hematol. 1998, 91, 2482–2490. [Google Scholar]
- Schwartz, G.K.; O’Reilly, E.; Ilson, D.; Saltz, L.; Sharma, S.; Tong, W.; Maslak, P.; Stoltz, M.; Eden, L.; Perkins, P.; et al. Phase I study of the cyclin-dependent kinase inhibitor flavopiridol in combination with paclitaxel in patients with advanced solid tumors. J. Clin. Oncol. 2002, 20, 2157–2170. [Google Scholar] [CrossRef]
- Lapenna, S.; Giordano, A. Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov. 2009, 8, 547–566. [Google Scholar] [CrossRef]
- Chen, R.; Keating, M.J.; Gandhi, V.; Plunkett, W. Transcription inhibition by flavopiridol: Mechanism of chronic lymphocytic leukemia cell death. Blood 2005, 106, 2513–2519. [Google Scholar] [CrossRef]
- Senderowicz, A.M.; Headlee, D.; Stinson, S.F.; Lush, R.M.; Kalil, N.; Villalba, L.; Hill, K.; Steinberg, S.M.; Figg, W.D.; Tompkins, A.; et al. Phase I trial of continuous infusion flavopiridol, a novel cyclin-dependent kinase inhibitor, in patients with refractory neoplasms. J. Clin. Oncol. 1998, 16, 2986–2999. [Google Scholar] [CrossRef]
- Senderowicz, A.M. Flavopiridol: The first cyclin-dependent kinase inhibitor in human clinical trials. Investig. New Drugs 1999, 17, 313–320. [Google Scholar] [CrossRef]
- Aklilu, M.; Kindler, H.L.; Donehower, R.C.; Mani, S.; Vokes, E.E. Phase II study of flavopiridol in patients with advanced colorectal cancer. Ann. Oncol. 2003, 14, 1270–1273. [Google Scholar] [CrossRef]
- Colevas, D.; Blaylock, B.; Gravell, A. Clinical trials referral resource. Flavopiridol. Oncology 2002, 16, 1204–1214. [Google Scholar] [PubMed]
- Jäger, W.; Zembsch, B.; Wolschann, P.; Pittenauer, E.; Senderowicz, A.M.; Sausville, E.A.; Sedlacek, H.H.; Graf, J.; Thalhammer, T. Metabolism of the anticancer drug flavopiridol, a new inhibitor of cyclin dependent kinases, in rat liver. Life Sci. 1998, 62, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Bharate, S.B.; Kumar, V.; Jain, S.K.; Mintoo, M.J.; Guru, S.K.; Nuthakki, V.K.; Sharma, M.; Bharate, S.S.; Gandhi, S.G.; Mondhe, D.M.; et al. Discovery and preclinical development of IIIM-290, an orally active potent cyclin-dependent kinase inhibitor. J. Med. Chem. 2018, 61, 1664–1687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yuan, X.; Wang, J.; Feng, Y.; Ji, F.; Li, Z.; Bian, J. A review on flavones targeting serine/threonine protein kinases for potential anticancer drugs. Bioorg. Med. Chem. 2019, 27, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Bonnet, P.; Brion, J.-D.; Peyrat, J.-F.; Bignon, J.; Levaique, H.; Josselin, B.; Robert, T.; Colas, P.; Bach, S.; et al. Identification of a new series of flavopiridol-like structures as kinase inhibitors with high cytotoxic potency. Eur. J. Med. Chem. 2020, 199, 112355. [Google Scholar] [CrossRef]
- Chao, S.-H.; Fujinaga, K.; Marion, J.E.; Taube, R.; Sausville, E.A.; Senderowicz, A.M.; Peterlin, B.M.; Price, D.H. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J. Biol. Chem. 2000, 275, 28345–28348. [Google Scholar] [CrossRef]
- Yamamoto, M.; Onogi, H.; Kii, I.; Yoshida, S.; Iida, K.; Sakai, H.; Abe, M.; Tsubota, T.; Ito, N.; Hosoya, T.; et al. CDK9 inhibitor FIT-039 prevents replication of multiple DNA viruses. J. Clin. Investig. 2014, 124, 3479–3488. [Google Scholar] [CrossRef]
- Perwitasari, O.; Yan, X.; O’Donnell, J.; Johnson, S.; Tripp, R.A.; Schor, S.; Einav, S.; Bloom, B.E.; Krouse, A.J.; Gray, L.; et al. Repurposing kinase inhibitors as antiviral agents to control influenza A virus replication. Assay Drug Dev. Technol. 2015, 13, 638–649. [Google Scholar] [CrossRef]
- Gu, L.; Li, C.; Peng, X.; Lin, H.; Niu, Y.; Zheng, H.; Zhao, G.; Lin, J. Flavopiridol Protects against Fungal Keratitis due to Aspergillus fumigatus by Alleviating Inflammation through the Promotion of Autophagy. ACS Infect. Dis. 2022, 8, 2362–2373. [Google Scholar] [CrossRef]
- Srikumar, T.; Padmanabhan, J. Potential use of flavopiridol in treatment of chronic diseases. In Drug Discovery from Mother Nature; Springer: Berlin/Heidelberg, Germany, 2016; pp. 209–228. [Google Scholar]
- AbAbotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Kaur, G.; Stetler-Stevenson, M.; Sebers, S.; Worland, P.; Sedlacek, H.; Myers, C.; Czech, J.; Naik, R.; Sausville, E. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. JNCI J. Natl. Cancer Inst. 1992, 84, 1736–1740. [Google Scholar] [CrossRef] [PubMed]
- Senderowicz, A.M.; Sausville, E.A. Preclinical and clinical development of cyclin-dependent kinase modulators. JNCI J. Natl. Cancer Inst. 2000, 92, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Murthi, K.K.; Dubay, M.; McClure, C.; Brizuela, L.; Boisclair, M.D.; Worland, P.J.; Mansuri, M.M.; Pal, K. Structure–activity relationship studies of flavopiridol analogues. Bioorg. Med. Chem. Lett. 2000, 10, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo, W.F., Jr.; Mueller-Dieckmann, H.-J.; Schulze-Gahmen, U.; Worland, P.J.; Sausville, E.; Kim, S.-H. Structural basis for specificity and potency of a flavonoid inhibitor of human CDK2, a cell cycle kinase. Proc. Natl. Acad. Sci. USA 1996, 93, 2735–2740. [Google Scholar] [CrossRef]
- Joshi, H.; Gupta, D.S.; Abjani, N.K.; Kaur, G.; Mohan, C.D.; Kaur, J.; Aggarwal, D.; Rani, I.; Ramniwas, S.; Abdulabbas, H.S.; et al. Genistein: A promising modulator of apoptosis and survival signaling in cancer. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 2893–2910. [Google Scholar] [CrossRef]
- Joshi, H.; Gupta, D.S.; Kaur, G.; Singh, T.; Ramniwas, S.; Sak, K.; Aggarwal, D.; Chhabra, R.S.; Gupta, M.; Saini, A.K.; et al. Nanoformulations of quercetin for controlled delivery: A review of preclinical anticancer studies. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 1–16. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Bhadra, K. A Mini Review on Molecules Inducing Caspase-Independent Cell Death: A New Route to Cancer Therapy. Molecules 2022, 27, 6401. [Google Scholar] [CrossRef]
- Sedlacek, H. Mechanisms of action of flavopiridol. Crit. Rev. Oncol./Hematol. 2001, 38, 139–170. [Google Scholar] [CrossRef]
- Javelaud, D.; Besançon, F. Inactivation of p21WAF1Sensitizes Cells to Apoptosis via an Increase of Both p14ARF and p53 Levels and an Alteration of the Bax/Bcl-2 Ratio. J. Biol. Chem. 2002, 277, 37949–37954. [Google Scholar] [CrossRef]
- Blagosklonny, M.V.; Darzynkiewicz, Z.; Figg, W., II. Flavopiridol inversely affects p21WAF1/CIP1 and p53 and protects p21-sensitive cells from paclitaxel. Cancer Biol. Ther. 2002, 1, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.E.; Cimica, V.; Chinni, S.; Challagulla, K.; Mani, S.; Kalpana, G.V.; Tan, B.K.; Tan, L.K.; Yu, K.; Tan, P.H.; et al. Rhabdoid tumor growth is inhibited by flavopiridol. Clin. Cancer Res. 2008, 14, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Matranga, C.B.; Cai, D.; Latham, V.M., Jr.; Zhang, X.; Lowell, A.M.; Martelli, F.; Shapiro, G.I. Flavopiridol-induced apoptosis during S phase requires E2F-1 and inhibition of cyclin A-dependent kinase activity. Cancer Res. 2003, 63, 7410–7422. [Google Scholar] [PubMed]
- Chen, L.; Fang, B.; Qiao, L.; Zheng, Y. Discovery of Anticancer Activity of Amentoflavone on Esophageal Squamous Cell Carcinoma: Bioinformatics, Structure-Based Virtual Screening, and Biological Evaluation. J. Microbiol. Biotechnol. 2022, 32, 718–729. [Google Scholar] [CrossRef]
- Byrd, J.C.; Shinn, C.; Waselenko, J.K.; Fuchs, E.J.; Lehman, T.A.; Nguyen, P.L.; Flinn, I.W.; Diehl, L.F.; Sausville, E.; Grever, M.R. Flavopiridol induces apoptosis in chronic lymphocytic leukemia cells via activation of caspase-3 without evidence of bcl-2 modulation or dependence on functional p53. Blood J. Am. Soc. Hematol. 1998, 92, 3804–3816. [Google Scholar]
- König, A.; Schwartz, G.K.; Mohammad, R.M.; Al-Katib, A.; Gabrilove, J.L. The novel cyclin-dependent kinase inhibitor flavopiridol downregulates Bcl-2 and induces growth arrest and apoptosis in chronic B-cell leukemia lines. Blood J. Am. Soc. Hematol. 1997, 90, 4307–4312. [Google Scholar]
- Kitada, S.; Zapata, J.M.; Andreeff, M.; Reed, J.C. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood J. Am. Soc. Hematol. 2000, 96, 393–397. [Google Scholar]
- Gojo, I.; Zhang, B.; Fenton, R.G. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clin. Cancer Res. 2002, 8, 3527–3538. [Google Scholar]
- Dai, Y.; Rahmani, M.; Grant, S. Proteasome inhibitors potentiate leukemic cell apoptosis induced by the cyclin-dependent kinase inhibitor flavopiridol through a SAPK/JNK-and NF-κB-dependent process. Oncogene 2003, 22, 7108–7122. [Google Scholar] [CrossRef]
- Wittmann, S.; Bali, P.; Donapaty, S.; Nimmanapalli, R.; Guo, F.; Yamaguchi, H.; Huang, M.; Jove, R.; Wang, H.G.; Bhalla, K. Flavopiridol down-regulates antiapoptotic proteins and sensitizes human breast cancer cells to epothilone B-induced apoptosis. Cancer Res. 2003, 63, 93–99. [Google Scholar]
- Wall, N.R.; O’Connor, D.S.; Plescia, J.; Pommier, Y.; Altieri, D.C. Suppression of survivin phosphorylation on Thr34 by flavopiridol enhances tumor cell apoptosis. Cancer Res. 2003, 63, 230–235. [Google Scholar] [PubMed]
- Pinto, N.; Prokopec, S.D.; Ghasemi, F.; Meens, J.; Ruicci, K.M.; Khan, I.M.; Mundi, N.; Patel, K.; Han, M.W.; Yoo, J.; et al. Flavopiridol causes cell cycle inhibition and demonstrates anti-cancer activity in anaplastic thyroid cancer models. PLoS ONE 2020, 15, e0239315. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bhuiyan, M.; Alhasan, S.; Senderowicz, A.M.; Sarkar, F.H. Induction of Apoptosis and Inhibition of c-erb B-2 in Breast Cancer Cells by Flavopiridol. Clin. Cancer Res. 2000, 6, 223–229. [Google Scholar] [PubMed]
- Rapoport, A.P.; Simons-Evelyn, M.; Chen, T.; Sidell, R.; Luhowskyj, S.; Rosell, K.; Obrig, T.; Hicks, D.; Hinkle, P.M.; Nahm, M.; et al. Flavopiridol induces apoptosis and caspase-3 activation of a newly characterized Burkitt’s lymphoma cell line containing mutant p53 genes. Blood Cells Mol. Dis. 2001, 27, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Cartee, L.; Smith, R.; Dai, Y.; Rahmani, M.; Rosato, R.; Almenara, J.; Dent, P.; Grant, S. Synergistic induction of apoptosis in human myeloid leukemia cells by phorbol 12-myristate 13-acetate and flavopiridol proceeds via activation of both the intrinsic and tumor necrosis factor-mediated extrinsic cell death pathways. Mol. Pharmacol. 2002, 61, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Newcomb, E.W. Flavopiridol: Pleiotropic biological effects enhance its anti-cancer activity. Anti-Cancer Drugs 2004, 15, 411–419. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Schrump, W.D.; Tsai, W.S.; Chen, A.; Stewart, J.H., IV; Steiner, F.; Schrump, D.S. Enhancement of depsipeptide-mediated apoptosis of lung or esophageal cancer cells by flavopiridol: Activation of the mitochondria-dependent death-signaling pathway. J. Thorac. Cardiovasc. Surg. 2003, 125, 1132–1142. [Google Scholar] [CrossRef]
- Saisomboon, S.; Kariya, R.; Vaeteewoottacharn, K.; Wongkham, S.; Sawanyawisuth, K.; Okada, S. Antitumor effects of flavopiridol, a cyclin-dependent kinase inhibitor, on human cholangiocarcinoma in vitro and in an in vivo xenograft model. Heliyon 2019, 5, e01675. [Google Scholar] [CrossRef]
- Motwani, M.; Jung, C.; Sirotnak, F.M.; She, Y.; A Shah, M.; Gonen, M.; Schwartz, G.K. Augmentation of apoptosis and tumor regression by flavopiridol in the presence of CPT-11 in Hct116 colon cancer monolayers and xenografts. Clin. Cancer Res. 2001, 7, 4209–4219. [Google Scholar]
- Achenbach, T.V.; Müller, R.; Slater, E.P. Bcl-2 Independence of Flavopiridol-induced Apoptosis: Mitochondrial depolarization in the absence of cytochromec release. J. Biol. Chem. 2000, 275, 32089–32097. [Google Scholar] [CrossRef]
- Alonso, M.; Tamasdan, C.; Miller, D.C.; Newcomb, E.W. Flavopiridol induces apoptosis in glioma cell lines independent of retinoblastoma and p53 tumor suppressor pathway alterations by a caspase-independent pathway. Mol. Cancer Ther. 2003, 2, 139–150. [Google Scholar] [PubMed]
- Newcomb, E.W.; Tamasdan, C.; Entzminger, Y.; Alonso, J.; Friedlander, D.; Crisan, D.; Miller, D.C.; Zagzag, D. Flavopiridol induces mitochondrial-mediated apoptosis in murine glioma GL261 cells via release of cytochrome c and apoptosis inducing factor. Cell Cycle 2003, 2, 242–249. [Google Scholar] [CrossRef]
- Yu, C.; Rahmani, M.; Dai, Y.; Conrad, D.; Krystal, G.; Dent, P.; Grant, S. The lethal effects of pharmacological cyclin-dependent kinase inhibitors in human leukemia cells proceed through a phosphatidylinositol 3-kinase/Akt-dependent process. Cancer Res. 2003, 63, 1822–1833. [Google Scholar] [PubMed]
- Decaudin, D.; Marzo, I.; Brenner, C.; Kroemer, G. Mitochondria in chemotherapy-induced apoptosis: A prospective novel target of cancer therapy. Int. J. Oncol. 1998, 12, 141–193. [Google Scholar] [CrossRef] [PubMed]
- Motwani, M.; Delohery, T.M.; Schwartz, G.K. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin. Cancer Res. 1999, 5, 1876–1883. [Google Scholar]
- Jung, C.P.; Motwani, M.V.; Schwartz, G.K. Flavopiridol increases sensitization to gemcitabine in human gastrointestinal cancer cell lines and correlates with down-regulation of ribonucleotide reductase M2 subunit. Clin. Cancer Res. 2001, 7, 2527–2536. [Google Scholar]
- Schwartz, G.K.; Farsi, K.; Maslak, P.; Kelsen, D.P.; Spriggs, D. Potentiation of apoptosis by flavopiridol in mitomycin-C-treated gastric and breast cancer cells. Clin. Cancer Res. 1997, 3, 1467–1472. [Google Scholar]
- Ang, C.; O’Reilly, E.M.; Carvajal, R.D.; Capanu, M.; Gonen, M.; Doyle, L.; Ghossein, R.; Schwartz, L.; Jacobs, G.; Ma, J.; et al. A nonrandomized, phase II study of sequential irinotecan and flavopiridol in patients with advanced hepatocellular carcinoma. Gastrointest. Cancer Res. GCR 2012, 5, 185. [Google Scholar]
- Joshi, H.; Kumar, G.; Tuli, H.S.; Mittal, S. Inhibition of Cancer Cell Metastasis by Nanotherapeutics: Current Achievements and Future Trends. In Nanotherapeutics in Cancer; Jenny Stanford Publishing: Singapore, 2023; pp. 161–209. [Google Scholar]
- Banerjee, D.; Cieslar-Pobuda, A.; Zhu, G.H.; Wiechec, E.; Patra, H.K. Adding nanotechnology to the metastasis treatment arsenal. Trends Pharmacol. Sci. 2019, 40, 403–418. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef]
- Kumar, G.; Tuli, H.S.; Mittal, S.; Shandilya, J.K.; Tiwari, A.; Sandhu, S.S. Isothiocyanates: A class of bioactive metabolites with chemopreventive potential. Tumor Biol. 2015, 36, 4005–4016. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Mittal, S.; Sak, K.; Singhal, P.; Tuli, H.S. Molecular mechanisms of action of quercetin in cancer: Recent advances. Tumor Biol. 2016, 37, 12927–12939. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Joshi, H.; Vashishth, K.; Ramniwas, S.; Varol, M.; Kumar, M.; Rani, I.; Rani, V.; Sak, K. Chemopreventive mechanisms of amentoflavone: Recent trends and advancements. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Cheng, R.; Wang, J.; Fang, Y.; Hwang, K.C. Nanomedicines inhibiting tumor metastasis and recurrence and their clinical applications. Nano Today 2021, 36, 101004. [Google Scholar] [CrossRef]
- Tuli, H.S.; Rath, P.; Chauhan, A.; Parashar, G.; Parashar, N.C.; Joshi, H.; Rani, I.; Ramniwas, S.; Aggarwal, D.; Kumar, M.; et al. Wogonin, as a potent anticancer compound: From chemistry to cellular interactions. Exp. Biol. Med. 2023, 248, 820–828. [Google Scholar] [CrossRef]
- Newcomb, E.W.; Ali, M.A.; Schnee, T.; Lan, L.; Lukyanov, Y.; Fowkes, M.; Miller, D.C.; Zagzag, D. Flavopiridol downregulates hypoxia-mediated hypoxia-inducible factor-1α expression in human glioma cells by a proteasome-independent pathway: Implications for in vivo therapy. Neuro-Oncology 2005, 7, 225–235. [Google Scholar] [CrossRef]
- Takada, Y.; Aggarwal, B.B. Flavopiridol inhibits NF-κB activation induced by various carcinogens and inflammatory agents through inhibition of IκBα kinase and p65 phosphorylation: Abrogation of cyclin D1, cyclooxygenase-2, and matrix metalloprotease-9. J. Biol. Chem. 2004, 279, 4750–4759. [Google Scholar] [CrossRef]
- Mason, K.A.; Hunter, N.R.; Raju, U.; Ariga, H.; Husain, A.; Valdecanas, D.; Neal, R.; Ang, K.K.; Milas, L. Flavopiridol increases therapeutic ratio of radiotherapy by preferentially enhancing tumor radioresponse. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1181–1189. [Google Scholar] [CrossRef]
- Zocchi, L.; Wu, S.C.; Wu, J.; Hayama, K.L.; Benavente, C.A. The cyclin-dependent kinase inhibitor flavopiridol (alvocidib) inhibits metastasis of human osteosarcoma cells. Oncotarget 2018, 9, 23505. [Google Scholar] [CrossRef]
- Dogan Turacli, I.; Demirtas Korkmaz, F.; Candar, T.; Ekmekci, A. Flavopiridol’s effects on metastasis in KRAS mutant lung adenocarcinoma cells. J. Cell. Biochem. 2019, 120, 5628–5635. [Google Scholar] [CrossRef]
- Heijkants, R.; Willekens, K.; Schoonderwoerd, M.; Teunisse, A.; Nieveen, M.; Radaelli, E.; Hawinkels, L.; Marine, J.-C.; Jochemsen, A. Combined inhibition of CDK and HDAC as a promising therapeutic strategy for both cutaneous and uveal metastatic melanoma. Oncotarget 2017, 9, 6174–6187. [Google Scholar] [CrossRef] [PubMed]
- Nilubol, N.; Boufraqech, M.; Zhang, L.; Gaskins, K.; Shen, M.; Zhang, Y.-Q.; Gara, S.K.; Austin, C.P.; Kebebew, E. Synergistic combination of flavopiridol and carfilzomib targets commonly dysregulated pathways in adrenocortical carcinoma and has biomarkers of response. Oncotarget 2018, 9, 33030–33042. [Google Scholar] [CrossRef] [PubMed]
- Holkova, B.; Perkins, E.B.; Ramakrishnan, V.; Tombes, M.B.; Shrader, E.; Talreja, N.; Wellons, M.D.; Hogan, K.T.; Roodman, G.D.; Coppola, D.; et al. Phase I Trial of Bortezomib (PS-341; NSC 681239) and Alvocidib (Flavopiridol; NSC 649890) in Patients with Recurrent or Refractory B-Cell Neoplasms Phase I Trial of Bortezomib and Alvocidib. Clin. Cancer Res. 2011, 17, 3388–3397. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, H.M.; Verschraegen, C.F.; Royce, M.; Smith, H.O.; Lee, F.C.; Rabinowitz, I. A phase I study of flavopiridol in combination with gemcitabine and irinotecan in patients with metastatic cancer. Am. J. Clin. Oncol. 2010, 33, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Nagaria, T.S.; Williams, J.L.; Leduc, C.; A Squire, J.; A Greer, P.; Sangrar, W. Flavopiridol synergizes with sorafenib to induce cytotoxicity and potentiate antitumorigenic activity in EGFR/HER-2 and mutant RAS/RAF breast cancer model systems. Neoplasia 2013, 15, 939–951, IN25–IN27. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Tammela, T.; Enholm, B.; Alitalo, K.; Paavonen, K. The biology of vascular endothelial growth factors. Cardiovasc. Res. 2005, 65, 550–563. [Google Scholar] [CrossRef]
- Carmeliet, P.; Dor, Y.; Herbert, J.-M.; Fukumura, D.; Brusselmans, K.; Dewerchin, M.; Neeman, M.; Bono, F.; Abramovitch, R.; Maxwell, P.; et al. Role of HIF-1α in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998, 394, 485–490. [Google Scholar] [CrossRef]
- MMelillo, G.; A Sausville, E.; Cloud, K.; Lahusen, T.; Varesio, L.; Senderowicz, A.M. Flavopiridol, a protein kinase inhibitor, down-regulates hypoxic induction of vascular endothelial growth factor expression in human monocytes. Cancer Res. 1999, 59, 5433–5437. [Google Scholar]
- Mukhopadhyay, D.; Tsiokas, L.; Sukhatme, V.P. Wild-type p53 and v-Src exert opposing influences on human vascular endothelial growth factor gene expression. Cancer Res. 1995, 55, 6161–6165. [Google Scholar]
- Marconcini, L.; Marchiò, S.; Morbidelli, L.; Cartocci, E.; Albini, A.; Ziche, M.; Bussolino, F.; Oliviero, S. c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 9671–9676. [Google Scholar] [CrossRef] [PubMed]
- Rapella, A.; Negrioli, A.; Melillo, G.; Pastorino, S.; Varesio, L.; Bosco, M.C. Flavopiridol inhibits vascular endothelial growth factor production induced by hypoxia or picolinic acid in human neuroblastoma. Int. J. Cancer 2002, 99, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.S.; Wexler, R.S.; A Mousa, S.; Robinson, C.S.; Wexler, E.J.; Mohamed, S.; E Voss, M.; Devenny, J.J.; Czerniak, P.M.; Gudzelak, A.; et al. Novel small molecule alpha v integrin antagonists: Comparative anti-cancer efficacy with known angiogenesis inhibitors. Anticancer Res. 1999, 19, 959–968. [Google Scholar] [PubMed]
- Yang, G.; Sun, H.; Kong, Y.; Hou, G.; Han, J. Diversity of RGD radiotracers in monitoring antiangiogenesis of flavopiridol and paclitaxel in ovarian cancer xenograft-bearing mice. Nucl. Med. Biol. 2014, 41, 856–862. [Google Scholar] [CrossRef]
- Reiner, T.; de las Pozas, A.; Perez-Stable, C. Sequential combinations of flavopiridol and docetaxel inhibit prostate tumors, induce apoptosis, and decrease angiogenesis in the Gγ/T-15 transgenic mouse model of prostate cancer. Prostate 2006, 66, 1487–1497. [Google Scholar] [CrossRef]
- Robinson, C.; Slee, A.; Kerr, J. Flavopiridol inhibits angiogenesis. FASEB J. 1997, 9650, 20814–23998. [Google Scholar]
- McFerrin, H.; Angelova, M.; Abboud, E.; Nelson, A.; Betancourt, A.; Morris, G.; Shelby, B.; Morris, C.; Sullivan, D. The angiogenic properties of Kaposi’s sarcoma-associated herpesvirus encoded G-protein coupled receptor are reduced by flavopiridol, an inhibitor of cyclin-dependent kinase 9. Infect. Agents Cancer 2010, 5, A75. [Google Scholar] [CrossRef]
- Han, Y.; Zhan, Y.; Hou, G.; Li, L. Cyclin-dependent kinase 9 may as a novel target in downregulating the atherosclerosis inflammation. Biomed. Rep. 2014, 2, 775–779. [Google Scholar] [CrossRef]
- Leitch, A.; Haslett, C.; Rossi, A. Cyclin-dependent kinase inhibitor drugs as potential novel anti-inflammatory and pro-resolution agents. Br. J. Pharmacol. 2009, 158, 1004–1016. [Google Scholar] [CrossRef]
- Krystof, V.; Baumli, S.; Furst, R. Perspective of cyclin-dependent kinase 9 (CDK9) as a drug target. Curr. Pharm. Des. 2012, 18, 2883–2890. [Google Scholar] [CrossRef]
- Schmerwitz, U.K.; Sass, G.; Khandoga, A.G.; Joore, J.; Mayer, B.A.; Berberich, N.; Totzke, F.; Krombach, F.; Tiegs, G.; Zahler, S.; et al. Flavopiridol protects against inflammation by attenuating leukocyte-endothelial interaction via inhibition of cyclin-dependent kinase 9. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhang, J.; Pei-Qian, Z.; Chang-Hua, M.; Lin-Hui, Y.; Jun, L.; Zhen-Zhong, L.U.O.; Guo-Hai, X.U. Intrathecal administration of flavopiridol promotes regeneration in experimental model of spinal cord injury. Turk. Neurosurg. 2016, 26, 922–929. [Google Scholar]
- Hou, T.; Ray, S.; Brasier, A.R. The functional role of an interleukin 6-inducible CDK9· STAT3 complex in human γ-fibrinogen gene expression. J. Biol. Chem. 2007, 282, 37091–37102. [Google Scholar] [CrossRef] [PubMed]
- Terashima, T.; Haque, A.; Kajita, Y.; Takeuchi, A.; Nakagawa, T.; Yokochi, T. Flavopiridol inhibits interferon-γ-induced nitric oxide production in mouse vascular endothelial cells. Immunol. Lett. 2012, 148, 91–96. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Haque, A.; Koide, N.; Iftakhar-E-Khuda, I.; Noman, A.S.M.; Odkhuu, E.; Badamtseren, B.; Naiki, Y.; Komatsu, T.; Yoshida, T.; Yokochi, T. Flavopiridol inhibits lipopolysaccharide-induced TNF-α production through inactivation of nuclear factor-κB and mitogen-activated protein kinases in the MyD88-dependent pathway. Microbiol. Immunol. 2011, 55, 160–167. [Google Scholar] [CrossRef]
- Yik, J.H.; Hu Za Kumari, R.; Christiansen, B.A.; Haudenschild, D.R. Cyclin-Dependent Kinase 9 Inhibition Protects Cartilage From the Catabolic Effects of Proinflammatory Cytokines. Arthritis Rheumatol. 2014, 66, 1537–1546. [Google Scholar] [CrossRef]
- Hu Za Chen, Y.; Song, L.; Yik, J.H.; Haudenschild, D.R.; Fan, S. Flavopiridol protects bone tissue by attenuating RANKL induced osteoclast formation. Front. Pharmacol. 2018, 9, 174. [Google Scholar]
- Brendan, F.; Boyce, M.; Xing, L. Functions of RANKL/RANK/OPG in bone modelling and remodelling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar]
- Xing, L.; Xiu, Y.; Boyce, B.F. Osteoclast fusion and regulation by RANKL-dependent and independent factors. World J. Orthop. 2012, 3, 212. [Google Scholar] [CrossRef]
- Ren, H.; Han, M.; Zhou, J.; Zheng, Z.-F.; Lu, P.; Wang, J.-J.; Wang, J.Q.; Mao, Q.J.; Gao, J.Q.; Ouyang, H.W. Repair of spinal cord injury by inhibition of astrocyte growth and inflammatory factor synthesis through local delivery of flavopiridol in PLGA nanoparticles. Biomaterials 2014, 35, 6585–6594. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Verma, A.; Soni, D.K. Impact of Microbial Genomics Approaches for Novel Antibiotic Target. In Microbial Genomics in Sustainable Agroecosystems; Springer: Berlin/Heidelberg, Germany, 2019; pp. 75–88. [Google Scholar]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.J.; D’agati, V.D.; Gries, J.-M.; Suarez, J.-R.; Gelman, I.H. Amelioration of nephropathy in mice expressing HIV-1 genes by the cyclin-dependent kinase inhibitor flavopiridol. J. Antimicrob. Chemother. 2003, 51, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Sandri-Goldin, R.M. Inhibition of cdk9 during herpes simplex virus 1 infection impedes viral transcription. PLoS ONE 2013, 8, e79007. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.S.M.; Eckshtain-Levi, M.; Sobrado, P. Identification of eukaryotic UDP-galactopyranose mutase inhibitors using the ThermoFAD assay. Biochem. Biophys. Res. Commun. 2017, 493, 58–63. [Google Scholar] [CrossRef]
- Leggio, G.M.; Catania, M.V.; Puzzo, D.; Spatuzza, M.; Pellitteri, R.; Gulisano, W.; Torrisi, S.A.; Giurdanella, G.; Piazza, C.; Impellizzeri, A.R.; et al. The antineoplastic drug flavopiridol reverses memory impairment induced by Amyloid-ß1-42 oligomers in mice. Pharmacol. Res. 2016, 106, 10–20. [Google Scholar] [CrossRef]
- Wang, F.; Corbett, D.; Osuga, H.; Osuga, S.; Ikeda, J.-E.; Slack, R.S.; Hogan, M.J.; Hakim, A.M.; Park, D.S. Inhibition of cyclin-dependent kinases improves CA1 neuronal survival and behavioral performance after global ischemia in the rat. J. Cereb. Blood Flow Metab. 2002, 22, 171–182. [Google Scholar] [CrossRef]
- Osuga, H.; Osuga, S.; Wang, F.; Fetni, R.; Hogan, M.J.; Slack, R.S.; Hakim, A.M.; Ikeda, J.-E.; Park, D.S. Cyclin-dependent kinases as a therapeutic target for stroke. Proc. Natl. Acad. Sci. USA 2000, 97, 10254–10259. [Google Scholar] [CrossRef]
- Wu, J.; Stoica, B.A.; Dinizo, M.; Pajoohesh-Ganji, A.; Piao, C.; Faden, A.I. Delayed cell cycle pathway modulation facilitates recovery after spinal cord injury. Cell Cycle 2012, 11, 1782–1795. [Google Scholar] [CrossRef]
- Padmanabhan, J.; Brown, K.R.; Padilla, A.; Shelanski, M.L. Functional role of RNA polymerase II and P70 S6 kinase in KCl withdrawal-induced cerebellar granule neuron apoptosis. J. Biol. Chem. 2015, 290, 5267–5279. [Google Scholar] [CrossRef]
- Di Giovanni, S.; Movsesyan, V.; Ahmed, F.; Cernak, I.; Schinelli, S.; Stoica, B.; Faden, A.I. Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc. Natl. Acad. Sci. USA 2005, 102, 8333–8338. [Google Scholar] [CrossRef] [PubMed]
- Jaschke, B.; Milz, S.; Vogeser, M.; Michaelis, C.; Vorpahl, M.; Schömig, A.; Kastrati, A.; Wessely, R. Local cyclin-dependent kinase inhibition by flavopiridol inhibits coronary artery smooth muscle cell proliferation and migration: Implications for the applicability on drug-eluting stents to prevent neointima formation following vascular injury. FASEB J. 2004, 18, 1285–1287. [Google Scholar] [CrossRef] [PubMed]
- Hassan, P.; Fergusson, D.; Grant, K.M.; Mottram, J.C. The CRK3 protein kinase is essential for cell cycle progression of Leishmania mexicana. Mol. Biochem. Parasitol. 2001, 113, 189–198. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Mitra, P.; Kolagani, A.; Gurupwar, R. Cdk-related kinase 9 regulates RNA polymerase II mediated transcription in Toxoplasma gondii. Biochim. Biophys. Acta (BBA)–Gene Regul. Mech. 2018, 1861, 572–585. [Google Scholar] [CrossRef]
- Graeser, R.; Wernli, B.; Franklin, R.M.; Kappes, B. Plasmodium falciparum protein kinase 5 and the malarial nuclear division cycles. Mol. Biochem. Parasitol. 1996, 82, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Oikonomakos, N.G.; Schnier, J.B.; Zographos, S.E.; Skamnaki, V.T.; Tsitsanou, K.E.; Johnson, L.N. Flavopiridol Inhibits Glycogen Phosphorylase by Binding at the Inhibitor Site. J. Biol. Chem. 2000, 275, 34566–34573. [Google Scholar] [CrossRef]
- Schoepfer, J.; Fretz, H.; Chaudhuri, B.; Muller, L.; Seeber, E.; Meijer, L.; Lozach, O.; Vangrevelinghe, E.; Furet, P. Structure-Based Design and Synthesis of 2-Benzylidene-benzofuran-3-ones as Flavopiridol Mimics. J. Med. Chem. 2002, 45, 1741–1747. [Google Scholar] [CrossRef]
- Kim, K.S.; Sack, J.S.; Tokarski, J.S.; Qian, L.; Chao, S.T.; Leith, L.; Kelly, Y.F.; Misra, R.N.; Hunt, J.T.; Kimball, S.D.; et al. Thio- and Oxoflavopiridols, Cyclin-Dependent Kinase 1-Selective Inhibitors: Synthesis and Biological Effects. J. Med. Chem. 2000, 43, 4126–4134. [Google Scholar] [CrossRef]
- Joshi, K.S.; Rathos, M.J.; Joshi, R.D.; Sivakumar, M.; Mascarenhas, M.; Kamble, S.; Lal, B.; Sharma, S. In vitro antitumor properties of a novel cyclin-dependent kinase inhibitor, P276-00. Mol. Cancer Ther. 2007, 6, 918–925. [Google Scholar] [CrossRef]
- Ali, A.; Ghosh, A.; Nathans, R.S.; Sharova, N.; O’Brien, S.; Cao, H.; Stevenson, M.; Rana, T.M. Identification of Flavopiridol Analogues that Selectively Inhibit Positive Transcription Elongation Factor (P-TEFb) and Block HIV-1 Replication. ChemBioChem 2009, 10, 2072–2080. [Google Scholar] [CrossRef]
- Ahn, Y.M.; Vogeti, L.; Liu, C.-J.; Santhapuram, H.K.; White, J.M.; Vasandani, V.; Mitscher, L.A.; Lushington, G.H.; Hanson, P.R.; Powell, D.R.; et al. Design, synthesis, and antiproliferative and CDK2-cyclin a inhibitory activity of novel flavopiridol analogues. Bioorg. Med. Chem. 2007, 15, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Dey, J.; Deckwerth, T.L.; Kerwin, W.S.; Casalini, J.R.; Merrell, A.J.; Grenley, M.O.; Burns, C.; Ditzler, S.H.; Dixon, C.P.; Beirne, E.; et al. Voruciclib, a clinical stage oral CDK9 inhibitor, represses MCL-1 and sensitizes high-risk diffuse large B-cell lymphoma to BCL2 inhibition. Sci. Rep. 2017, 7, 18007. [Google Scholar] [CrossRef] [PubMed]
- Messmann, R.A.; Ullmann, C.D.; Lahusen, T.; Kalehua, A.; Wasfy, J.; Melillo, G.; Ding, I.; Headlee, D.; Figg, W.D.; A Sausville, E.; et al. Flavopiridol-related proinflammatory syndrome is associated with induction of interleukin-6. Clin. Cancer Res. 2003, 9, 562–570. [Google Scholar]
- Connors, J.M.; Kouroukis, C.; Belch, A.; Crump, M.; Imrie, K. Flavopiridol for mantle cell lymphoma: Moderate activity and frequent disease stabilization. Blood 2001, 98, 3355. [Google Scholar]
- Burdette-Radoux, S.; Tozer, R.; Lohmann, R.; Quirt, I.; Ernst, D.; Walsh, W.; Wainman, N.; Colevas, D.; Eisenhauer, E. A. NCIC CTG phase II study of flavopiridol in patients with previously untreated metastatic malignant melanoma. Proc. Am. Soc. Clin. Oncol. 2002, 21, 346. [Google Scholar]
- Mahoney, E.; Byrd, J.C.; Johnson, A.J. Autophagy and ER stress play an essential role in the mechanism of action and drug resistance of the cyclin-dependent kinase inhibitor flavopiridol. Autophagy 2013, 9, 434–435. [Google Scholar] [CrossRef]
- Li, X.; Lu, J.; Kan, Q.; Li, X.; Fan, Q.; Li, Y.; Huang, R.; Slipicevic, A.; Dong, H.P.; Eide, L.; et al. Metabolic reprogramming is associated with flavopiridol resistance in prostate cancer DU145 cells. Sci. Rep. 2017, 7, 5081. [Google Scholar] [CrossRef]
- Zeidner, J.F.; Karp, J.E. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk. Res. 2015, 39, 1312–1318. [Google Scholar] [CrossRef]
- Wiernik, P.H. Alvocidib (flavopiridol) for the treatment of chronic lymphocytic leukemia. Expert Opin. Investig. Drugs 2016, 25, 729–734. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, X.; Phelps, M.A.; Piao, L.; Rozewski, D.M.; Liu, Q.; Lee, L.J.; Marcucci, G.; Grever, M.R.; Byrd, J.C.; et al. A novel liposomal formulation of flavopiridol. Int. J. Pharm. 2009, 365, 170–174. [Google Scholar] [CrossRef]
- Chen, K.T.; Militao, G.G.; Anantha, M.; Witzigmann, D.; Leung, A.W.; Bally, M.B. Development and characterization of a novel flavopiridol formulation for treatment of acute myeloid leukemia. J. Control. Release 2021, 333, 246–257. [Google Scholar] [CrossRef] [PubMed]






| S. No. | Therapeutics | Diseases | Mechanisms | Dose | Route | Experimental Models | Refs. |
|---|---|---|---|---|---|---|---|
| 1 | Anti-Alzheimer | Alzheimer | Rescue from memory impairment and cell cycle reactivation caused by Aβ1-42 oligomers; Aβ-treated mice had improved long-term memory response | 0.5–1 mg/kg | i.p. | CD1 mice | [119] |
| 2 | Neuroprotective | Ischaemic stroke | Inhibits the phosphorylation of Rb; increased levels of E2F1; inhibits CDK activation and prevents CA1 neuronal cell death | 500 µmol/L 500 µM | i.c.v. | Wistar rats | [120,121] |
| Spinal cord injury | Reduces expression of cyclin D1, pRb, CDK4, E2F1, and PCNA; increases expression of endogenous CDK inhibitor p27; reduces levels of galactin-3 and Iba-1, leading to decreased number of Iba-1+ microglial cells; increases CC1+ oligodendrocytes and white matter myelinated area; abrogates RNA Pol II phosphorylation and induction to promote neuronal survival | 1 mg/kg | i.p. | Male Sprague-Dawley rats | [122,123] | ||
| Traumatic brain injury | Increases neuronal survival post DNA damage and inactivated astroglial proliferation, microglial activation, and scar formation by blocking cell cycle progression proteins | 250 µM | i.c.v. | Male Sprague-Dawley rats | [124] | ||
| 3 | Anti-vasoproliferative | In-stent restenosis | Anti-proliferative effects were observed in human coronary artery smooth muscle cells (HCASMC) by cell cycle arrest at G1/S and G2/M phases; increases levels of p21, p27, and p53; abrogation of Rb hyperphosphorylation; reduces apoptosis; prevents neointima formation | 0.1 µM, 25 mg/mL | - | HCASMC, human coronary artery endothelial cells, rat | [125] |
| 4 | Anti-hepatitis | Concavalin A-induced hepatitis | Inhibits ConA-induced hepatitis and neutrophil infiltration; suppresses TNF-α-induced leukocyte–endothelial cell interaction; abrogates the levels of ICAM-1, VCAM-1, E-selectin, and NF-κB by inhibition of CDK9 activity | 44 ng | i.v. | C57BL/6 male mice | [103] |
| 5 | Anti-viral | Acquired immunodeficiency syndrome | Inhibits the phosphorylation induced by P-TEFb at the C-terminus region of RNA Pol II large subunit; abrogates Tat transactivation and HIV-1 viral replication | 6–12 nM | - | Transfecting 293T cells with the HIV-1HXB2 provirus, HIV-1NL4–3 viral particles, Jurkat cells | [26] |
| Human-immunodeficiency-virus-associated nephropathy | Inhibition of HIV-1 transcript levels in infected glomerular visceral epithelial cells; improved nephropathy in mouse model | 2.5 mg/kg | i.p. | HIV-1 NL4-3 transgenic mouse model | [116] | ||
| Herpes simplex virus 1 infection | Inhibition of P-TEFb/CDK9 complex by blocking the phosphorylation at serine-2 residue on the C-terminus region of RNA Pol II; suppresses the replication of HSV-1 and HSV-2 through inhibition of mRNA transcription | 450 nM 30 mg | - | HeLa cells, Wistar rats, BALB/c mice | [27,117] | ||
| Human cytomegalovirus infection | Suppresses the replication of HCMV through inhibition of mRNA transcription by CDK9 inhibition | 1–5 µM | - | A549, Vero cells | [27] | ||
| Human adenovirus infection | Suppresses the replication of HAdV5 and HAdV53 through inhibition of mRNA transcription by CDK9 inhibition; decreases expression of an early gene of adenovirus E1A | 1–10 µM | - | A549, Vero cells | [27] | ||
| Influenza A virus infection | Suppresses the replication of H1N1, H3N2, and H7N9 through inhibition of mRNA transcription by CDK9 inhibition | 0.59 µM 0.70 µM 0.24 µM | - | A549 cells | [28] | ||
| 6 | Anti-fungal | Fungal keratitis | Downregulation of IL-1β, IL-6, and TNF-α expression and induction of IL-10 expression; increases expression of LC3, Beclin-1, and Atg7 proteins; promotes the phagocytosis of RAW264.7 cells; suppresses biofilm formation, growth, and attachment of Aspergillus fumigatus; decreases inflammation in fungal keratitis disease by inducing autophagy | 5 µM | s.c.i | RAW 264.7 cells, C57BL/6 female mice | [29] |
| Aspergillosis | Acts as a non-competitive inhibitor of UDP-galactopyranose mutase (UGM) to treat Aspergillus fumigatus infection | 200 µM | - | AfUGM | [118] | ||
| 7 | Anti-leishmanial | Leishmaniasis | Inhibition of CRK3 kinase; inhibition of in vitro growth of Leishmania Mexicana promastigotes; suppresses cell cycle progression at G2 and G2/M phase | 2.5 µM | - | Leishmania Mexicana promastigotes | [126] |
| 8 | Anti-parasitic | Toxoplasma gondii infection | Inhibition of TgCRK9 kinase activity led to the abrogation of RNA pol II dependent transcription elongation; inhibition of parasite multiplication and proliferation | 4 nM, 8 nM | - | HFF cells, Toxoplasma gondii culture | [127] |
| 9 | Anti-malarial | Malaria | Abrogates the activity of PfPK5 kinase in Plasmodium falciparum; inhibition of DNA synthesis | 0.06 µM, 2 µM | - | Red blood cells, Plasmodium falciparum culture | [128] |
| 10 | Anti-diabetic | Diabetes | Inhibition of glycogen phosphorylase a and b enzymes | 15.5 µM | - | Rabbit skeletal muscle, A549 cells | [129] |
| 11 | Anti-arthritis | Osteoarthritis | Abolishes the activation of iNOS by IL-1β; inhibits a broad range of inflammatory mediators; inhibits the induction of MMP1,3, 9, and 13; protects the cartilage from the harmful effects of pro-inflammatory cytokines | 300 nM | - | Human chondrocytes, human cartilage explants | [109] |
| Derivatives | IC50 (in µM) | Refs. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CDK | GSK3β | |||||||||
| 1 | 2 | 4 | 6 | 7 | 9 | 10 | ||||
| 1 | 4,6-Dihydroxy-2-[1-[4-(4-methyl-piperazin-1-yl)-phenyl]-meth-(E)-ylidene]-7-(1-methyl-piperidin-4-yl)-benzofuran-3-one | 0.80 | 2.98 | 1.92 | - | - | - | - | 1.75 | [130] |
| 2 | 4-[4,6-Dihydroxy-7-(1-methyl-piperidin-4-yl)-3-oxo-3Hbenzofuran-(2E)-ylidenemethyl] benzenesulfonamide | 0.009 | 0.03 | 1.87 | - | - | - | - | 3.7 | |
| 3 | 4,6-Dihydroxy-7-(1-methyl-piperidin-4-yl)-2-[1-(4-nitrophenyl)-meth-(E)-ylidene]-benzofuran-3-one | 0.06 | 0.31 | 2.21 | - | - | - | - | 4.8 | |
| 4 | 2-[1-(2-Chloro-phenyl)-meth-(E)-ylidene]-4,6-dihydroxy-7-(1-methyl-piperidin-4-yl)-benzofuran-3-one | 0.60 | 3.97 | 25.25 | - | - | - | - | 10 | |
| 5 | 4,6-Dihydroxy-7-(1-methyl-piperidin-4-yl)-2-[1-phenylmeth-(E)-ylidene]-benzofuran-3-one | 0.11 | 1.28 | 4.41 | - | - | - | - | 4.2 | |
| R1-N-[2-(2-Chlorophenyl)-5,7-dihydroxy-4-oxo-4Hchromen-8-yl]-R2 | [134] | |||||||||
| 6 | R2 = benzamide | - | 91 | - | - | - | - | - | - | |
| 7 | R2 = 4-methylbenzamide | - | 90 | - | - | - | - | - | - | |
| 8 | R2 = 4-methoxybenzamide | - | 54 | - | - | - | - | - | - | |
| 9 | R1 = 3,5-Dichloro; R2 = 2- hydroxyl benzene sulfonamide | - | 94 | - | - | - | - | - | - | |
| R-5,7-dihydroxy-8-(3-hydroxy-1-methyl-4-piperidinyl)-4H-1-benzopyran-4-one | [131] | |||||||||
| 10 | R = (±)-(3SR,4RS)-2-(Ethylthio) | 0.46 | 3.93 | 2.06 | - | - | - | - | - | |
| 11 | R = (3S,4R)-2-[(2-Chlorophenyl)thio] | 0.11 | 2.10 | 16.2 | - | - | - | - | - | |
| 12 | R = (3S,4R)-2-(2-Chlorophenoxy) | 0.13 | 2.11 | 6.15 | - | - | - | - | - | |
| 13 | R = (±)-(3SR,4RS)-2-(Phenylthio) | 0.44 | 6.59 | 4.10 | - | - | - | - | - | |
| 14 | R = (±)-(3SR,4RS)-2-(tert-Butylthio) | 0.08 | 1.07 | 2.07 | - | - | - | - | - | |
| 15 | R = (±)-(3SR,4RS)-2-[(4,6-Dimethylpyrimidin-2-yl)thio] | 6.40 | 40.4 | 82.5 | - | - | - | - | - | |
| 16 | R = (±)-(3SR,4RS)-2-(Phenylamino) | 16.3 | >25 | >25 | - | - | - | - | - | |
| 17 | R = (±)-(3SR,4RS)-2-N-Piperidyl | 2.50 | 9.69 | 3.70 | - | - | - | - | - | |
| cis-5,7-dihydroxy-2-(R1)-8-[R2-piperidinyl]-1-benzopyran-4-one | [34] | |||||||||
| 18 | R1 = 2-chlorophenyl; R2 = 4-(3-one-1-methyl) | 10 | - | 8 | - | - | - | - | - | |
| 19 | R1 = 2-chlorophenyl; R2 = cis-8-2-hydroxycyclohexyl | 12 | - | 31 | - | - | - | - | - | |
| 20 | R1 = 2-chlorophenyl; R2 = 4-(3-en) | 1.1 | - | 0.8 | - | - | - | - | - | |
| 21 | R1 = 3-chlorophenyl; R2 = 4-(3-en) | 1.2 | - | 2.4 | - | - | - | - | - | |
| 22 | R1 = 4-chlorophenyl; R2 = 4-(3-en) | 1.7 | - | 0.55 | - | - | - | - | - | |
| 23 | R1 = 2-florophenyl; R2 = 4-(3-en) | 2.3 | - | 1.8 | - | - | - | - | - | |
| 24 | R1 = 2-bromophenyl; R2 = 4-(3-en) | 0.98 | - | 0.65 | - | - | - | - | - | |
| 25 | R1 = 2-florophenyl; R2 = 4-(3-en) | - | - | 2.5 | - | - | - | - | - | |
| 26 | R1 = 2-phenyl; R2 = 4-(3-en) | - | - | 1.0 | - | - | - | - | - | |
| 27 | R1 = 2, 4-dichlorophenyl; R2 = 4-(3-en) | 1.7 | - | 1.2 | - | - | - | - | - | |
| 28 | R1 = 4-pyridyl; R2 = 4-(3-en) | - | - | 0.8 | - | - | - | - | - | |
| 29 | R1 = cyclohexyl; R2 = 4-(3-en) | - | - | 7 | - | - | - | - | - | |
| 30 | 2-(2-chlorophenyl)-5,7-dihydroxy-8-((2R,3S)-2-(hydroxymethyl)-1-methylpyrrolidin-3-yl)-4H-chromen-4-one hydrochloride (P-276-00) | 0.079 | 0.224 | 0.063 | 0.396 | 2.870 | 0.020 | - | 2.771 | [132] |
| 31 | 2-(2-Chloro-4-(trifluoromethyl)phenyl)-5,7-dihydroxy-8-((2R,3S)-2-(hydroxymethyl)-1-methylpyrrolidin-3-yl)-4H-1-benzopyran-4-one (Voruciclib) | 0.0054 | - | 0.0039 | 0.0029 | - | 0.0017 | - | - | [135] |
| 32 | 2-(2,6-dichlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]chromen-4-one (IIIM-290) | 0.0049 | 0.0155 | 0.0225 | 0.045 | 0.711 | 0.0019 | - | - | [23] |
| 33 | 2-(4-((1H-benzo[d]imidazol-2-yl)thio)phenyl)-5,7-dihydroxy-8-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-4H-chromen-4-one | - | 0.162 | - | - | - | 0.008 | - | 0.117 | [25] |
| 34 | 5,7-Dihydroxy-8-(1-methyl-1,2,3,6-tetrahydro pyridin-4-yl)-2-(4-((1-methyl-1H-benzo[d] imidazol-2-yl)thio)phenyl)-4Hchromen-4-one | - | 0.349 | - | - | - | 0.014 | - | 0.160 | |
| 35 | 5,7-Dihydroxy-8-(1-methyl-1,2,3,6-tetrahydro pyridin-4-yl)-2-(4-((5-phenyl-1H-imidazol-2-yl)thio) phenyl)-4H-chromen-4-one | - | 0.469 | - | - | - | 0.009 | - | 0.356 | |
| 36 | 2-(4-((1H-benzo[d]imidazol-2-yl)thio)-2-chloro phenyl)-5,7-dihydroxy-8-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-4H-chromen-4-one | - | 1.556 | - | - | - | 0.015 | - | 0.216 | |
| 37 | 2-(2-Chloro-4-((1-methyl-1H-benzo[d] imidazol-2-yl)thio) phenyl)-5,7-dihydroxy-8-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-4H-chromen-4-one | - | 1.725 | - | - | - | 0.064 | 0.149 | 0.059 | |
| 2-R-5,7-dihydroxy-8[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]chromen-4-one | [133] | |||||||||
| 38 | R = phenyl | - | 0.196 | - | - | - | 0.009 | - | - | |
| 39 | R = 3-chlorophenyl | - | 0.164 | - | - | - | 0.004 | - | - | |
| 40 | R = 4-chlorophenyl | - | 0.287 | - | - | - | 0.012 | - | - | |
| 41 | R = 2-fluorophenyl | 0.123 | 0.356 | - | - | >10 | 0.003 | - | >1 | |
| 42 | R = 4-fluorophenyl | - | 0.129 | - | - | - | 0.002 | - | - | |
| 43 | R = 4-bromophenyl | - | 0.223 | - | - | - | 0.005 | - | - | |
| 44 | R = 4-tert-butylphenyl | - | 0.567 | - | - | - | 0.019 | - | - | |
| 45 | R = 4-trifluoromethylphenyl | - | 0.302 | - | - | - | 0.019 | - | - | |
| 46 | R = 4-hydroxyphenyl | - | 0.196 | - | - | - | 0.010 | - | - | |
| 47 | R = 2-pyridyl | - | 0.886 | - | - | - | 0.011 | - | - | |
| 48 | R = 3-pyridyl | - | 0.247 | - | - | - | 0.005 | - | - | |
| 49 | R = 4-pyridyl | - | 0.208 | - | - | - | 0.006 | - | - | |
| 50 | R = 2-chloro-3-pyridyl | - | 0.314 | - | - | - | 0.013 | - | - | |
| 51 | R = 5-methylisoxazole | - | 0.238 | - | - | - | 0.020 | - | - | |
| 52 | R = 3-vinylphenyl | - | 0.130 | - | - | - | 0.010 | - | - | |
| 53 | R = 4-vinylphenyl | - | 0.206 | - | - | - | 0.010 | - | - | |
| 54 | R = 4-fluorophenyl | - | 0.208 | - | - | - | 0.006 | - | - | |
| 55 | R = 2-bromophenyl | - | 0.639 | - | - | - | 0.005 | - | - | |
| 56 | R = 3-pyridyl | - | 1.023 | - | - | - | 0.012 | - | - | |
| S. No. | Derivatives | Experimental Models | Dose (IC50) in µM | Refs. |
|---|---|---|---|---|
| cis-5,7-dihydroxy-2-(R)-8-[4-(3-en)-piperidinyl]-1-benzopyran-4-one | [34] | |||
| 1 | R = 2-chlorophenyl | MCF-7 | 0.75 | |
| 2 | R = 3-chlorophenyl | MCF-7 | 1 | |
| 3 | R = 4-chlorophenyl | MCF-7 | 3 | |
| 4 | R = 2-florophenyl | MCF-7 | 1 | |
| 5 | R = 2-chlorophenyl | MCF-7 | 1 | |
| 6 | R = 2-bromophenyl | MCF-7 | 1.5 | |
| 7 | (3S,4R)-2-[(2-Chlorophenyl)thio]-5,7-dihydroxy-8-(3-hydroxy-1-methyl-4-piperidinyl)-4H-1-benzopyran-4-one | PC3, Mia PaCa-2, HCT116, A2780 | 0.02, 0.03, 0.21, 0.87 | [131] |
| 8 | 4,6-Dihydroxy-7-(1-methyl-piperidin-4-yl)-2-[1-phenylmeth-(E)-ylidene]-benzofuran-3-one | HCT-116 | >50 | [130] |
| 9 | 2-[1-(2-Chloro-phenyl)-meth-(E)-ylidene]-4,6-dihydroxy-7-(1-methyl-piperidin-4-yl)-benzofuran-3-one | HCT-116 | 20.1 | |
| 10 | 4,6-Dihydroxy-7-(1-methyl-piperidin-4-yl)-2-[1-(4-nitrophenyl)-meth-(E)-ylidene]-benzofuran-3-one | HCT-116 | 35.6 | |
| 11 | 4-[4,6-Dihydroxy-7-(1-methyl-piperidin-4-yl)-3-oxo-3Hbenzofuran-(2E)-ylidenemethyl]-benzene sulfonamide | HCT-116 | >50 | |
| 12 | 4,6-Dihydroxy-2-[1-[4-(4-methyl-piperazin-1-yl)-phenyl]-meth-(E)-ylidene]-7-(1-methyl-piperidin-4-yl)-benzofuran-3-one | HCT-116 | 24.8 | |
| R1-N-[2-(2-Chlorophenyl)-5,7-dihydroxy-4-oxo-4Hchromen-8-yl]-R2 | [134] | |||
| 13 | R2 = benzamide | MCF-7 | 8.5 | |
| 14 | R2 = 4-methylbenzamide | MCF-7 | 9.7 | |
| 15 | R2 = 4-methoxybenzamide | MCF-7 | 13 | |
| 16 | R1 = 3,5-Dichloro; R2 = 2-hydroxyl benzene sulfonamide | ID-8, MCF-7 | 24, 17 | |
| 17 | 2-(2-chlorophenyl)-5,7-dihydroxy-8-((2R,3S)-2-(hydroxymethyl)-1-methylpyrrolidin-3-yl)-4H-chromen-4-one hydrochloride (P-276-00) | HCT-116, T-24, U2OS, SiHa, MCF-7, PC-3, HT-29, Colo-205, Caco-2, HL-60, SW-480, H-460, MRC-5, WI-38 | 0.31, 0.39, 0.4, 0.42, 0.52, 0.56, 0.6, 0.65, 0.65, 0.75, 0.76, 0.8, 11.5, 16.5 | [132] |
| 18 | 2-(2-Chloro-4-(trifluoromethyl)phenyl)-5,7-dihydroxy-8-((2R,3S)-2-(hydroxymethyl)-1-methylpyrrolidin-3-yl)-4H-1-benzopyran-4-one (Voruciclib) | RIVA | 56.3% | [135] |
| 19 | 2-(2,6-dichlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]chromen-4-one (IIIM-290) | HL60, MOLT-4, MIAPaCa-2, Panc-1, PC-3, DU145, MCF-7, MDAMB-231, MDAMB-468, BT-549, T47D, Caco-2, SW630, Colo-205, HCT116, A549, NCIH322, NCIH522, HOP62, HOP92, NCIH-226, 786-O, A431, LOXIMVI, OVCAR-3, OVCAR-4, OVCAR-5, mouse adenocarcinoma, HGF, fR2, HEK293 | 0.9, 0.5, 1, 4, 6, 5, 4, 4, 4, 5, 6, 7, 0.3, 7, 5, 4, 2, 5, 7, 3, 4, 6, 8, 4, 8, 9, 7, 1.2, 18, 19, 22 | [23] |
| 20 | 2-(4-((1H-benzo[d]imidazol-2-yl)thio)phenyl)-5,7-dihydroxy-8-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-4H-chromen-4-one | NCI-N87, K562, SKBR3, HCT116, SKOV3, PC3, MiaPaCa-2 | 0.183, 0.194, 0.254, 0.293, 0.595, 0.742, 0.852 | [25] |
| 21 | 5,7-Dihydroxy-8-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-2-(4-((1-methyl-1H-benzo[d]imidazol-2-yl)thio)phenyl)-4Hchromen-4-one | HCT116, SKBR3, SKOV3, K562, SKBR3, MiaPaCa-2, NCI-N87 | 0.173, 0.243, 0.295, 0.300, 0.352, 0.448, 4.796 | |
| 22 | 5,7-Dihydroxy-8-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-2-(4-((5-phenyl-1H-imidazol-2-yl)thio)phenyl)-4H-chromen-4-one | HCT116, SKBR3, PC3, SKOV3, K562, MiaPaCa-2, NCI-N87 | 0.219, 0.249, 0.276, 0.344, 0.345, 0.361, 0.391 | |
| 23 | 2-(4-((1H-benzo[d]imidazol-2-yl)thio)-2-chlorophenyl)-5,7-dihydroxy-8-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-4H-chromen-4-one | NCI-N87, K562, SKBR3, HCT116, PC3, SKOV3, MiaPaCa-2 | 0.012, 0.036, 0.066, 0.170, 0.326, 0.333, 0.361 | |
| 24 | 2-(2-Chloro-4-((1-methyl-1H benzo[d]imidazol-2-yl)thio) phenyl)-5,7-dihydroxy-8-(1-methyl-1,2,3,6-tetrahydropyridin-4-yl)-4H-chromen-4-one | NCI-N87, K562, MiaPaCa-2, SKBR3, PC3, SKOV3, HCT116 | 0.049, 0.051, 0.053, 0.054, 0.085, 0.094, 0.181 | |
| 2-R-5,7-dihydroxy-8[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]chromen-4-one | [133] | |||
| 25 | R = phenyl | HeLa | 0.190 | |
| 26 | R = 3-chlorophenyl | HeLa | 0.170 | |
| 27 | R = 4-chlorophenyl | HeLa | 0.200 | |
| 28 | R = 2-fluorophenyl | HeLa | 0.274 | |
| 29 | R = 4-fluorophenyl | HeLa | 0.200 | |
| 30 | R = 4-bromophenyl | HeLa | 0.280 | |
| 31 | R = 4-tert-butylphenyl | HeLa | 0.660 | |
| 32 | R = 4-trifluoromethylphenyl | HeLa | 1.200 | |
| 33 | R = 4-hydroxyphenyl | HeLa | 20.920 | |
| 34 | R = 2-pyridyl | HeLa | 1.490 | |
| 35 | R = 3-pyridyl | HeLa | 3.300 | |
| 36 | R = 4-pyridyl | HeLa | 3.350 | |
| 37 | R = 2-chloro-3-pyridyl | HeLa | 0.177 | |
| 38 | R = 5-methylisoxazole | HeLa | 0.645 | |
| 39 | R = 3-vinylphenyl | HeLa | 0.195 | |
| 40 | R = 4-vinylphenyl | HeLa | 0.219 | |
| 41 | R = 4-fluorophenyl | HeLa | 0.266 | |
| 42 | R = 2-bromophenyl | HeLa | 0.331 | |
| 43 | R = 3-pyridyl | HeLa | 1.440 | |
| Clinical Trial | Cancer | Phase | Status | Sample Size | Treatment |
|---|---|---|---|---|---|
| NCT00003256 | Recurrent prostate cancer | Phase II | Completed | 40 | FP |
| NCT00007917 | Adult solid tumors | Phase I | Completed | 58 | FP and gemcitabine hydrochloride |
| NCT00016939 | Renal cell carcinoma | Phase II | Completed | 35 | FP |
| NCT00070239 | Hematopoietic, adult solid tumors, and lymphoid cancer | Phase I | Terminated | 100 | FP and fludeoxyglucose F 18 |
| NCT00006245 | Metastatic esophageal cancer | Phase II | Completed | 37 | FP and paclitaxel |
| NCT00006485 | Adult solid tumors | Phase I | Completed | 50 | FP and irinotecan hydrochloride |
| NCT00112684 | Metastatic solid tumors | Phase I | Terminated | 25 | FP |
| NCT00324480 | Adult solid tumors | Phase I | Completed | 60 | FP and vorinostat |
| NCT00020332 | Metastatic breast cancer | Phase I/II | Completed | 49 | FP and docetaxel |
| NCT00331682 | Recurrent pancreatic cancer and pancreatic adenocarcinoma | Phase II | Completed | 10 | FP and docetaxel |
| NCT00080990 | Adult solid tumors | Phase I | Completed | 46 | FP, fluorouracil, oxaliplatin, and leucovorin calcium |
| NCT00039455 | Metastatic breast cancer | Phase I | Terminated | 50 | FP and trastuzumab |
| NCT00087282 | Advanced liver cancer | Phase II | Completed | 32 | FP and irinotecan hydrochloride |
| NCT00072436 | Adult solid tumors | Phase I | Completed | 58 | FP and gemcitabine hydrochloride |
| NCT00045448 | Advanced solid tumors | Phase I | Completed | 56 | FP and docetaxel |
| NCT00046917 | Adult solid tumors | Phase I | Completed | 13 | FP, irinotecan hydrochloride, and cisplatin |
| NCT00019344 | Adult solid tumors, lymphoma, prostate cancer, and small intestine cancer | Phase I | Completed | 36 | FP |
| NCT00047307 | Locally advanced and unresectable pancreatic cancer | Phase I | Completed | 46 | FP, gemcitabine hydrochloride, and radiation therapy |
| NCT00023894 | Recurrent or persistent endometrial cancer | Phase II | Completed | 51 | FP |
| NCT00016185 | Advanced solid tumors | Phase I | Completed | 24 | FP and docetaxel |
| NCT00003690 | Breast cancer, melanoma, prostate cancer, and adult solid tumors | Phase I | Completed | 48 | FP |
| NCT00042874 | Metastatic solid tumors | Phase I | Completed | 77 | FP, irinotecan hydrochloride, fluorouracil, and leucovorin calcium |
| NCT00003004 | Refractory or recurrent solid tumors | Phase I | Completed | 73 | FP, cisplatin, and paclitaxel |
| NCT00079352 | Metastatic solid tumors | Phase I | Completed | 24 | FP, gemcitabine hydrochloride, and irinotecan hydrochloride |
| NCT00020189 | Metastatic head and neck cancer, Thromboembolism | Phase II | Completed | 37 | FP, acetylsalicylic acid, and clopidogrel bisulfate |
| NCT00094978 | Small cell carcinoma, non-small cell lung cancer, esophageal neoplasms, and mesothelioma | Phase I | Terminated | 23 | FP and depsipeptide |
| NCT00021073 | Unspecified adult solid tumors | Phase I | Completed | 90 | FP, leucovorin calcium, fluorouracil, and irinotecan hydrochloride |
| NCT00012181 | Recurrent childhood solid tumors, recurrent neuroblastoma, recurrent osteosarcoma, and recurrent retinoblastoma | Phase I | Completed | 30 | FP |
| NCT00957905 | Recurrent or relapsed germ cell tumors | Phase II | Completed | 36 | FP, leucovorin calcium, fluorouracil, and oxaliplatin |
| NCT00064285 | Leukemia | Phase I | Completed | 22 | FP and imatinib mesylate |
| NCT00991952 | Advanced stomach and gastroesophageal junction cancer | Phase II | Completed | 19 | FP and irinotecan hydrochloride |
| NCT00083122 | Advanced ovarian epithelial cancer and primary peritoneal cancer | Phase II | Completed | 45 | FP and cisplatin |
| NCT00082784 | Recurrent or refractory indolent B-cell neoplasms | Phase I | Completed | 93 | FP and bortezomib |
| NCT00098579 | Metastatic or recurrent sarcoma | Phase I | Completed | 36 | FP and doxorubicin hydrochloride |
| NCT03441555 | Relapsed or refractory acute myeloid leukemia | Phase I | Completed | 36 | FP and venetoclax |
| NCT00047203 | Relapsed or refractory multiple myeloma | Phase II | Completed | 35 | FP |
| NCT03298984 | Acute myeloid leukemia | Phase I | Completed | 32 | FP, cytarabine, and daunorubicin |
| NCT03969420 | Acute myeloid leukemia | Phase II | Terminated | 11 | FP and cytarabine |
| NCT03563560 | Acute myeloid leukemia | Phase I | Completed | 10 | FP, cytarabine, mitoxantrone, and daunorubicin |
| NCT00005974 | Recurrent and metastatic soft tissue sarcoma | Phase II | Completed | 18 | FP |
| NCT02520011 | Acute myeloid leukemia | Phase II | Terminated | 104 | FP, cytarabine, and mitoxantrone |
| NCT03593915 | Myelodysplastic syndromes | Phase I/II | Terminated | 20 | FP and decitabine or azacitidine |
| NCT00470197 | Relapsed or refractory acute leukemia | Phase I | Completed | 35 | FP, cytarabine, and mitoxantrone hydrochloride |
| NCT00112723 | Relapsed or refractory lymphoma and multiple myeloma | Phase I/II | Terminated | 46 | FP |
| NCT00445341 | Relapsed mantle cell lymphoma and diffuse large B-cell lymphoma | Phase I/II | Completed | 28 | FP |
| NCT01349972 | Acute myeloid leukemia | Phase II | Completed | 172 | FP, mitoxantrone hydrochloride, cytarabine, and daunorubicin hydrochloride |
| NCT00795002 | Acute myeloid leukemia | Phase II | Completed | 78 | FP, mitoxantrone hydrochloride, and cytarabine |
| NCT00634244 | Relapsed or refractory acute myeloid leukemia | Phase II | Completed | 92 | FP, mitoxantrone hydrochloride, carboplatin, cytarabine, sirolimus, etoposide, and topotecan hydrochloride |
| NCT00003039 | Lymphoma | Phase II | Completed | 40 | FP |
| NCT00058240 | Chronic lymphocytic leukemia and lymphocytic lymphoma | Phase I/II | Completed | 52 | FP |
| NCT00005971 | Metastatic malignant melanoma | Phase II | Completed | 17 | FP |
| NCT00101231 | Relapsed or refractory acute myeloid leukemia, acute lymphoblastic leukemia, and chronic myelogenous leukemia | Phase I | Terminated | 88 | FP |
| NCT00058227 | Lymphoproliferative disorders or mantle cell lymphoma | Phase I | Completed | 37 | FP, fludarabine phosphate, and rituximab |
| NCT00098371 | Chronic lymphocytic leukemia and prolymphocytic leukemia | Phase II | Terminated | 64 | FP |
| NCT00003620 | Chronic lymphocytic leukemia | Phase II | Completed | 37 | FP |
| NCT00464633 | Chronic lymphocytic leukemia | Phase II | Completed | 165 | FP |
| NCT00278330 | Relapsed or refractory acute leukemia, chronic myelogenous leukemia, and refractory anemia | Phase I | Completed | 24 | FP and vorinostat |
| NCT00735930 | Relapsed or refractory B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma | Phase I | Completed | 39 | FP and lenalidomide |
| NCT00377104 | B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma | Phase I | Terminated | 24 | FP |
| NCT00016016 | Acute leukemia | Phase I/II | Completed | 53 | FP, cytarabine, and mitoxantrone hydrochloride |
| NCT01076556 | B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma | Phase I | Terminated | 9 | FP, cyclophosphamide, and rituximab |
| NCT00407966 | Acute myeloid leukemia | Phase II | Completed | 45 | FP, cytarabine, and mitoxantrone hydrochloride |
| NCT00005074 | Relapsed or untreated mantle cell lymphoma | Phase II | Completed | 33 | FP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, H.; Tuli, H.S.; Ranjan, A.; Chauhan, A.; Haque, S.; Ramniwas, S.; Bhatia, G.K.; Kandari, D. The Pharmacological Implications of Flavopiridol: An Updated Overview. Molecules 2023, 28, 7530. https://doi.org/10.3390/molecules28227530
Joshi H, Tuli HS, Ranjan A, Chauhan A, Haque S, Ramniwas S, Bhatia GK, Kandari D. The Pharmacological Implications of Flavopiridol: An Updated Overview. Molecules. 2023; 28(22):7530. https://doi.org/10.3390/molecules28227530
Chicago/Turabian StyleJoshi, Hemant, Hardeep Singh Tuli, Anuj Ranjan, Abhishek Chauhan, Shafiul Haque, Seema Ramniwas, Gurpreet Kaur Bhatia, and Divya Kandari. 2023. "The Pharmacological Implications of Flavopiridol: An Updated Overview" Molecules 28, no. 22: 7530. https://doi.org/10.3390/molecules28227530