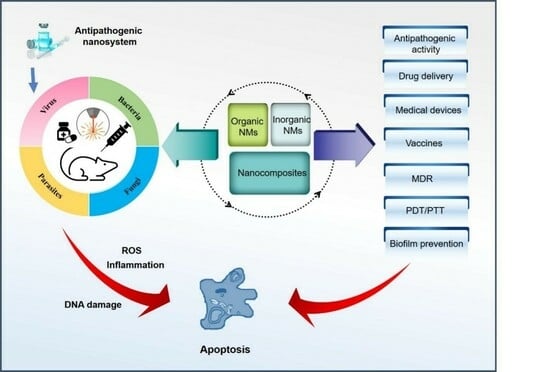
Recent Updates on Multifunctional Nanomaterials as Antipathogens in Humans and Livestock: Classification, Application, Mode of Action, and Challenges
Abstract
:1. Introduction
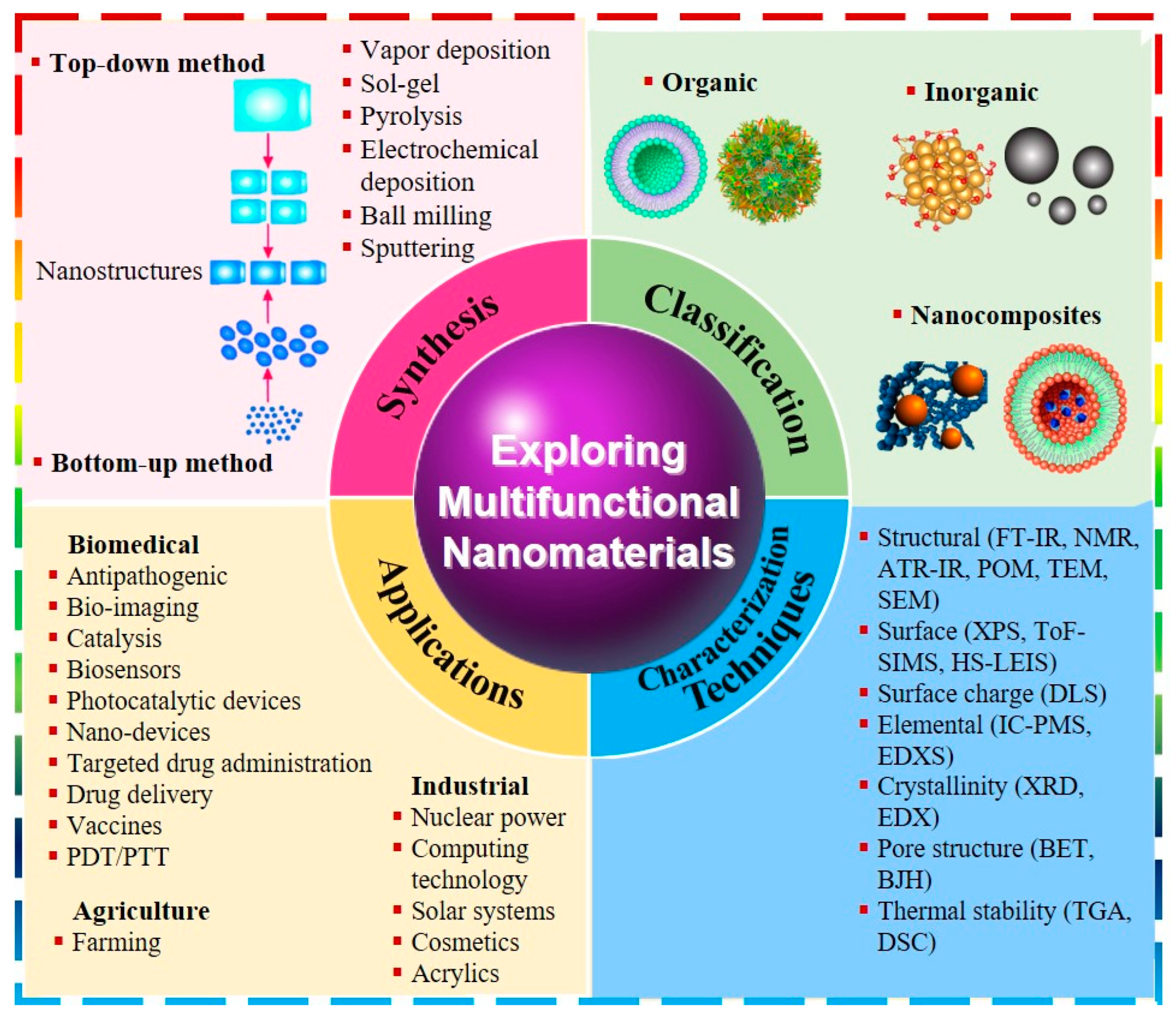
2. Overview of Nanomaterials
2.1. Classification of Nanomaterials
2.1.1. Organic Nanomaterials
2.1.2. Inorganic Nanomaterials
2.1.3. Nanocomposites
2.2. Synthesis and Characterization of Nanomaterials
2.3. Multiple Functions of Nanomaterials
2.4. Role of Nanomaterials as Delivery Systems That Enhance the Antimicrobial Activity of Potential Agents
3. Research Advances of Nanomaterials as Antipathogens
3.1. Nanomaterials for Antibacterial Applications
3.1.1. Organic NMs
3.1.2. Inorganic NMs
3.1.3. Hybrid NMs
3.2. Nanomaterials for Antiviral Applications
3.2.1. Organic NMs
3.2.2. Inorganic NMs
3.2.3. Hybrid NMs
3.3. Nanomaterials for Antifungal Applications
3.3.1. Organic NMs
3.3.2. Inorganic NMs
3.3.3. Hybrid NMs
3.4. Nanomaterials for Antiparasitic Applications
3.4.1. Organic NMs
3.4.2. Inorganic NMs
3.4.3. Hybrid NMs
4. The Mechanism of Nanomaterials for Antipathogens
4.1. Mode of Action of Nanomaterials for Antibacterial Activity
4.1.1. Disruption to the Cell Membranes
4.1.2. Production of Reactive Oxygen Species (ROS)
4.1.3. Interaction with Cell Contents and Damage to DNA
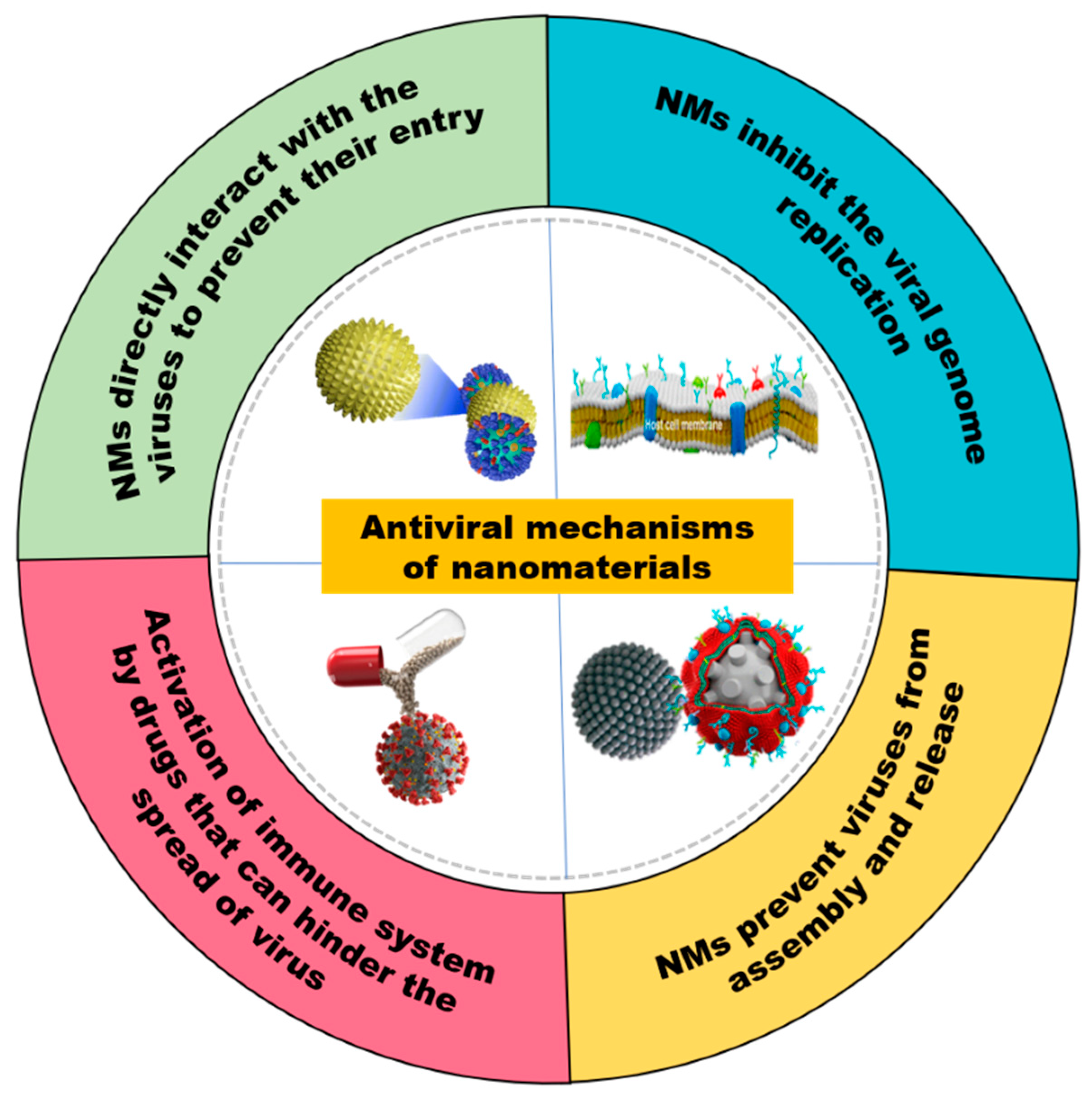
4.2. Mode of Action of Nanomaterials for Antiviral Activity
4.2.1. NMs Directly Interact with the Viruses to Prevent Their Entry
4.2.2. NMs Inhibit Viral Genome Replication
4.2.3. NMs Prevent Viruses Assembly and Release
4.2.4. Activation of Immune System by Drugs That Can Hinder the Spread of Viruses
4.3. Mode of Action of NMs for Antifungal Activity
4.4. Mode of Action of NMs for Antiparasitic Activity
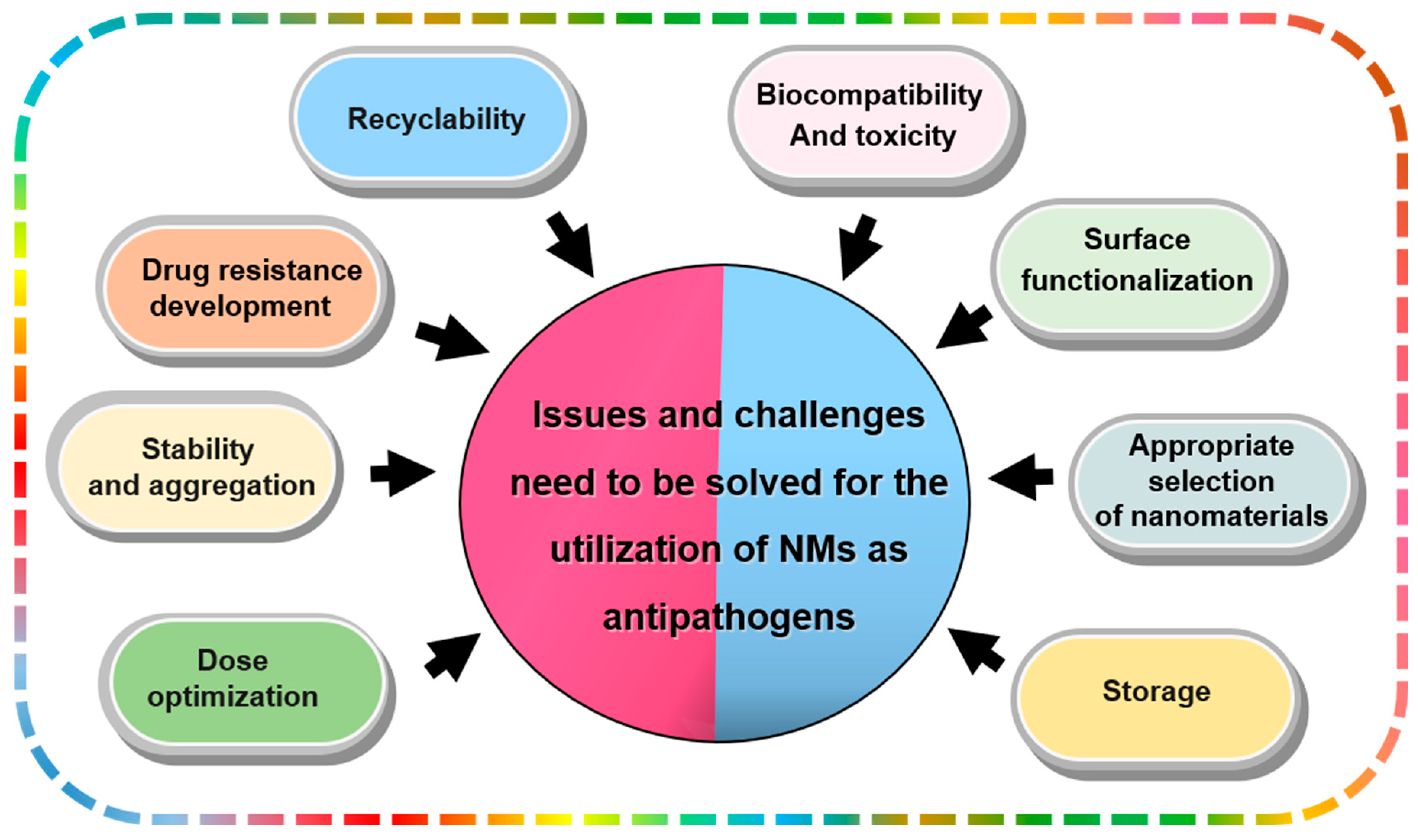
5. Issues and Challenges Need to Be Solved for the Utilizations of NMs as Antipathogens
5.1. Biocompatibility and Toxicity
5.2. Appropriate Selection of Nanomaterials
5.3. Surface Functionalization
5.4. Storage
5.5. Dose Optimization
5.6. Stability and Aggregation
5.7. Drug resistance Development
5.8. Recyclability
6. Prospects
7. Conclusions
Funding
Conflicts of Interest
References
- Chen, Z.K.; Lin, S.; Wu, Y.X.; Zhao, Z.M.; Zhou, X.M.; Sadiq, S.; Zhang, Z.D.; Guo, X.J.; Wu, P. Hsp90 could promote BmNPV proliferation by interacting with Actin-4 and enhance its expression. Dev. Comp. Immunol. 2023, 142, 104667. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Kang, K.; Khan, A.; Jiyuan, G.; Khan, S.; Khan, S.; Basir, A.; Sadiq, S. Efficient CO2 conversion and organic pollutants degradation over Sm3+ doped and rutile TiO2 nanorods decorated-GdFeO3 nanorods. Int. J. Hydrogen Energy 2023, 48, 32756–32770. [Google Scholar] [CrossRef]
- Khan, I.; Yuan, A.; Khan, S.; Khan, A.; Khan, S.; Shah, S.; Luo, M.S.; Yaseen, W.; Shen, X.; Yaseen, M. Graphitic Carbon Nitride Composites with Gold and ZIF-67 Nanoparticles as Visible-Light-Promoted Catalysts for CO2 Conversion and Bisphenol A Degradation. ACS Appl. Nano Mater. 2022, 5, 13404–13416. [Google Scholar] [CrossRef]
- Zaman, S.; Khan, I.; Zhang, F.-M.; Khan, S.; Khan, A.; Khan, S.; Sadiq, S.; Rafiq, M.; Saghir, S.; Sun, X.-J. Synthesis of mediator free hollow BiFeO3 spheres/porous g-C3N4 Z-scheme photocatalysts for CO2 conversion and Alizarin Red S degradation. Mater. Sci. Semicond. Process. 2023, 162, 107534. [Google Scholar] [CrossRef]
- Ullah, S.; Khalid, R.; Rehman, M.F.; Irfan, M.I.; Abbas, A.; Alhoshani, A.; Anwar, F.; Amin, H.M.A. Biosynthesis of phyto-functionalized silver nanoparticles using olive fruit extract and evaluation of their antibacterial and antioxidant properties. Front. Chem. 2023, 11, 1202252. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.L.; Rausalu, K.; Savest, N.; Žusinaite, E.; Vasiliev, G.; Viirsalu, M.; Plamus, T.; Krumme, A.; Merits, A.; Bondarenko, O. Antibacterial and Antiviral Effects of Ag, Cu and Zn Metals, Respective Nanoparticles and Filter Materials Thereof against Coronavirus SARS-CoV-2 and Influenza A Virus. Pharmaceutics 2022, 14, 2549. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Khan, S.; Qi, K.; Khan, I.; Wang, A.; Liu, J.; Humayun, M.; Khan, A.; Bahadur, A.; Alanazi, A.F.; Bououdina, M. Eco-friendly graphitic carbon nitride nanomaterials for the development of innovative biomaterials: Preparation, properties, opportunities, current trends, and future outlook. J. Saudi Chem. Soc. 2023, 27, 101753. [Google Scholar] [CrossRef]
- Khan, S.S.; Ullah, I.; Ullah, S.; An, R.; Xu, H.; Nie, K.; Liu, C.; Liu, L. Recent Advances in the Surface Functionalization of Nanomaterials for Antimicrobial Applications. Materials 2021, 14, 6932. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, X.; Li, C. Challenges in cell membrane-camouflaged drug delivery systems: Development strategies and future prospects. Chin. Chem. Lett. 2021, 32, 2347–2358. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.; Jayanthi, S. Synthesis of gold nanoparticles using Platycodon grandiflorum extract and its antipathogenic activity under optimal conditions. Nanomater. Nanotechnol. 2020, 10, 1847980420961697. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Verma, M.; Karandikar, P.; Furin, J.; Langer, R.; Traverso, G. Nanotechnology approaches for global infectious diseases. Nat. Nanotechnol. 2021, 16, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, S.; Salar, U.; Khan, K.; Rehman, G.; Gul, N.; Khan, I. Biology-Oriented Drug Synthesis (BIODS), Structural Characterization and Bioactivities of Novel Albendazole Derivatives. Lett. Drug Des. Discov. 2019, 16, 1329–1338. [Google Scholar] [CrossRef]
- Jabbar, A.; Abbas, A.; Assad, N.; Naeem-ul-Hassan, M.; Alhazmi, H.A.; Najmi, A.; Zoghebi, K.; Al Bratty, M.; Hanbashi, A.; Amin, H.M.A. A highly selective Hg2+ colorimetric sensor and antimicrobial agent based on green synthesized silver nanoparticles using Equisetum diffusum extract. RSC Adv. 2023, 13, 28666–28675. [Google Scholar] [CrossRef] [PubMed]
- Asghar, H.; Khan, I.; Saeed, M.; Wu, P.; Khan, A. Synthesis of g-C3N4/SmFeO3 nanosheets Z-scheme based nanocomposites as efficient visible light photocatalysts for CO2 reduction and Congo red degradation. J. Mater. Res. 2023, 38, 2986–2997. [Google Scholar] [CrossRef]
- Khan, I.; Luo, M.s.; Khan, S.; Asghar, H.; Saeed, M.; Khan, S.; Khan, A.; Humayun, M.; Guo, L.; Shi, B. Green synthesis of SrO bridged LaFeO3/g-C3N4 nanocomposites for CO2 conversion and bisphenol A degradation with new insights into mechanism. Environ. Res. 2022, 207, 112650. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Adeel, M.; Khan, I.; Akram, N.; Muneer, M. Synthesis of p-n CoO-ZnO Heterojunction for Enhanced Visible-Light Assisted Photodegradation of Methylene Blue. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Fang, F.; Li, M.; Zhang, J.; Lee, C.-S. Different Strategies for Organic Nanoparticle Preparation in Biomedicine. ACS Mater. Lett. 2020, 2, 531–549. [Google Scholar] [CrossRef]
- Khan, I.; Wang, C.; Khan, S.; Chen, J.; Khan, A.; Shah, S.; Yuan, A.; Khan, S.; Butt, M.; Asghar, H. Bio-capped and green synthesis of ZnO/g-C3N4 nanocomposites and its improved antibiotic and photocatalytic activities: An exceptional approach towards environmental remediation. Chin. J. Chem. Eng. 2022, 56, 215–224. [Google Scholar] [CrossRef]
- Shah, S.; Khan, I.; Yuan, A. MoS2 asv a Co-Catalyst for Photocatalytic Hydrogen Production: A Mini Review. Molecules 2022, 27, 3289. [Google Scholar] [CrossRef]
- Ghosh, S. Chapter 12—Promising inorganic nanomaterials for future generation. In Applications of Multifunctional Nanomaterials; Thomas, S., Kalarikkal, N., Abraham, A.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 247–263. [Google Scholar] [CrossRef]
- Song, J.; Zhao, K.; Yin, X.; Liu, Y.; Khan, I.; Liu, S.-Y. Photocatalytic degradation of tetracycline hydrochloride with g-C3N4/Ag/AgBr composites. Front. Chem. 2022, 10, 1069816. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, M.; Guo, H.; Rahman, M.S.; Wang, D.; Pfeil, C.J.; Hager, S.; Tian, L. Moldable and transferrable conductive nanocomposites for epidermal electronics. npj Flex. Electron. 2022, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, Structure, Properties and New Application Opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Khan, I.; Luo, M.S.; Lin, G.; Khan, S.; Shah, S.; Khan, I.; Khan, A.; Chunjuan, W.; Ai, B.; Zaman, S. Synthesis of phosphate-bridged g-C3N4/LaFeO3 nanosheets Z-scheme nanocomposites as efficient visible photocatalysts for CO2 reduction and malachite green degradation. Appl. Catal. A Gen. 2021, 629, 118418. [Google Scholar] [CrossRef]
- Qi, K.; Lv, W.; Khan, I.; Liu, S.-Y. Photocatalytic H2 generation via CoP quantum-dot-modified g-C3N4 synthesized by electroless plating. Chin. J. Catal. 2020, 41, 114–121. [Google Scholar] [CrossRef]
- Zhao, K.; Khan, I.; Qi, K.; Liu, Y.; Khataee, A. Ionic liquid assisted preparation of phosphorus-doped g-C3N4 photocatalyst for decomposition of emerging water pollutants. Mater. Chem. Phys. 2020, 253, 123322. [Google Scholar] [CrossRef]
- Khan, S.; Wan, C.; Chen, J.; Khan, I.; Luo, M.S.; Wang, C. Eriobotrya japonica assisted green synthesis of g-C3N4 nanocomposites and its exceptional photoactivities for doxycycline and rhodamine B degradation with mechanism insight. J. Chin. Chem. Soc. 2021, 68, 2093–2102. [Google Scholar] [CrossRef]
- Yaseen, W.; Meng, S.; Li, W.; Xie, M.; Rafiq, M.; Yusuf, B.; Shah, S.; Khan, I.; Xie, J.; Xu, Y. Facile synthesis of CoMoO4/CoMoB/boron-doped carbon nanocomposite as a highly durable bifunctional electrocatalyst for overall water splitting. Int. J. Hydrogen Energy, 2023, in press. [CrossRef]
- Khan, I.; Sun, N.; Wang, Y.; Zhijun, L.; Qu, Y.; Jing, L. Synthesis of SnO2/yolk-shell LaFeO3 nanocomposites as efficient visible-light photocatalysts for 2,4-dichlorophenol degradation. Mater. Res. Bull. 2020, 127, 110857. [Google Scholar] [CrossRef]
- Saeed, M.; Albalawi, K.; Khan, I.; Akram, N.; Abd El-Rahim, I.; Al-hag, S. Synthesis of p-n NiO-ZnO heterojunction for photodegradation of crystal violet dye. Alex. Eng. J. 2022, 65, 561–574. [Google Scholar] [CrossRef]
- Hayat, A.; Rahman, M.; Khan, I.; Khan, J.; Sohail, M.; Yasmeen, H.; Liu, S.; Qi, K.; Lv, W. Conjugated electron donor-acceptor hybrid polymeric carbon nitride as a photocatalyst for CO2 reduction. Molecules 2019, 24, 1779. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Saeed, M.; Khan, I.; Muneer, M.; Akram, N. Synthesis and Characterization of Co–ZnO and Evaluation of Its Photocatalytic Activity for Photodegradation of Methyl Orange. ACS Omega 2021, 6, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cui, Y.; Li, X.; Khan, I.; Liu, X.; Xu, Y.; Song, Y.; Xie, H. Single-atom Pt anchored thiophene ring doped carbon nitride nanosheets for enhanced visible-light photocatalytic H2 evolution and ciprofloxacin degradation. Int. J. Hydrogen Energy, 2023, in press. [CrossRef]
- Geetha, K.; Chellapandian, M.; Arulnathan, N.; Ramanathan, A. Nano zinc oxide—An alternate zinc supplement for livestock. Vet. World 2020, 13, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Ai, B.; Luo, M.s.; Khan, I. Effective Malachite Green Degradation over the Noble Metal-Doped and MOF-Coupled CsSnBr3 Nanocomposite Catalyst. Processes 2023, 11, 1398. [Google Scholar] [CrossRef]
- Taha, T.B.; Barzinjy, A.A.; Hussain, F.H.S.; Nurtayeva, T. Nanotechnology and Computer Science: Trends and advances. Mem.—Mater. Devices Circuits Syst. 2022, 2, 100011. [Google Scholar] [CrossRef]
- Kang, K.; Hu, Y.; Khan, I.; He, S.; Fetahi, P. Recent advances in the synthesis and application of magnetic biochar for wastewater treatment. Bioresour. Technol. 2023, 390, 129786. [Google Scholar] [CrossRef]
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456. [Google Scholar] [CrossRef]
- Karnwal, A.; Kumar, G.; Pant, G.; Hossain, K.; Ahmad, A.; Alshammari, M.B. Perspectives on Usage of Functional Nanomaterials in Antimicrobial Therapy for Antibiotic-Resistant Bacterial Infections. ACS Omega 2023, 8, 13492–13508. [Google Scholar] [CrossRef]
- Liu, Y.; Busscher, H.J.; Zhao, B.; Li, Y.; Zhang, Z.; van der Mei, H.C.; Ren, Y.; Shi, L. Surface-Adaptive, Antimicrobially Loaded, Micellar Nanocarriers with Enhanced Penetration and Killing Efficiency in Staphylococcal Biofilms. ACS Nano 2016, 10, 4779–4789. [Google Scholar] [CrossRef]
- Hoque, J.; Adhikary, U.; Yadav, V.; Samaddar, S.; Konai, M.M.; Prakash, R.G.; Paramanandham, K.; Shome, B.R.; Sanyal, K.; Haldar, J. Chitosan Derivatives Active against Multidrug-Resistant Bacteria and Pathogenic Fungi: In Vivo Evaluation as Topical Antimicrobials. Mol. Pharm. 2016, 13, 3578–3589. [Google Scholar] [CrossRef]
- Ramachandraiah, K.; Hong, G.-P. Polymer Based Nanomaterials for Strategic Applications in Animal Food Value Chains. Food Rev. Int. 2022, 38, 1577–1606. [Google Scholar] [CrossRef]
- Singh, H. Nanotechnology Applications in Functional Foods; Opportunities and Challenges. Prev. Nutr. Food Sci. 2016, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Watarai, S.; Iwasaki, T.; Kodama, H. Suppression of Salmonella enterica serovar Enteritidis excretion by intraocular vaccination with fimbriae proteins incorporated in liposomes. Dev. Comp. Immunol. 2004, 28, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan, S.E.-D.; Eid, A.M.; Abdel-Rahman, M.A.; Hamza, M.F. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, Penicillium crustosum EP-1. Sci. Rep. 2022, 12, 11834. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Z.; Yang, D.; Zhu, L.; Liang, Z.; Pang, Y.; Zhou, L. Novel Microbial Palladium Nanoparticles with a High Photothermal Effect for Antibacterial Applications. ACS Omega 2023, 8, 1534–1541. [Google Scholar] [CrossRef]
- Mohd Yusof, H.; Abdul Rahman, N.A.; Mohamad, R.; Hasanah Zaidan, U.; Samsudin, A.A. Antibacterial Potential of Biosynthesized Zinc Oxide Nanoparticles against Poultry-Associated Foodborne Pathogens: An In Vitro Study. Animals 2021, 11, 2093. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.; Sayed El-Ahl, R.M.H.; Oraby, N.H.; El-Hamaky, A.M.A.; Mansour, M.K. Chapter 25—Zinc nanomaterials: Toxicological effects and veterinary applications. In Zinc-Based Nanostructures for Environmental and Agricultural Applications; Abd-Elsalam, K.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 509–541. [Google Scholar] [CrossRef]
- Tsakmakidis, I.; Samaras, T.; Anastasiadou, S.; Basioura, A.; Ntemka, A.; Michos, I.A.; Simeonidis, K.; Karagiannis, I.; Tsousis, G.; Angelakeris, M.; et al. Toxic and Microbiological Effects of Iron Oxide and Silver Nanoparticles as Additives on Extended Ram Semen. Animals 2021, 11, 1011. [Google Scholar] [CrossRef]
- Kot, M.; Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Smulski, S.; Gołębiewski, M. In Vitro Studies of Nanoparticles as a Potentially New Antimicrobial Agent for the Prevention and Treatment of Lameness and Digital Dermatitis in Cattle. Int. J. Mol. Sci. 2023, 24, 6146. [Google Scholar] [CrossRef]
- Manhas, P.K.; Quintela, I.A.; Wu, V.C.H. Enhanced Detection of Major Pathogens and Toxins in Poultry and Livestock With Zoonotic Risks Using Nanomaterials-Based Diagnostics. Front. Vet. Sci. 2021, 8, 673718. [Google Scholar] [CrossRef]
- Hill, E.K.; Li, J. Current and future prospects for nanotechnology in animal production. J. Anim. Sci. Biotechnol. 2017, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- McCollum, C.R.; Levy, M.; Bertram, J.R.; Nagpal, P.; Chatterjee, A. Photoexcited Quantum Dots as Efficacious and Nontoxic Antibiotics in an Animal Model. ACS Biomater. Sci. Eng. 2021, 7, 1863–1875. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, S.; Pinnaka, A.K.; Soni, S.; Singhal, N.K. Antibody assisted graphene oxide coated gold nanoparticles for rapid bacterial detection and near infrared light enhanced antibacterial activity. Sens. Actuators B Chem. 2021, 329, 129141. [Google Scholar] [CrossRef]
- Ahghari, M.A.; Ahghari, M.R.; Kamalzare, M.; Maleki, A. Design, synthesis, and characterization of novel eco-friendly chitosan-AgIO3 bionanocomposite and study its antibacterial activity. Sci. Rep. 2022, 12, 10491. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Li, C.; Liu, R.; Lin, L. Fabrication of chitosan nanofibers containing tea tree oil liposomes against Salmonella spp. in chicken. LWT 2018, 96, 671–678. [Google Scholar] [CrossRef]
- Amjadi, S.; Nazari, M.; Alizadeh, S.A.; Hamishehkar, H. Multifunctional betanin nanoliposomes-incorporated gelatin/chitosan nanofiber/ZnO nanoparticles nanocomposite film for fresh beef preservation. Meat Sci. 2020, 167, 108161. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, I.; Yadavalli, T.; Wu, D.; Shukla, D. Plasma Membrane-Derived Liposomes Exhibit Robust Antiviral Activity against HSV-1. Viruses 2022, 14, 799. [Google Scholar] [CrossRef]
- Kandeel, M.; Al-Taher, A.; Park, B.K.; Kwon, H.J.; Al-Nazawi, M. A pilot study of the antiviral activity of anionic and cationic polyamidoamine dendrimers against the Middle East respiratory syndrome coronavirus. J. Med. Virol. 2020, 92, 1665–1670. [Google Scholar] [CrossRef]
- Bavananthasivam, J.; Alkie, T.N.; Astill, J.; Abdul-Careem, M.F.; Wootton, S.K.; Behboudi, S.; Yitbarek, A.; Sharif, S. In ovo administration of Toll-like receptor ligands encapsulated in PLGA nanoparticles impede tumor development in chickens infected with Marek’s disease virus. Vaccine 2018, 36, 4070–4076. [Google Scholar] [CrossRef]
- Dhakal, S.; Goodman, J.; Bondra, K.; Lakshmanappa, Y.S.; Hiremath, J.; Shyu, D.L.; Ouyang, K.; Kang, K.I.; Krakowka, S.; Wannemuehler, M.J.; et al. Polyanhydride nanovaccine against swine influenza virus in pigs. Vaccine 2017, 35, 1124–1131. [Google Scholar] [CrossRef]
- Innocenzi, P.; Stagi, L. Carbon-based antiviral nanomaterials: Graphene, C-dots, and fullerenes. A perspective. Chem. Sci. 2020, 11, 6606–6622. [Google Scholar] [CrossRef]
- Huy, T.Q.; Hien Thanh, N.T.; Thuy, N.T.; Chung, P.V.; Hung, P.N.; Le, A.-T.; Hong Hanh, N.T. Cytotoxicity and antiviral activity of electrochemical—Synthesized silver nanoparticles against poliovirus. J. Virol. Methods 2017, 241, 52–57. [Google Scholar] [CrossRef]
- Talebian, S.; Wallace, G.G.; Schroeder, A.; Stellacci, F.; Conde, J. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat. Nanotechnol. 2020, 15, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Thi Ngoc Dung, T.; Nang Nam, V.; Thi Nhan, T.; Ngoc, T.T.B.; Minh, L.Q.; Nga, B.T.T.; Phan Le, V.; Viet Quang, D. Silver nanoparticles as potential antiviral agents against African swine fever virus. Mater. Res. Express 2019, 6, 1250g9. [Google Scholar] [CrossRef]
- Zeedan, G.; El-Razik, K.; Allam, A.; Abdalhamed, A.; Abou-Zeina, H. Evaluations of Potential Antiviral Effects of Green Zinc Oxide and Silver Nanoparticles against Bovine Herpesvirus-1. Adv. Anim. Vet. Sci. 2020, 8, 433–443. [Google Scholar] [CrossRef]
- Bai, M.; Dong, H.; Su, X.; Jin, Y.; Sun, S.; Zhang, Y.; Yang, Y.; Guo, H. Hollow mesoporous silica nanoparticles as delivery vehicle of foot-and-mouth disease virus-like particles induce persistent immune responses in guinea pigs. J. Med. Virol. 2019, 91, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, M.; Khairy, G.M.; Hesham, A.; Rabaan, A.A.; El-Shamy, A.G.; Nagy, A. Nanoparticles as a novel and promising antiviral platform in veterinary medicine. Arch. Virol. 2021, 166, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tong, T.; Jiang, X.; Fang, L.; Wu, Y.; Liang, J.; Xiao, S. GSH-ZnS Nanoparticles Exhibit High-Efficiency and Broad-Spectrum Antiviral Activities via Multistep Inhibition Mechanisms. ACS Appl. Bio Mater. 2020, 3, 4809–4819. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, X.; Tong, T.; Fang, L.; Wu, Y.; Liang, J.; Xiao, S. High antiviral activity of mercaptoethane sulfonate functionalized Te/BSA nanostars against arterivirus and coronavirus. RSC Adv. 2020, 10, 14161–14169. [Google Scholar] [CrossRef]
- Du, P.; Liu, R.; Sun, S.; Dong, H.; Zhao, R.; Tang, R.; Dai, J.; Yin, H.; Luo, J.; Liu, Z.; et al. Biomineralization improves the thermostability of foot-and-mouth disease virus-like particles and the protective immune response induced. Nanoscale 2019, 11, 22748–22761. [Google Scholar] [CrossRef]
- Leal, A.F.; Leite, M.C.; Medeiros, C.S.; Cavalcanti, I.M.; Wanderley, A.G.; Magalhães, N.S.; Neves, R.P. Antifungal activity of a liposomal itraconazole formulation in experimental Aspergillus flavus keratitis with endophthalmitis. Mycopathologia 2015, 179, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.A.; LaMothe, R.A.; Ferrari, J.D.; Zhang, A.H.; Rossi, R.J.; Kolte, P.N.; Griset, A.P.; O’Neil, C.; Altreuter, D.H.; Browning, E.; et al. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc. Natl. Acad. Sci. USA 2015, 112, E156–E165. [Google Scholar] [CrossRef] [PubMed]
- Siopi, M.; Mouton, J.W.; Pournaras, S.; Meletiadis, J. In Vitro and In Vivo Exposure-Effect Relationship of Liposomal Amphotericin B against Aspergillus fumigatus. Antimicrob. Agents Chemother. 2019, 63, e02673-18. [Google Scholar] [CrossRef]
- Gignone, A.; Delle Piane, M.; Corno, M.; Ugliengo, P.; Onida, B. Simulation and Experiment Reveal a Complex Scenario for the Adsorption of an Antifungal Drug in Ordered Mesoporous Silica. J. Phys. Chem. C 2015, 119, 13068–13079. [Google Scholar] [CrossRef]
- El-Tawab, A.A.A.; El-Hofy, F.I.; Metwally, A. Comparative study on antifungal activity of Fe2O3, and Fe3O4 nanoparticles. Int. J. Adv. Res. 2018, 6, 189–194. [Google Scholar] [CrossRef]
- Nabawy, G.A.; Hassan, A.A.; El-Ahl, R.H.S.; Refai, M.K. Effect of metal nanoparticles in comparison with commercial antifungal feed additives on the growth of aspergillus flavus and aflatoxin b1 production. J. Glob. Biosci. 2014, 3, 954–971. [Google Scholar]
- Reda, A.E.; Fayed, B. The synthesis of calcium doped zinc oxide ceramic nanoparticles via sol–gel effective against the emerging multidrug-resistant Candida auris. J. Aust. Ceram. Soc. 2023. [Google Scholar] [CrossRef]
- Hamad, K.M.; Mahmoud, N.N.; Al-Dabash, S.; Al-Samad, L.A.; Abdallah, M.; Al-Bakri, A.G. Fluconazole conjugated-gold nanorods as an antifungal nanomedicine with low cytotoxicity against human dermal fibroblasts. RSC Adv. 2020, 10, 25889–25897. [Google Scholar] [CrossRef]
- Hernandez, R.; Jiménez Chávez, J.; De Vizcaya-Ruiz, A.; Lozano-Alvarez, J.; Escalante, K.; Medina Ramirez, I. Synthesis of TiO2-Cu2+/CuI Nanocomposites and Evaluation of Antifungal and Cytotoxic Activity. Nanomaterials 2023, 13, 1900. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; de Souza Neto, F.N.; Lima, B.H.R.; de Camargo, E.R.; Ramage, G.; Delbem, A.C.B.; Monteiro, D.R. Novel nanocarrier of miconazole based on chitosan-coated iron oxide nanoparticles as a nanotherapy to fight Candida biofilms. Colloids Surf. B Biointerfaces 2020, 192, 111080. [Google Scholar] [CrossRef]
- Kalińska, A.; Jaworski, S.; Wierzbicki, M.; Gołębiewski, M. Silver and Copper Nanoparticles—An Alternative in Future Mastitis Treatment and Prevention? Int. J. Mol. Sci. 2019, 20, 1672. [Google Scholar] [CrossRef] [PubMed]
- Moles, E.; Urbán, P.; Jiménez-Díaz, M.B.; Viera-Morilla, S.; Angulo-Barturen, I.; Busquets, M.A.; Fernàndez-Busquets, X. Immunoliposome-mediated drug delivery to Plasmodium-infected and non-infected red blood cells as a dual therapeutic/prophylactic antimalarial strategy. J. Control. Release 2015, 210, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Moles, E.; Galiano, S.; Gomes, A.; Quiliano, M.; Teixeira, C.; Aldana, I.; Gomes, P.; Fernàndez-Busquets, X. ImmunoPEGliposomes for the targeted delivery of novel lipophilic drugs to red blood cells in a falciparum malaria murine model. Biomaterials 2017, 145, 178–191. [Google Scholar] [CrossRef]
- Hiszczyńska-Sawicka, E.; Li, H.; Xu, J.; Akhtar, M.; Holec-Gąsior, L.; Kur, J.; Bickerstaffe, R.; Stankiewicz, M. Induction of immune responses in sheep by vaccination with liposome-entrapped DNA complexes encoding Toxoplasma gondii MIC3 gene. Pol. J. Vet. Sci. 2012, 15, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, G.; Gadora, K.; Cheng, H.; Peng, J.; Ma, Y.; Guo, Y.; Chi, C.; Zhou, J.; Ding, Y. Dual-sensitive chitosan derivative micelles for site-specific drug release in the treatment of chicken coccidiosis. RSC Adv. 2018, 8, 14515–14526. [Google Scholar] [CrossRef]
- Smith, L.; Serrano, D.R.; Mauger, M.; Bolás-Fernández, F.; Dea-Ayuela, M.A.; Lalatsa, A. Orally Bioavailable and Effective Buparvaquone Lipid-Based Nanomedicines for Visceral Leishmaniasis. Mol. Pharm. 2018, 15, 2570–2583. [Google Scholar] [CrossRef]
- Kojom Foko, L.P.; Hawadak, J.; Verma, V.; Belle Ebanda Kedi, P.; Eboumbou Moukoko, C.E.; Kamaraju, R.; Pande, V.; Singh, V. Phytofabrication and characterization of Alchornea cordifolia silver nanoparticles and evaluation of antiplasmodial, hemocompatibility and larvicidal potential. Front. Bioeng. Biotechnol. 2023, 11, 1109841. [Google Scholar] [CrossRef]
- Prasanna, P.; Kumar, P.; Kumar, S.; Rajana, V.K.; Kant, V.; Prasad, S.R.; Mohan, U.; Ravichandiran, V.; Mandal, D. Current status of nanoscale drug delivery and the future of nano-vaccine development for leishmaniasis—A review. Biomed. Pharmacother. 2021, 141, 111920. [Google Scholar] [CrossRef]
- Neves Borgheti-Cardoso, L.; San Anselmo, M.; Lantero, E.; Lancelot, A.; Serrano, J.L.; Hernández-Ainsa, S.; Fernàndez-Busquets, X.; Sierra, T. Promising nanomaterials in the fight against malaria. J. Mater. Chem. B 2020, 8, 9428–9448. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Ishii, K.; Kato, K. L-tryptophan-titanium oxide nanoparticles showed selective anti-Toxoplasma gondii activity and improved host biocompatibility. Biomed. Pharmacother. 2023, 162, 114597. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Murata, Y.; Sugi, T.; Kato, K. Inorganic nanoparticles kill Toxoplasma gondii via changes in redox status and mitochondrial membrane potential. Int. J. Nanomed. 2017, 12, 1647–1661. [Google Scholar] [CrossRef]
- Jahani, Z.; Meshgi, B.; Rajabi-Bzl, M.; Jalousian, F.; Hasheminasab, S. Improved serodiagnosis of hydatid cyst disease using gold nanoparticle labeled antigen B in naturally infected sheep. Iran. J. Parasitol. 2014, 9, 218–225. [Google Scholar]
- Tomar, R.S.; Preet, S. Evaluation of anthelmintic activity of biologically synthesized silver nanoparticles against the gastrointestinal nematode, Haemonchus contortus. J. Helminthol. 2017, 91, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Aydin, A.; Göz, Y.; Yüksek, N.; Ayaz, E. Prevalence of Toxocara vitulorum in Hakkari eastern region of Turkey. Bull. Vet. Inst. Pulawy 2006, 50, 51–54. [Google Scholar]
- Yu, Z.; Cao, W.; Gao, X.; Aleem, M.T.; Liu, J.; Luo, J.; Yan, R.; Xu, L.; Song, X.; Li, X. With Chitosan and PLGA as the Delivery Vehicle, Toxoplasma gondii Oxidoreductase-Based DNA Vaccines Decrease Parasite Burdens in Mice. Front. Immunol. 2021, 12, 726615. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, A.A.L.; Arenas Velásquez, A.M.; Patiño Linares, I.A.; de Almeida, L.; Fontana, C.R.; Garcia, C.; Graminha, M.A.S. Efficacy of photodynamic therapy using TiO2 nanoparticles doped with Zn and hypericin in the treatment of cutaneous Leishmaniasis caused by Leishmania amazonensis. Photodiagnosis Photodyn. Ther. 2020, 30, 101676. [Google Scholar] [CrossRef]
- Elfeky, A.S.; Salem, S.S.; Elzaref, A.S.; Owda, M.E.; Eladawy, H.A.; Saeed, A.M.; Awad, M.A.; Abou-Zeid, R.E.; Fouda, A. Multifunctional cellulose nanocrystal /metal oxide hybrid, photo-degradation, antibacterial and larvicidal activities. Carbohydr. Polym. 2020, 230, 115711. [Google Scholar] [CrossRef]
- Shehu, Z.; Abba, E.; Danbature, W.; Yoriyo, K.; Abubakar, Z.; Kenneth, Z.; Usiju, Z.; Abubakar, A. Bio-Fabrication of ZnO-CuO Nanoporous Composite and Its Application as Nanolarvicidal Agent for Malaria Vectors. J. Pharm. Res. Int. 2020, 32, 31–39. [Google Scholar] [CrossRef]
- Jiang, Y.; Zheng, W.; Tran, K.; Kamilar, E.; Bariwal, J.; Ma, H.; Liang, H. Hydrophilic nanoparticles that kill bacteria while sparing mammalian cells reveal the antibiotic role of nanostructures. Nat. Commun. 2022, 13, 197. [Google Scholar] [CrossRef]
- Da Costa, D.; Exbrayat-Héritier, C.; Rambaud, B.; Megy, S.; Terreux, R.; Verrier, B.; Primard, C. Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J. Nanobiotechnol. 2021, 19, 12. [Google Scholar] [CrossRef]
- Wu, J.; Guan, R.; Cao, G.; Liu, Z.; Wang, Z.; Shen, H.; Xia, Q. Antioxidant and Antimicrobial Effects of Catechin Liposomes on Chinese Dried Pork. J. Food Prot. 2018, 81, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Ghaffar, S.; Abbas, A.; Naeem-ul-Hassan, M.; Assad, N.; Sher, M.; Ullah, S.; Alhazmi, H.A.; Najmi, A.; Zoghebi, K.; Al Bratty, M.; et al. Improved Photocatalytic and Antioxidant Activity of Olive Fruit Extract-Mediated ZnO Nanoparticles. Antioxidants 2023, 12, 1201. [Google Scholar] [CrossRef] [PubMed]
- Groß, S.; Panter, F.; Pogorevc, D.; Seyfert, C.E.; Deckarm, S.; Bader, C.D.; Herrmann, J.; Müller, R. Improved broad-spectrum antibiotics against Gram-negative pathogens via darobactin biosynthetic pathway engineering. Chem. Sci. 2021, 12, 11882–11893. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Yang, Y.; Zheng, W.; Huang, Y.; Xu, F.; Bao, Z. Synergistic photothermal antibacterial therapy enabled by multifunctional nanomaterials: Progress and perspectives. Mater. Chem. Front. 2023, 7, 355–380. [Google Scholar] [CrossRef]
- Adegbeye, M.; Elghandour, M.; Barbabosa-Pliego, A.; Monroy, J.; Mellado, M.; Reddy, P.R.K.; Salem, A.Z. Nanoparticles in Equine Nutrition: Mechanism of Action and Application as Feed Additives. J. Equine Vet. Sci. 2019, 78, 29–37. [Google Scholar] [CrossRef]
- Michalak, I.; Dziergowska, K.; Alagawany, M.; Farag, M.R.; El-Shall, N.A.; Tuli, H.S.; Emran, T.B.; Dhama, K. The effect of metal-containing nanoparticles on the health, performance and production of livestock animals and poultry. Vet. Q. 2022, 42, 68–94. [Google Scholar] [CrossRef]
- Ding, R.; Chen, Y.; Wang, Q.; Wu, Z.; Zhang, X.; Li, B.; Lin, L. Recent advances in quantum dots-based biosensors for antibiotics detection. J. Pharm. Anal. 2022, 12, 355–364. [Google Scholar] [CrossRef]
- Mukherjee, A.; Bose, S.; Shaoo, A.; Das, S.K. Nanotechnology based therapeutic approaches: An advanced strategy to target the biofilm of ESKAPE pathogens. Mater. Adv. 2023, 4, 2544–2572. [Google Scholar] [CrossRef]
- Mobed, A.; Hasanzadeh, M.; Seidi, F. Anti-bacterial activity of gold nanocomposites as a new nanomaterial weapon to combat photogenic agents: Recent advances and challenges. RSC Adv. 2021, 11, 34688–34698. [Google Scholar] [CrossRef]
- Asem, H.; Zheng, W.; Nilsson, F.; Zhang, Y.; Hedenqvist, M.S.; Hassan, M.; Malmström, E. Functional Nanocarriers for Drug Delivery by Surface Engineering of Polymeric Nanoparticle Post-Polymerization-Induced Self-Assembly. ACS Appl. Bio Mater. 2021, 4, 1045–1056. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, D.; Sui, G.; Wang, D.; Wu, M.; Han, L.; Mu, H.; Duan, J. Gentamicin decorated phosphatidylcholine-chitosan nanoparticles against biofilms and intracellular bacteria. Int. J. Biol. Macromol. 2020, 156, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Yuan, L.; Lin, L. Novel chitosan film embedded with liposome-encapsulated phage for biocontrol of Escherichia coli O157:H7 in beef. Carbohydr. Polym. 2017, 177, 156–164. [Google Scholar] [CrossRef]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- Huang, L.; Teng, W.; Cao, J.; Wang, J. Liposomes as Delivery System for Applications in Meat Products. Foods 2022, 11, 3017. [Google Scholar] [CrossRef] [PubMed]
- Patoo, T.S.; Khanday, F.; Qurashi, A. Prospectus of advanced nanomaterials for antiviral properties. Mater. Adv. 2022, 3, 2960–2970. [Google Scholar] [CrossRef]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef]
- Ullah, A.; Qazi, J.; Rahman, L.; Kanaras, A.G.; Khan, W.S.; Hussain, I.; Rehman, A. Nanoparticles-assisted delivery of antiviral-siRNA as inhalable treatment for human respiratory viruses: A candidate approach against SARS-CoV-2. Nano Sel. 2020, 1, 612–621. [Google Scholar] [CrossRef]
- Akbar, H.; Fasick, J.J.; Ponnuraj, N.; Jarosinski, K.W. Purinergic signaling during Marek’s disease in chickens. Sci. Rep. 2023, 13, 2044. [Google Scholar] [CrossRef]
- Singh, S.M.; Alkie, T.N.; Abdelaziz, K.T.; Hodgins, D.C.; Novy, A.; Nagy, É.; Sharif, S. Characterization of Immune Responses to an Inactivated Avian Influenza Virus Vaccine Adjuvanted with Nanoparticles Containing CpG ODN. Viral Immunol. 2016, 29, 269–275. [Google Scholar] [CrossRef]
- Dhakal, S.; Renu, S.; Ghimire, S.; Shaan Lakshmanappa, Y.; Hogshead, B.T.; Feliciano-Ruiz, N.; Lu, F.; HogenEsch, H.; Krakowka, S.; Lee, C.W.; et al. Mucosal Immunity and Protective Efficacy of Intranasal Inactivated Influenza Vaccine Is Improved by Chitosan Nanoparticle Delivery in Pigs. Front. Immunol. 2018, 9, 934. [Google Scholar] [CrossRef]
- Huang, J.; Liu, H.; Wang, M.; Bai, X.; Cao, J.; Zhang, Z.; Wang, Q. Mannosylated gelatin nanoparticles enhanced inactivated PRRSV targeting dendritic cells and increased T cell immunity. Vet. Immunol. Immunopathol. 2021, 235, 110237. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Yoon, S.W.; Jung, W.N.; Chung, M.H.; Kim, H.; Jeong, H.; Yoo, K.H. Photothermal inactivation of universal viral particles by localized surface plasmon resonance mediated heating filter membrane. Sci. Rep. 2022, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.C.F.; Poletto, F.; Eberhardt, M.J.; Domingues, S.C.; De Sousa, F.B.; Tebaldi, M.L. Polymer-hybrid nanosystems for antiviral applications: Current advances. Biomed. Pharmacother. 2022, 146, 112249. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, H.; Tavakoli, A.; Moradi, A.; Tabarraei, A.; Bokharaei-Salim, F.; Zahmatkeshan, M.; Farahmand, M.; Javanmard, D.; Kiani, S.J.; Esghaei, M.; et al. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: Another emerging application of nanomedicine. J. Biomed. Sci. 2019, 26, 70. [Google Scholar] [CrossRef] [PubMed]
- Hodek, J.; Zajícová, V.; Lovětinská-Šlamborová, I.; Stibor, I.; Müllerová, J.; Weber, J. Protective hybrid coating containing silver, copper and zinc cations effective against human immunodeficiency virus and other enveloped viruses. BMC Microbiol. 2016, 16 (Suppl. 1), 56. [Google Scholar] [CrossRef]
- Smith, N.; Bade, A.N.; Soni, D.; Gautam, N.; Alnouti, Y.; Herskovitz, J.; Ibrahim, I.M.; Wojtkiewicz, M.S.; Dyavar Shetty, B.L.; McMillan, J.; et al. A long acting nanoformulated lamivudine ProTide. Biomaterials 2019, 223, 119476. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Bar, H.M.; Abdallah, I.A.; Fayed, M.A.A.; Moatasim, Y.; Mostafa, A.; El-Behairy, M.F.; Elimam, H.; Elshaier, Y.; Abouzid, K.A.M. Lipid polymer hybrid nanocarriers as a combinatory platform for different anti-SARS-CoV-2 drugs supported by computational studies. RSC Adv. 2021, 11, 28876–28891. [Google Scholar] [CrossRef]
- Chen, Y.N.; Hsueh, Y.H.; Hsieh, C.T.; Tzou, D.Y.; Chang, P.L. Antiviral Activity of Graphene-Silver Nanocomposites against Non-Enveloped and Enveloped Viruses. Int. J. Environ. Res. Public Health 2016, 13, 430. [Google Scholar] [CrossRef]
- Yazdani, S.; Mozaffarian, M.; Pazuki, G.; Hadidi, N.; Gallego, I.; Puras, G.; Pedraz, J.L. Design of double functionalized carbon nanotube for amphotericin B and genetic material delivery. Sci. Rep. 2022, 12, 21114. [Google Scholar] [CrossRef]
- Helal, S.H.; Abdel-Aziz, H.M.M.; El-Zayat, M.M.; Hasaneen, M.N.A. Preparation, characterization and properties of three different nanomaterials either alone or loaded with nystatin or fluconazole antifungals. Sci. Rep. 2022, 12, 22110. [Google Scholar] [CrossRef]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef]
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; El-Matbouli, M.; Saleh, M. In vitro assessment of the antimicrobial efficacy of chitosan nanoparticles against major fish pathogens and their cytotoxicity to fish cell lines. J. Fish Dis. 2020, 43, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Fozia; Khattak, B.; Alotaibi, A.; Qasim, M.; Ahmad, I.; Ullah, R.; Bourhia, M.; Gul, A.; Zahoor, S.; et al. Green Synthesis of Copper Oxide Nanoparticles Using Aerva javanica Leaf Extract and Their Characterization and Investigation of In Vitro Antimicrobial Potential and Cytotoxic Activities. Evid.-Based Complement. Altern. Med. 2021, 2021, 5589703. [Google Scholar] [CrossRef]
- Chen, J.-N.; Wu, L.-T.; Song, K.; Zhu, Y.-S.; Ding, W. Nonphytotoxic copper oxide nanoparticles are powerful “nanoweapons” that trigger resistance in tobacco against the soil-borne fungal pathogen Phytophthora nicotianae. J. Integr. Agric. 2022, 21, 3245–3262. [Google Scholar] [CrossRef]
- Hassan, A.A.; Sayed-Elahl, R.M.; Oraby, N.H.; El-Hamaky, A.M.A. Chapter 11—Metal nanoparticles for management of mycotoxigenic fungi and mycotoxicosis diseases of animals and poultry. In Nanomycotoxicology; Rai, M., Abd-Elsalam, K.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 251–269. [Google Scholar] [CrossRef]
- Alagawany, M.; Qattan, S.Y.A.; Attia, Y.A.; El-Saadony, M.T.; Elnesr, S.S.; Mahmoud, M.A.; Madkour, M.; Abd El-Hack, M.E.; Reda, F.M. Use of Chemical Nano-Selenium as an Antibacterial and Antifungal Agent in Quail Diets and Its Effect on Growth, Carcasses, Antioxidant, Immunity and Caecal Microbes. Animals 2021, 11, 3027. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanoparticle Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Pathakumari, B.; Liang, G.; Liu, W. Immune defence to invasive fungal infections: A comprehensive review. Biomed. Pharmacother. 2020, 130, 110550. [Google Scholar] [CrossRef]
- Mohapatra, B.; Sharma, N. Molecular insights into the antifungal activity of biosynthesized Ag-ZnO hybrid nanostructures against Schizosaccharomyces pombe. Mater. Today Commun. 2023, 36, 106597. [Google Scholar] [CrossRef]
- Al Shap, N.F.; El-Sherbeny, E.M.E.; El Masry, D.M.A. The efficacy of metal nanocomposite (Fe3O4/CuO/ZnO) to ameliorate the toxic effects of ochratoxin in broilers. BMC Vet. Res. 2022, 18, 312. [Google Scholar] [CrossRef]
- Hassan, A.; Abo-Zaid, K.; Oraby, N. Molecular and Conventional Detection of Antimicrobial Activity of Zinc Oxide Nanoparticles and Cinnamon Oil against Escherichia coli and Aspergillus flavus. Adv. Anim. Vet. Sci. 2020, 8, 839–847. [Google Scholar] [CrossRef]
- Nafari, A.; Cheraghipour, K.; Sepahvand, M.; Shahrokhi, G.; Gabal, E.; Mahmoudvand, H. Nanoparticles: New agents toward treatment of leishmaniasis. Parasite Epidemiol. Control 2020, 10, e00156. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, M.; Yang, J.; Li, J. Cell Membrane-Coated Nanoparticles for Management of Infectious Diseases: A Review. Ind. Eng. Chem. Res. 2022, 61, 12867–12883. [Google Scholar] [CrossRef]
- Uwimana, A.; Legrand, E.; Stokes, B.H.; Ndikumana, J.M.; Warsame, M.; Umulisa, N.; Ngamije, D.; Munyaneza, T.; Mazarati, J.B.; Munguti, K.; et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat. Med. 2020, 26, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Singh, M.; Wasnik, K.; Pareek, D.; Gupta, P.S.; Mukherjee, S.; Paik, P. Polymeric Nanoparticle Based Diagnosis and Nanomedicine for Treatment and Development of Vaccines for Cerebral Malaria: A Review on Recent Advancement. ACS Appl. Bio Mater. 2021, 4, 7342–7365. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Lymperaki, E.; Egwu, C.O.; Pouroutzidou, G.K.; Kazeli, K.; Reybier, K.; Bourgeade-Delmas, S.; Valentin, A.; Kontonasaki, E. Effect of Silica Based Nanoparticles against Plasmodium falciparum and Leishmania infantum parasites. J. Xenobiotics 2021, 11, 155–162. [Google Scholar] [CrossRef]
- Bahaaeldine, M.A.; El Garhy, M.; Fahmy, S.R.; Mohamed, A.S. In vitro anti-Toxocara vitulorum effect of silver nanoparticles. J. Parasit. Dis. 2022, 46, 409–420. [Google Scholar] [CrossRef]
- Asthana, S.; Jaiswal, A.K.; Gupta, P.K.; Dube, A.; Chourasia, M.K. Th-1 biased immunomodulation and synergistic antileishmanial activity of stable cationic lipid–polymer hybrid nanoparticle: Biodistribution and toxicity assessment of encapsulated amphotericin B. Eur. J. Pharm. Biopharm. 2015, 89, 62–73. [Google Scholar] [CrossRef]
- Zaheer, T.; Pal, K.; Zaheer, I. Topical review on nano-vaccinology: Biochemical promises and key challenges. Process Biochem. 2021, 100, 237–244. [Google Scholar] [CrossRef]
- Yang, X.; Wu, F.; Chen, D.-Z.; Lin, H.-W. An electrochemical immunosensor for rapid determination of clenbuterol by using magnetic nanocomposites to modify screen printed carbon electrode based on competitive immunoassay mode. Sens. Actuators B Chem. 2014, 192, 529–535. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef] [PubMed]
- Strahl, H.; Errington, J. Bacterial Membranes: Structure, Domains, and Function. Annu. Rev. Microbiol. 2017, 71, 519–538. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Qin, W.; Panunzio, M.; Biondi, S. Antibacterial Agents Targeting the Bacterial Cell Wall. Curr. Med. Chem. 2020, 27, 2902–2926. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Amro, N.A.; Kotra, L.P.; Wadu-Mesthrige, K.; Bulychev, A.; Mobashery, S.; Liu, G.-Y. High-Resolution Atomic Force Microscopy Studies of the Escherichia coli Outer Membrane: Structural Basis for Permeability. Langmuir 2000, 16, 2789–2796. [Google Scholar] [CrossRef]
- Zhang, Y.; Shareena Dasari, T.P.; Deng, H.; Yu, H. Antimicrobial Activity of Gold Nanoparticles and Ionic Gold. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 286–327. [Google Scholar] [CrossRef]
- Dasari, T.P.S.; Zhang, Y.; Yu, H. Antibacterial Activity and Cytotoxicity of Gold (I) and (III) Ions and Gold Nanoparticles. Biochem. Pharmacol. Open Access 2015, 4, 199. [Google Scholar] [CrossRef]
- Gualdi, S.; Agnoli, K.; Vitale, A.; Higgins, S.; Eberl, L. Identification of genes required for gold and silver tolerance in Burkholderia cenocepacia H111 by transposon sequencing. Environ. Microbiol. 2022, 24, 737–751. [Google Scholar] [CrossRef]
- Mourenza, Á.; Gil, J.A.; Mateos, L.M.; Letek, M. Oxidative Stress-Generating Antimicrobials, a Novel Strategy to Overcome Antibacterial Resistance. Antioxidants 2020, 9, 361. [Google Scholar] [CrossRef]
- Hegemann, D.; Hanselmann, B.; Zuber, F.; Pan, F.; Gaiser, S.; Rupper, P.; Maniura-Weber, K.; Ruffieux, K.; Ren, Q. Plasma-deposited AgOx-doped TiOx coatings enable rapid antibacterial activity based on ROS generation. Plasma Process. Polym. 2022, 19, 2100246. [Google Scholar] [CrossRef]
- Yoo, Y.; Park, J.-C.; Cho, M.-H.; Yang, J.; Kim, C.-Y.; Jung, K.-H.; Jeon, J.-S.; An, G.; Lee, S.-W. Lack of a Cytoplasmic RLK, Required for ROS Homeostasis, Induces Strong Resistance to Bacterial Leaf Blight in Rice. Front. Plant Sci. 2018, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Oves, M.; Khan, A.U. Obliteration of bacterial growth and biofilm through ROS generation by facilely synthesized green silver nanoparticles. PLoS ONE 2017, 12, e0181363. [Google Scholar] [CrossRef] [PubMed]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial Response to Oxidative Stress and RNA Oxidation. Front. Genet. 2022, 12, 821535. [Google Scholar] [CrossRef] [PubMed]
- Elwakil, B.H.; Toderas, M.; El-Khatib, M. Arc discharge rapid synthesis of engineered copper oxides nano shapes with potent antibacterial activity against multi-drug resistant bacteria. Sci. Rep. 2022, 12, 20209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Rock, C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008, 6, 222–233. [Google Scholar] [CrossRef]
- Kashef, N.; Huang, Y.Y.; Hamblin, M.R. Advances in antimicrobial photodynamic inactivation at the nanoscale. Nanophotonics 2017, 6, 853–879. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Karunakaran, S.; Pandit, S.; Basu, B.; De, M. Simultaneous Exfoliation and Functionalization of 2H-MoS2 by Thiolated Surfactants: Applications in Enhanced Antibacterial Activity. J. Am. Chem. Soc. 2018, 140, 12634–12644. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2017, 43, 3940–3961. [Google Scholar] [CrossRef]
- Eckhardt, S.; Brunetto, P.S.; Gagnon, J.; Priebe, M.; Giese, B.; Fromm, K.M. Nanobio Silver: Its Interactions with Peptides and Bacteria, and Its Uses in Medicine. Chem. Rev. 2013, 113, 4708–4754. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial silver nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Holt, K.B.; Bard, A.J. Interaction of Silver(I) Ions with the Respiratory Chain of Escherichia coli: An Electrochemical and Scanning Electrochemical Microscopy Study of the Antimicrobial Mechanism of Micromolar Ag+. Biochemistry 2005, 44, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef]
- Lee, H.; Lee, D.G. Gold nanoparticles induce a reactive oxygen species-independent apoptotic pathway in Escherichia coli. Colloids Surf. B Biointerfaces 2018, 167, 1–7. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Takayama, K.; Tuñón-Molina, A.; Seyran, M.; Hassan, S.S.; Pal Choudhury, P.; Uversky, V.N.; Lundstrom, K.; Adadi, P.; Palù, G.; et al. Carbon-Based Nanomaterials: Promising Antiviral Agents to Combat COVID-19 in the Microbial-Resistant Era. ACS Nano 2021, 15, 8069–8086. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1. [Google Scholar] [CrossRef]
- Park, S.; Park, H.H.; Kim, S.Y.; Kim, S.J.; Woo, K.; Ko, G. Antiviral properties of silver nanoparticles on a magnetic hybrid colloid. Appl. Environ. Microbiol. 2014, 80, 2343–2350. [Google Scholar] [CrossRef]
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.N.R.; Topno, R.K.; Bhawna; Madhukar, M.; et al. Iron oxide nanoparticles based antiviral activity of H1N1 influenza A virus. J. Infect. Chemother. 2019, 25, 325–329. [Google Scholar] [CrossRef]
- Abo-Zeid, Y.; Ismail, N.S.M.; McLean, G.R.; Hamdy, N.M. A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, J.; Das, S.; Aich, S.; Bhattacharyya, P.; Acharya, K. Antiviral potential of nanoparticles for the treatment of Coronavirus infections. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2022, 72, 126977. [Google Scholar] [CrossRef]
- Bryaskova, R.; Pencheva, D.; Nikolov, S.; Kantardjiev, T. Synthesis and comparative study on the antimicrobial activity of hybrid materials based on silver nanoparticles (AgNps) stabilized by polyvinylpyrrolidone (PVP). J. Chem. Biol. 2011, 4, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Shao, K.; Li, Z.; Guo, N.; Zuo, Y.; Li, Q.; Lu, Z.; Chen, L.; He, Q.; Han, H. Antiviral Activity of Graphene Oxide: How Sharp Edged Structure and Charge Matter. ACS Appl. Mater. Interfaces 2015, 7, 21571–21579. [Google Scholar] [CrossRef] [PubMed]
- Broglie, J.J.; Alston, B.; Yang, C.; Ma, L.; Adcock, A.F.; Chen, W.; Yang, L. Antiviral Activity of Gold/Copper Sulfide Core/Shell Nanoparticles against Human Norovirus Virus-Like Particles. PLoS ONE 2015, 10, e0141050. [Google Scholar] [CrossRef]
- Ramos-Zúñiga, J.; Bruna, N.; Pérez-Donoso, J.M. Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. Int. J. Mol. Sci. 2023, 24, 10503. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Gao, X.; Wang, X.; Zhang, L. 2′- and 3′-Ribose Modifications of Nucleotide Analogues Establish the Structural Basis to Inhibit the Viral Replication of SARS-CoV-2. J. Phys. Chem. Lett. 2022, 13, 4111–4118. [Google Scholar] [CrossRef]
- Dimitrov, D.S. Virus entry: Molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004, 2, 109–122. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Sengupta, A.; Hinkula, J.; Slater, N.K.H.; Patra, H.K. Nano toolbox in immune modulation and nanovaccines. Trends Biotechnol. 2022, 40, 1195–1212. [Google Scholar] [CrossRef]
- Mahajan, S.; Choudhary, S.; Kumar, P.; Tomar, S. Antiviral strategies targeting host factors and mechanisms obliging +ssRNA viral pathogens. Bioorganic Med. Chem. 2021, 46, 116356. [Google Scholar] [CrossRef]
- Dungdung, R.; Bayal, M.; Valliyott, L.; Unniyampurath, U.; Nair, S.S.; Pilankatta, R. A slow, efficient and safe nanoplatform of tailored ZnS QD-mycophenolic acid conjugates for in vitro drug delivery against dengue virus 2 genome replication. Nanoscale Adv. 2020, 2, 5777–5789. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.; Guduru, R.; Liang, P.; Hong, J.; Sagar, V.; Khizroev, S. Externally-controlled on-demand release of anti-HIV drug AZTTP using magneto-electric nanoparticles as carriers. Nat. Commun. 2013, 4, 1707. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Wang, C.-Y.; Zare, E.N.; Borzacchiello, A.; Niu, L.-N.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Farooq, M.; Ilyas, N.; Ilyas, N.; Khan, I.; Saboor, A.; Khan, S.M.; Khan, M.N.; Qayum, A.; Bakhtiar, M.T. Antifungal activity of plant extracts and Silver nano particles against Citrus brown spot pathogen (Alternaria citri). Int. J. Environ. Agric. Res. 2018, 4, 118–125. [Google Scholar]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an antimicrobial agent: Recent advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef]
- Matras, E.; Gorczyca, A.; Przemieniecki, S.W.; Oćwieja, M. Surface properties-dependent antifungal activity of silver nanoparticles. Sci. Rep. 2022, 12, 18046. [Google Scholar] [CrossRef]
- Munir, S.; Asghar, F.; Younis, F.; Tabassum, S.; Shah, A.; Khan, S.B. Assessing the potential biological activities of TiO2 and Cu, Ni and Cr doped TiO2 nanoparticles. RSC Adv. 2022, 12, 3856–3861. [Google Scholar] [CrossRef]
- Morsy, E.A.; Hussien, A.M.; Ibrahim, M.A.; Farroh, K.Y.; Hassanen, E.I. Cytotoxicity and Genotoxicity of Copper oxide Nanoparticles in chickens. Biol. Trace Elem. Res. 2021, 199, 4731–4745. [Google Scholar] [CrossRef]
- Khatami, M.; Alijani, H.Q.; Mousazadeh, F.; Hashemi, N.; Mahmoudi, Z.; Darijani, S.; Bamorovat, M.; Keyhani, A.; Abdollahpour-Alitappeh, M.; Borhani, F. Calcium carbonate nanowires: Greener biosynthesis and their leishmanicidal activity. RSC Adv. 2020, 10, 38063–38068. [Google Scholar] [CrossRef]
- de Villiers, K.A.; Egan, T.J. Heme Detoxification in the Malaria Parasite: A Target for Antimalarial Drug Development. Acc. Chem. Res. 2021, 54, 2649–2659. [Google Scholar] [CrossRef]
- El-khadragy, M.; Alolayan, E.M.; Metwally, D.M.; El-Din, M.F.S.; Alobud, S.S.; Alsultan, N.I.; Alsaif, S.S.; Awad, M.A.; Abdel Moneim, A.E. Clinical Efficacy Associated with Enhanced Antioxidant Enzyme Activities of Silver Nanoparticles Biosynthesized Using Moringa oleifera Leaf Extract, Against Cutaneous Leishmaniasis in a Murine Model of Leishmania major. Int. J. Environ. Res. Public Health 2018, 15, 1037. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Torres, M.; Trujillo-Ferrara, J.G.; Godínez-Victoria, M.; Jarillo-Luna, R.A.; Tsutsumi, V.; Sánchez-Monroy, V.; Posadas-Mondragón, A.; Cuevas-Hernández, R.I.; Santiago-Cruz, J.A.; Pacheco-Yépez, J. Riluzole, a Derivative of Benzothiazole as a Potential Anti-Amoebic Agent against Entamoeba histolytica. Pharmaceuticals 2023, 16, 896. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.; Karimi, N.; Valadbeigi, T. Antibacterial, Antibiofilm, Antiquorum Sensing, Antimotility, and Antioxidant Activities of Green Fabricated Ag, Cu, TiO2, ZnO, and Fe3O4 NPs via Protoparmeliopsis muralis Lichen Aqueous Extract against Multi-Drug-Resistant Bacteria. ACS Biomater. Sci. Eng. 2019, 5, 4228–4243. [Google Scholar] [CrossRef] [PubMed]
- Ghobashy, M.M.; Elkodous, M.A.; Shabaka, S.H.; Younis, S.A.; Alshangiti, D.M.; Madani, M.; Al-Gahtany, S.A.; Elkhatib, W.F.; Noreddin, A.M.; Nady, N.; et al. An overview of methods for production and detection of silver nanoparticles, with emphasis on their fate and toxicological effects on human, soil, and aquatic environment. Nanotechnol. Rev. 2021, 10, 954–977. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Docter, D.; Stauber, R.H.; Leong, D.T. Understanding and exploiting nanoparticles intimacy with the blood vessel and blood. Chem. Soc. Rev. 2015, 44, 8174–8199. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Kumar, R.; Anand, J.; Gupta, R.; Gupta, A.; Pant, K.; Dohare, S.; Tiwari, P.; Kesari, K.K.; Krishnan, S.; et al. Nanotechnology-Based Solutions for Antibiofouling Applications: An Overview. ACS Appl. Nano Mater. 2023, 6, 12828–12848. [Google Scholar] [CrossRef]
- Jayakumar, A.; Mathew, S.; Radoor, S.; Kim, J.T.; Rhim, J.-W.; Siengchin, S. Recent advances in two-dimensional nanomaterials: Properties, antimicrobial, and drug delivery application of nanocomposites. Mater. Today Chem. 2023, 30, 101492. [Google Scholar] [CrossRef]
- Zein, R.; Sharrouf, W.; Selting, K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020, 2020, 5194780. [Google Scholar] [CrossRef]
- Gorshkov, K.; Susumu, K.; Chen, J.; Xu, M.; Pradhan, M.; Zhu, W.; Hu, X.; Breger, J.C.; Wolak, M.; Oh, E. Quantum Dot-Conjugated SARS-CoV-2 Spike Pseudo-Virions Enable Tracking of Angiotensin Converting Enzyme 2 Binding and Endocytosis. ACS Nano 2020, 14, 12234–12247. [Google Scholar] [CrossRef]
- Khan, I.; Sun, N.; Zhang, Z.; Zhijun, L.; Humayun, M.; Ali, S.; Qu, Y.; Jing, L. Improved visible-light photoactivities of porous LaFeO3 by coupling with nanosized alkaline earth metal oxides and its mechanism insight. Catal. Sci. Technol. 2019, 9, 3149–3157. [Google Scholar] [CrossRef]
- Sidhu, V.; Marchi, J.; Borges, R.; Ahmadi, E. Surface modification of metallic orthopedic implants for anti-pathogenic characteristics. J. Compos. Compd. 2022, 4, 51–60. [Google Scholar] [CrossRef]
- Khan, I.; Yuan, A.; Khan, A.; Khan, S.; Khan, S.; Shah, S.; Yaseen, W.; Cui, Y.; Shen, X.; Wang, X. Efficient Visible-Light Activities of TiO2 decorated and Cr3+ incorporated-porous SmFeO3 for CO2 conversion and 4-chlorophenol degradation. Surf. Interfaces 2022, 34, 102358. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, B.; Kumar, R.; Chhabra, D.; Ghosh, M.; Manuja, M.; Brar, B.; Pal, Y.; Tripathi, B.N.; Prasad, M. Metal/metal oxide nanoparticles: Toxicity concerns associated with their physical state and remediation for biomedical applications. Toxicol. Rep. 2021, 8, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Khan, S.; Chen, J.; Shah, S.; Yuan, A. Biological Inspired Green Synthesis of TiO2 Coupled g-C3N4 Nanocomposites and Its Improved Activities for Sulfadiazine and Bisphenol A Degradation. J. Clust. Sci. 2022, 34, 1453–1464. [Google Scholar] [CrossRef]
- Graham, U.M.; Jacobs, G.; Yokel, R.A.; Davis, B.H.; Dozier, A.K.; Birch, M.E.; Tseng, M.T.; Oberdörster, G.; Elder, A.; DeLouise, L. From Dose to Response: In Vivo Nanoparticle Processing and Potential Toxicity. Adv. Exp. Med. Biol. 2017, 947, 71–100. [Google Scholar] [CrossRef]
- Pazos-Ortiz, E.; Roque-Ruiz, J.H.; Hinojos-Márquez, E.A.; López-Esparza, J.; Donohué-Cornejo, A.; Cuevas-González, J.C.; Espinosa-Cristóbal, L.F.; Reyes-López, S.Y. Dose-Dependent Antimicrobial Activity of Silver Nanoparticles on Polycaprolactone Fibers against Gram-Positive and Gram-Negative Bacteria. J. Nanomater. 2017, 2017, 4752314. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Albasha, A.; Hikmat, S.; Hamadneh, L.; Zaza, R.; Shraideh, Z.; Khalil, E.A. Nanoparticle size and chemical modification play a crucial role in the interaction of nano gold with the brain: Extent of accumulation and toxicity. Biomater. Sci. 2020, 8, 1669–1682. [Google Scholar] [CrossRef]
- Hu, W.; Wang, C.; Gao, D.; Liang, Q. Toxicity of transition metal nanoparticles: A review of different experimental models in the gastrointestinal tract. J. Appl. Toxicol. 2023, 43, 32–46. [Google Scholar] [CrossRef]
- Kim, J.K.; Jo, M.S.; Kim, Y.; Kim, T.G.; Shin, J.H.; Kim, B.W.; Kim, H.P.; Lee, H.K.; Kim, H.S.; Ahn, K.; et al. 28-Day inhalation toxicity study with evaluation of lung deposition and retention of tangled multi-walled carbon nanotubes. Nanotoxicology 2020, 14, 250–262. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, L. Nanomaterials arising amid antibiotic resistance. Nat. Rev. Microbiol. 2021, 19, 5–6. [Google Scholar] [CrossRef]
- Zhang, S.; Khan, I.; Qin, X.; Qi, K.; Liu, Y.; Bai, S. Construction of 1D Ag-AgBr/AlOOH Plasmonic Photocatalyst for Degradation of Tetracycline Hydrochloride. Front. Chem. 2020, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Amaro, F.; Morón, Á.; Díaz, S.; Martín-González, A.; Gutiérrez, J.C. Metallic Nanoparticles-Friends or Foes in the Battle against Antibiotic-Resistant Bacteria? Microorganisms 2021, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Song, H.; Lu, J.; Yuan, Z.; Guo, J. Copper nanoparticles and copper ions promote horizontal transfer of plasmid-mediated multi-antibiotic resistance genes across bacterial genera. Environ. Int. 2019, 129, 478–487. [Google Scholar] [CrossRef]
- Gupta, R.; Xie, H. Nanoparticles in Daily Life: Applications, Toxicity and Regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef]
- Furxhi, I.; Costa, A.; Vázquez-Campos, S.; Fito-López, C.; Hristozov, D.; Tamayo Ramos, J.A.; Resch, S.; Cioffi, M.; Friedrichs, S.; Rocca, C.; et al. Status, implications and challenges of European safe and sustainable by design paradigms applicable to nanomaterials and advanced materials. RSC Sustain. 2023, 1, 234–250. [Google Scholar] [CrossRef]
- Khan, I.; Luo, M.s.; Guo, L.; Khan, S.; Wang, C.; Saeed, M.; Zaman, S.; Qi, K.; Liu, Q.; Khan, A. Enhanced visible-light photoactivities of porous LaFeO3 by synchronously doping Ni2+ and coupling TS-1 for CO2 reduction and 2,4,6-Trinitrophenol degradation. Catal. Sci. Technol. 2021, 11, 6793–6803. [Google Scholar] [CrossRef]

| NMs | Nature | Antipathogens | Mode of Action | Therapeutic Outcome | Ref. |
|---|---|---|---|---|---|
| Polymeric micelles | Organic | S. aureus | Membrane lipases breakdown. | Multi-resistance drugs, biofilms | [41] |
| Chitosan HCL | Organic | Gram-negative and Gram-positive bacteria | Depolarizing the cell membrane. | Multi-resistance drug | [42] |
| Chitosan NPs | Organic | E. coli | Generate ROS production. | Antibacterial activity, meat preservation | [43] |
| Liposome | Organic | Gram-negative and Gram-positive bacteria | Break down cell membrane. | Antimicrobial activity, meat preservatives | [44] |
| Liposome | Organic | Salmonella enterica | Targeting viral cells; modified liposomes impair cellular processes. | Reduce microbial contaminants in poultry feed | [45] |
| Se-NPs | Inorganic | Gram-negative and Gram-positive bacteria | Increase ROS production. | Antimicrobial activity | [46] |
| Pd-NPs | Inorganic | S. aureus, E. coli | ROS induction via NIR. | Photothermal activity | [47] |
| ZnO-NPs | Inorganic | Gram-negative and Gram-positive bacteria | Induce ROS to disrupt essential proteins. | Multidrug-resistant bacteria in the poultry | [48,49] |
| Ag-NPs, Cu-NPs, Au-NPs, Pt-NPs, and Fe-NPs | Inorganic | Treponema bacteria | Oxidative stress damages cellular components. | Combating hoof disorders in cows | [50,51] |
| Au-NPs | Inorganic | Bacillus anthracis | Induce ROS to disrupt cell membrane. | Diagnostic marker in poultry and livestock | [52] |
| QDs | Inorganic | Gram positive and Gram-negative bacteria | The biochemical process is disrupted by damage to the plasma membrane and the cell wall. | Drug-resistant topical infections in livestock | [53,54] |
| PEG-GO-AuNPs | Hybrid | E. coli, S. typhimurium | Disrupt vital biomolecules by inducing ROS. | Biosensor, antibacterial agent | [55] |
| Chitosan-AgIO3 | Hybrid | P. aeruginosa, K. pneumoniae, S. saprophyticus, E. coli, S. aureus | Oxidative stress damages cellular components. | Antibacterial activity | [56] |
| Liposome-loaded chitosan | Hybrid | Salmonella spp. | Activate reactive oxygen species, causing membrane breakdown when exposed to UV light. | Livestock food production | [57] |
| Betanin nanoliposomes (G/CH NF/ZnO NPs/B NLPs) | Hybrid | E. coli | Cellular components are damaged by oxidative stress. | Meat preservation, antibacterial effects | [58] |
| Liposomes | Organic | HSV-1 | Modified liposomes target viral cells, disrupt cellular machinery. | Multi-resistance drug/biofilms | [59] |
| Dendrimers/PLL | Organic | H1N1, HIV, SARS, Ebola, MERS-CoV | Dendrimers interact with spike protein to inhibit DNA synthesis. | Antiviral drug delivery modulates the immune response | [60] |
| Polymeric lipid NPs | Organic | MDV | Modified polymeric lipids specifically target viral cells and interfere with biological processes. | Eliminate viral re-emergence | [61] |
| Polyanhydride-NPs | Organic | SwIAV | NPs enhance antigen adsorption, uptake, processing, maturation, immune response regulation, and are easily phagocytosed by APCs. | Lymphocyte proliferation, vaccines for pigs | [62] |
| Graphene, fullerenes, and CNTs | Inorganic | HSV-1, HIV, RSV | Electrostatic interactions with viral proteins to generate oxidative stress and immune responses. | Inhibiting viral replication, photothermal activity | [63] |
| Ag-NPs | Inorganic | H1N1, H3N2, enterovirus 71, HSV-1/HSV-2, DENV, HIV poliovirus | Plasma membrane rupturing and cell wall disruption, disturbs the biochemical process. | Eradicate viral replication | [64] |
| Cu, Ag, TiO2,graphene | Inorganic | SARS-CoV-2 | Release toxic ions and ROS and UV-induced membrane destruction. | PDT, PTT, PPE, antiviral activity | [65] |
| Ag-NPs | Inorganic | ASFV | Damage to membranes due to free radicals and ROS. | Disinfectant | [66] |
| ZnO-NPs, Ag-NPs | Inorganic | BoHV-1 | Cellular damage from oxidative stress | Antiviral agents | [67] |
| Mesoporous Si-NPs, Au-NPs | Inorganic | FMDV | ROS from ions disrupt homeostasis and permeate cells. | Vaccines | [68,69] |
| GSH-ZnS NPs, | Hybrid | PRRSV | Oxidative stress damages cellular components due to glycosylation and immunodominant decoy epitopes. | Antiviral activity | [70] |
| MES-coated tellurium NPs (Te/BSA NPs) | Hybrid | PRRSV | Te/BSA nanostars inhibit PRRSV proliferation and prophylactic effect. | Antiviral activity | [71] |
| Ca3(PO4)2 biomineralized core immunogen shell NPs | Hybrid | FMDV | The addition of polar amino acids to VLPs can enhance their stability in extreme environments, potentially improving their heat resistance. | Vaccines | [72] |
| Liposomes | Organic | A. flavus | Interact with the membrane, causing destabilization, cellular leakage. | Drug delivery, antifungal agent | [73] |
| Polymeric NPs | Organic | Streptomyces hygroscopicus | Antifungal activity involves cell membrane damage, causing cell death. | Drug delivery. treating allergies, autoimmune diseases | [74] |
| Liposomes | Organic | A. fumigatus | Liposome binding affinity for fungal cell walls ensuring stability and preventing toxicity. | Antimycotic infections, drug delivery | [75] |
| Si-NPs | Inorganic | C. auris | Ion’s release generates ROS disrupt homeostasis cause cell leakage. | Drug delivery, MDR | [76] |
| Fe2O3, Fe3O4, ZnO NPs | Inorganic | A. flavus | ROS induces mitochondrial dysfunctional apoptosis. | Antifungal activity | [77,78] |
| ZnO-CaO | Hybrid | C. auris | Zn2+ disrupts zinc-mediated protein activity, generates oxidative stress. | MDR | [79] |
| Chol-PEG-SH, PEG-Fluc-GNR | Hybrid | C. albicans | Opsonization and phagocytosis inhibit DNA/RNA synthesis. | Drug delivery | [80] |
| TiO2-Cu2CuI | Hybrid | A. Niger, C. parapsilosis | Restrict enzyme function, release of Cu2+, alter NADPH generation. | MDR | [81] |
| Iron oxide and chitosan NPs | Hybrid | Candida albicans and Candida glabrata | ROS generation occurs when antifungal NMs attach to antifungal effect cells, elaborating O2 and metal ions. | Antifungal activity | [82] |
| Ag@Cu-NPs | Hybrid | Candida albicans | Release ions cause oxidative stress, cell wall damage, enzymatic activity inhibition. | Antifungal activity | [83] |
| Liposomes | Organic | Plasmodium spp. | Liposomes interact with ligands or antibodies and release encapsulated drugs. | Antiparasitic activity, drug delivery | [84] |
| PEG-liposomes | Organic | P. falciparum | Preventing immune system recognition and eliminating parasites through drug cellular uptake. | Conjugated therapy, drug delivery, MDR | [85] |
| Liposome | Organic | Toxoplasma gondii | Destabilizing membranes through acidic pH, disulfide bonding cleaving, and degradation. | Vaccines | [86] |
| Chitosan | Organic | Eimeria spp. | Chitosan destabilizes hydrophobic scaffolds in tertiary amines and degrades in response to intracellular environment. | Drug delivery | [87] |
| Chitosan | Organic | Leishmania | Chitosan destabilizes cellular membrane. | Drug delivery, antiparasitic activity | [88] |
| Ag-NPs | Inorganic | P. falciparum | Induce ROS causing cellular contents leakage. | Antiprotozoal activity | [89] |
| Au, Ag, Cu-NPs | Inorganic | T. gondii, malaria, leishmaniasis | Release ions, generate oxidative stress to kill parasites. | Biomarkers | [90,91,92] |
| Au, Ag, Pt NPs | Inorganic | T. gondii | Adsorption, permeation, and cytotoxicity of NPs with electrically charged substances. | Antiparasitic activity | [93] |
| Au-NPs | Inorganic | Echinococcus granulosus | AuNPs on hydatid cyst protoscoleces, assessing their effects on cell wall and caspase-3 activation. | Diagnostic marker | [94] |
| Ag-NPs | Inorganic | Haemonchus contortus, Leishmania | Free radicals induce oxidative stress. | Antiprotozoal activity | [95] |
| ZnO and FeO-NPs | Inorganic | Toxocara vitulorum | Oxidative stress and ROS generation increasing antioxidant enzyme activity. | Antiprotozoal activity | [96] |
| PLGA@chitosan | Hybrid | T. gondii | Acidic environment causes PLGA degradation, releasing drugs, and targeting parasites. | Vaccines | [97] |
| TiO2/Zn-HY | Hybrid | L. amazonensis | Oxidative stress inhibits DNA/RNA synthesis. | PDT, photosensitizer, and cutaneous leishmaniasis therapy | [98] |
| CNC/ZnO/CuO | Hybrid | Anopheles stephensi | Generation of hydroxyl ions and ROS leads to membrane disruption. | Photodegradation and larvicidal activities | [99] |
| ZnO-CuO nanocomposite | Hybrid | Culex quinquefasciatus | Generation of ROS antioxidant property of enzymes. | Antiprotozoal activity | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadiq, S.; Khan, I.; Shen, Z.; Wang, M.; Xu, T.; Khan, S.; Zhou, X.; Bahadur, A.; Rafiq, M.; Sohail, S.; et al. Recent Updates on Multifunctional Nanomaterials as Antipathogens in Humans and Livestock: Classification, Application, Mode of Action, and Challenges. Molecules 2023, 28, 7674. https://doi.org/10.3390/molecules28227674
Sadiq S, Khan I, Shen Z, Wang M, Xu T, Khan S, Zhou X, Bahadur A, Rafiq M, Sohail S, et al. Recent Updates on Multifunctional Nanomaterials as Antipathogens in Humans and Livestock: Classification, Application, Mode of Action, and Challenges. Molecules. 2023; 28(22):7674. https://doi.org/10.3390/molecules28227674
Chicago/Turabian StyleSadiq, Samreen, Iltaf Khan, Zhenyu Shen, Mengdong Wang, Tao Xu, Sohail Khan, Xuemin Zhou, Ali Bahadur, Madiha Rafiq, Sumreen Sohail, and et al. 2023. "Recent Updates on Multifunctional Nanomaterials as Antipathogens in Humans and Livestock: Classification, Application, Mode of Action, and Challenges" Molecules 28, no. 22: 7674. https://doi.org/10.3390/molecules28227674