Development of Capillary Zone Electrophoresis Method for the Simultaneous Separation and Quantification of Metformin and Pioglitazone in Dosage Forms; and Comparison with HPLC Method
Abstract
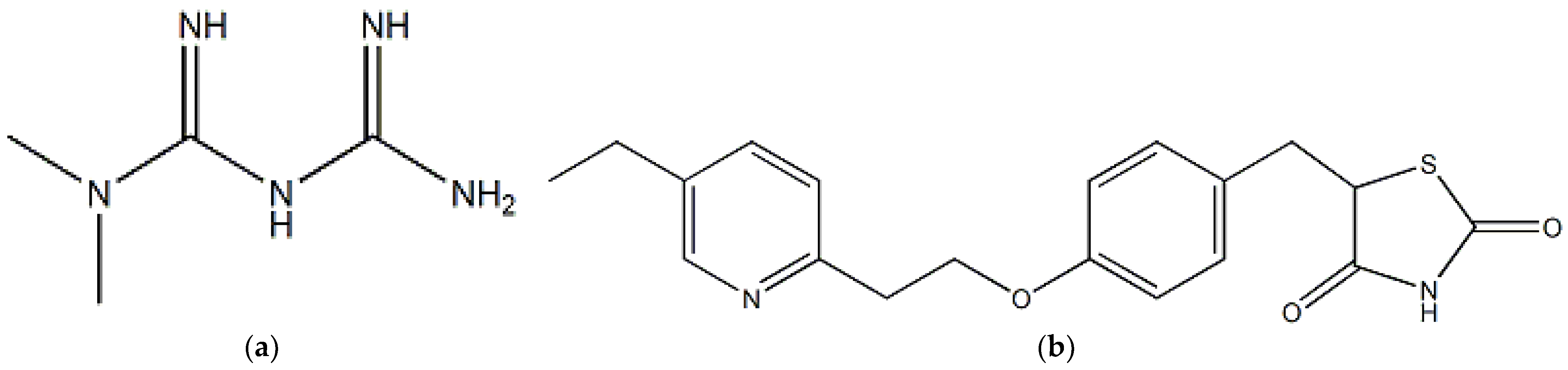
:1. Introduction
2. Results and Discussion
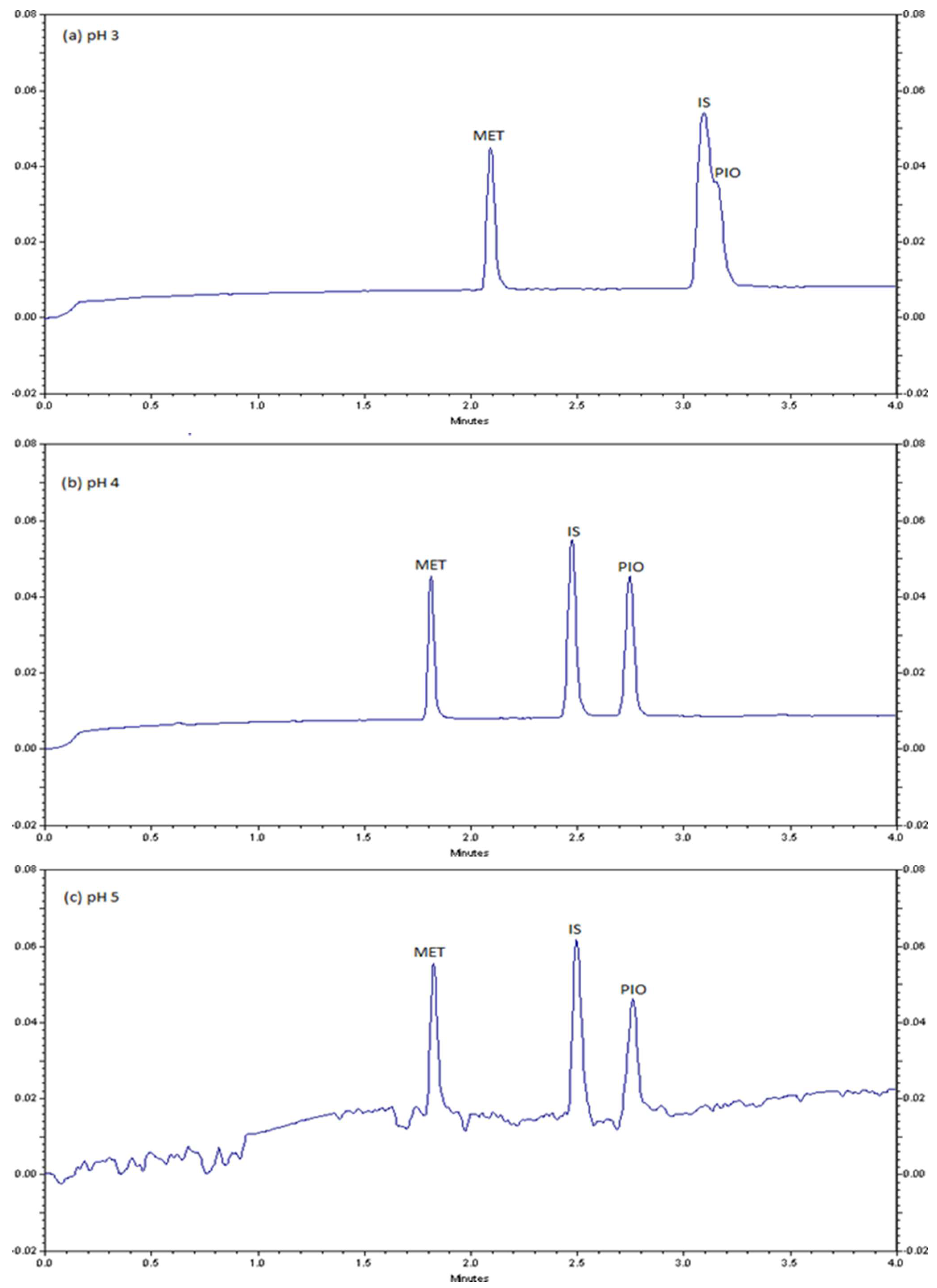
2.1. CZE method Development and Optimization
2.2. CZE Method Validation
2.3. CZE Method Application
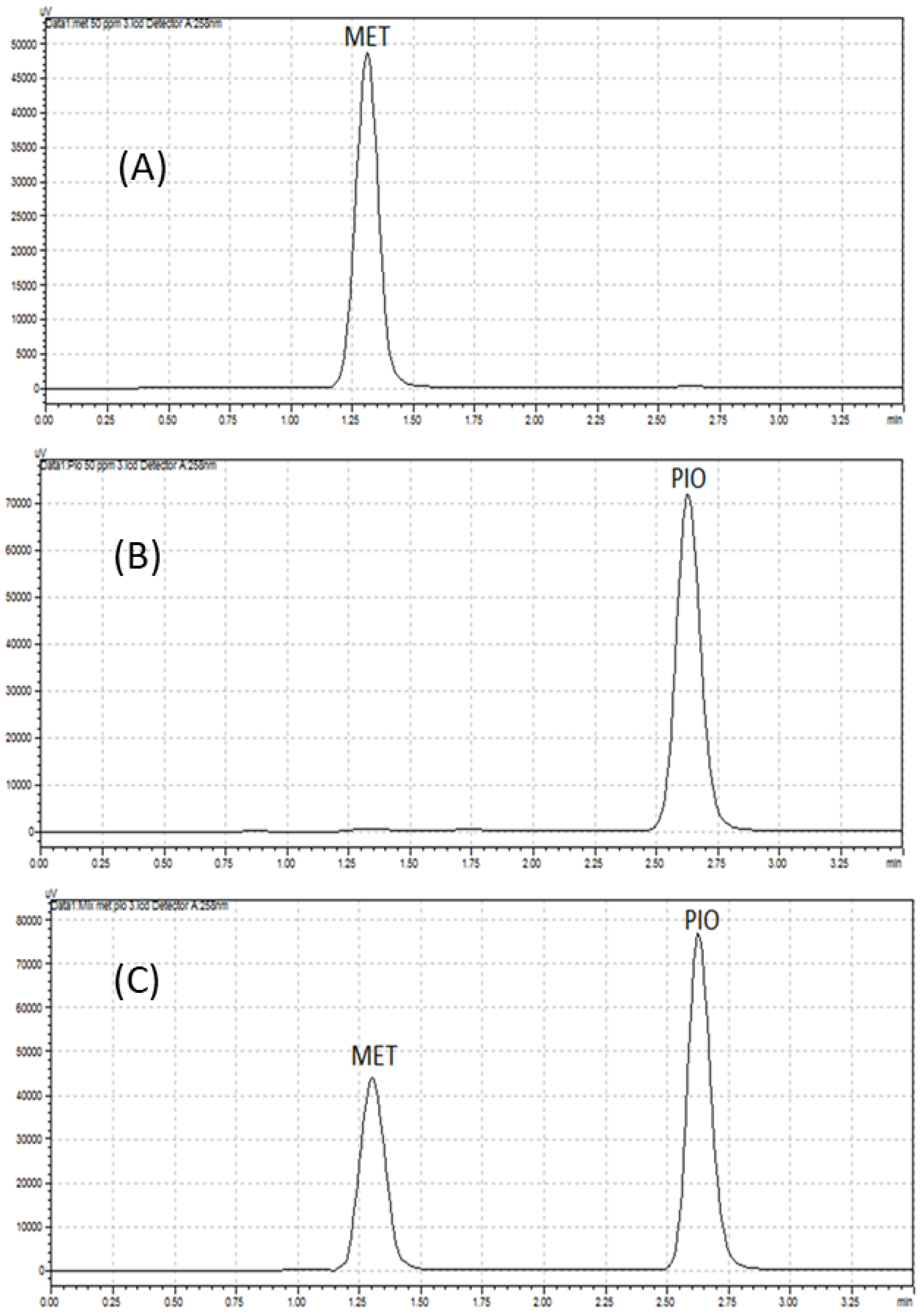
2.4. Comparison of CZE Method with HPLC Method
3. Experimental
3.1. Chemicals and Reagents
3.2. Instrumentation
3.3. Preparation of Standard Solutions
3.4. Preparation of Running Buffer
3.5. Preparation of Pharmaceutical Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Xu, X.; Du, M.; Zhao, T.; Wang, J. A preclinical overview of metformin for the treatment of type 2 diabetes. Biomed. Pharmacother. 2018, 106, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. Oxidative stress and glycemic regulation. Metabolism 2000, 49, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Baena-Diez, J.M.; Penafiel, J.; Subirana, I.; Ramos, R.; Elosua, R.; Marin-Ibanez, A.; Guembe, M.J.; Rigo, F.; Tormo-Diaz, M.J.; Moreno-Iribas, C.; et al. Risk of cause-specific death in individuals with diabetes: A Competing risks analysis. Diabetes Care 2016, 39, 1987–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekoé, J.-M. Diagnosis and classification of diabetes mellitus. In Encyclopedia of Endocrine Diseases, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019; Volume 1, pp. 105–109. [Google Scholar]
- Al Dawish, M.A.; Robert, A.A.; Braham, R.; Al Hayek, A.A.; Al Saeed, A.; Ahmed, R.A.; Al Sabaan, F.S. Diabetes mellitus in Saudi Arabia: A review of the recent literature. Curr. Diabetes Rev. 2016, 12, 359–368. [Google Scholar] [CrossRef]
- American Diabetes, A. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42 (Suppl. S1), S13–S28. [Google Scholar] [CrossRef] [Green Version]
- Lebovitz, H.E. Treating hyperglycemia in type 2 diabetes: New goals and strategies. Clevel. Clin. J. Med. 2002, 69, 809–820. [Google Scholar] [CrossRef] [Green Version]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [Green Version]
- Ruggiero-Lopez, D.; Lecomte, M.; Moinet, G.; Patereau, G.; Lagarde, M.; Wiernsperger, N. Reaction of metformin with dicarbonyl compounds. Possible implication in the inhibition of advanced glycation end product formation. Biochem. Pharmacol. 1999, 58, 1765–1773. [Google Scholar] [CrossRef]
- Tanaka, Y.; Uchino, H.; Shimizu, T.; Yoshii, H.; Niwa, M.; Ohmura, C.; Mitsuhashi, N.; Onuma, T.; Kawamori, R. Effect of metformin on advanced glycation endproduct formation and peripheral nerve function in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 1999, 376, 17–22. [Google Scholar] [CrossRef]
- Beisswenger, P.J.; Howell, S.K.; Touchette, A.D.; Lal, S.; Szwergold, B.S. Metformin reduces systemic methylglyoxal levels in type 2 diabetes. Diabetes 1999, 48, 198–202. [Google Scholar] [CrossRef]
- Simonson, G.; Cuddihy, R.; Reader, D.; Bergenstal, R. International Diabetes Center treatment of type 2 diabetes glucose algorithm. Diabetes Manag. 2011, 1, 175–189. [Google Scholar] [CrossRef]
- Mishra, K.; Soni, H.; Nayak, G.; Patel, S.S.; Singhai, A. Method development and validation of metformin hydrochloride in tablet dosage form. e-J. Chem. 2011, 8, 1309–1313. [Google Scholar] [CrossRef] [Green Version]
- Sen, A.K.; Hinsu, D.; Sen, D.; Zanwar, A.; Maheshwari, R.; Chandrakar, V. Analytical method development and validation for simultaneous estimation of Teneligliptin hydrobromide hydrate and Metformin hydrochloride from it’s pharmaceutical dosage form by three different UV spectrophotometric methods. J. Appl. Pharm. Sci. 2016, 6, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Reddy, N.P.; Chevela, N.T. RP-HPLC Method development and validation for the Simultaneous Estimation of Metformin and Canagliflozin in Tablet Dosage Form. Int. J. Pharma Sci. 2015, 5, 1155–1159. [Google Scholar]
- Mahrouse, M.A.; Lamie, N.T. Experimental design methodology for optimization and robustness determination in ion pair RP-HPLC method development: Application for the simultaneous determination of metformin hydrochloride, alogliptin benzoate and repaglinide in tablets. Microchem. J. 2019, 147, 691–706. [Google Scholar] [CrossRef]
- Sayed, S.; Thomas, A.; Kotapali, L. RP-HPLC method development for determination of pioglitazone hydrochloride from tablets. J. Pharm. Res. 2009, 2, 1479–1480. [Google Scholar]
- Srinivasulu, D.; Sastry, B.; Omprakash, G. Development and validation of new RPHPLC method for determination of pioglitazone hcl in pharmaceutical dosage forms. Int. J. Chem. Res. 2010, 1, 18–20. [Google Scholar]
- Lin, Z.J.; Ji, W.; Desai-Krieger, D.; Shum, L. Simultaneous determination of pioglitazone and its two active metabolites in human plasma by LC–MS/MS. J. Pharm. Biomed. Anal. 2003, 33, 101–108. [Google Scholar] [CrossRef]
- Kelani, K.M.; Rezk, M.R.; Badran, O.M.; Elghobashy, M.R. Determination of pioglitazone, its metabolite and alogliptin in human plasma by a novel LC-MS/MS method; application to a pharmacokinetic study. J. Chromatogr. B 2019, 1132, 121803. [Google Scholar] [CrossRef]
- Ahmad, R.; Hailat, M.; Jaber, M.; Alkhawaja, B.; Rasras, A.; Al-Shdefat, R.; Mallah, E.; Abu Dayyih, W. RP-HPLC method development for simultaneous estimation of empagliflozin, pioglitazone, and metformin in bulk and tablet dosage forms. Acta Pol. Pharm. Drug Res. 2021, 78, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Nirupa, G.; Tripathi, U.M. RP-HPLC analytical method development and validation for simultaneous estimation of three drugs: Glimepiride, pioglitazone, and metformin and its pharmaceutical dosage forms. J. Chem. 2013, 2013, 726235. [Google Scholar] [CrossRef] [Green Version]
- Peraman, R.; Mallikarjuna, S.; Ammineni, P.; Kondreddy, V.K. RP-HPLC method development and validation for simultaneous estimation of Atorvastatin calcium and Pioglitazone hydrochloride in pharmaceutical dosage form. J. Chromatogr. Sci. 2014, 52, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Alnajjar, A.O.; Idris, A.M.; Attimarad, M.V.; Aldughaish, A.M.; Elgorashe, R.E. Capillary electrophoresis assay method for metoprolol and hydrochlorothiazide in their combined dosage form with multivariate optimization. J. Chromatogr. Sci. 2013, 51, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Alnajjar, A.O.; Idris, A.M.; Attimarad, M.V.; Elgorashe, R.E. Quadruple Response Factorial Design Optimization of Capillary Zone Electrophoresis Assay Procedure for Metformin and Sitagliptin Combination. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1379–1383. [Google Scholar] [CrossRef]
- Alnajjar, A.O.; Idris, A.M. Development of a CZE Method for the Quantification of Pseudoephedrine and Cetirizine. J. Chromatogr. Sci. 2014, 52, 1104–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kartsova, L.; Makeeva, D.; Bessonova, E. Current Status of Capillary Electrophoresis. J. Anal. Chem. 2020, 75, 1497–1513. [Google Scholar] [CrossRef]
- Bernardo-Bermejo, S.; Sánchez-López, E.; Castro-Puyana, M.; Marina, M.L. Chiral capillary electrophoresis. TrAC Trends Anal. Chem. 2020, 124, 115807. [Google Scholar] [CrossRef]
- Farid, J.; Mostafa, N.; Fayez, Y.; Essam, H. Capillary zone electrophoresis as a quality assessment tool of paracetamol and phenylephrine hydrochloride in presence of paracetamol impurities. Turk. J. Chem. 2022, 46, 217–223. [Google Scholar]
- Maher, H.M.; Abdelrahman, A.E.; Alzoman, N.Z.; Aljohar, H.I. Stability-indicating capillary electrophoresis method for the simultaneous determination of metformin hydrochloride, saxagliptin hydrochloride, and dapagliflozin in pharmaceutical tablets. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 161–171. [Google Scholar] [CrossRef]
- Attimarad, M. Multivariate optimization of a capillary zone electrophoresis assay method for simultaneous quantification of metformin and vildagliptin from a formulation. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 401–407. [Google Scholar] [CrossRef]
- Strugaru, A.-M.; Mircea, C.; Agoroaei, L.; Botnariu, G.; Grigoriu, I.-C.; Marti, T.D.; Butnaru, E. Quantitative Determination of Metformin by Capillary Electrophoresis with UV Detection. Rev. Chim. 2015, 66, 1448–1451. [Google Scholar]
- Calixto, L.A.; Bonato, P.S. Combination of hollow-fiber liquid-phase microextraction and capillary electrophoresis for pioglitazone and its main metabolites determination in rat liver microsomal fraction. Electrophoresis 2013, 34, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Swapna, J.; Madhu, C.; Srivani, M.; Sumalatha, M.; Nehalatha, Y.; Anusha, Y. Analytical method development and method validation for the simultaneous estimation of metformin hydrochloride and pioglitazone hydrochloride in tablet dosage form by RP-HPLC. Asian J. Pharm. Anal. 2012, 2, 85–89. [Google Scholar]
- Salim, M.; El-Enany, N.; Belal, F.; Walash, M.; Patonay, G. Simultaneous determination of sitagliptin and metformin in pharmaceutical preparations by capillary zone electrophoresis and its application to human plasma analysis. Anal. Chem. Insights 2012, 7, ACI.S9940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spudeit, D.A.; Gonçalves, S.; Bretanha, L.C.; Claumann, C.A.; Machado, R.A.; Micke, G.A. A systematic procedure to develop a capillary electrophoresis method using a minimal experimental data. J. Braz. Chem. Soc. 2016, 27, 1974–1979. [Google Scholar] [CrossRef]
- Gul, W. Metformin: Methods of analysis and its role in lowering the risk of cancer. J. Bioequivalence Bioavailab. 2016, 8, 254–259. [Google Scholar] [CrossRef]
- Altria, K.D. Capillary Electrophoresis Guidebook: Principles, Operation, and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1996; Volume 52. [Google Scholar]
- Riekkola, M.-L.; Jussila, M.; Porras, S.P.; Valkó, I.E. Non-aqueous capillary electrophoresis. J. Chromatogr. A 2000, 892, 155–170. [Google Scholar] [CrossRef]
- Alnajjar, A.O.; Ahmed Elbashir, A.; Elgorashe, R.E.; Ebrahim, A.M.; Idris, A.M.; Abd El-Lateef, H.M. Utilization of 4-fluoro-7-nitro-2, 1, 3-benzoxadiazole (NBD-F) as a fluorogenic reagent for the development of a spectrofluorometric assay method for taurine in energy drinks. J. Chem. Res. 2022, 46, 17475198221114760. [Google Scholar] [CrossRef]
- Konieczka, P.; Namieśnik, J. Quality Assurance and Quality Control in the Analytical Chemical Laboratory: A Practical Approach; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Şanlı, S.; Kılıçarslan, S.; Şanlı, N. Evaluation of natamycin in commercial dairy products by a green capillary zone electrophoresis method and confirmation with a Liquid Chromatography-Mass Spectrometry. Food Biosci. 2022, 50, 102114. [Google Scholar] [CrossRef]
- Mlinarić, Z.; Turković, L.; Begović, I.; Nigović, B.; Sertić, M. Rapid Capillary Electrophoresis Method for Simultaneous Determination of Abemaciclib, Ribociclib, and Palbociclib in Pharmaceutical Dosage Forms: A Green Approach. Molecules 2022, 27, 7603. [Google Scholar] [CrossRef] [PubMed]
- Kotak, V.; Tanna, N.; Patel, M.; Patel, R. Determination of Asenapine Maleate in Pharmaceutical and Biological Matrices: A Critical Review of Analytical Techniques over the Past Decade. Crit. Rev. Anal. Chem. 2022, 52, 1755–1771. [Google Scholar] [CrossRef] [PubMed]
- Knoll, S.; Rösch, T.; Huhn, C. Trends in sample preparation and separation methods for the analysis of very polar and ionic compounds in environmental water and biota samples. Anal. Bioanal. Chem. 2020, 412, 6149–6165. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, M.; Quirino, J.P. Can we replace liquid chromatography with the greener capillary electrophoresis? Curr. Opin. Green Sustain. Chem. 2021, 31, 100515. [Google Scholar] [CrossRef]

| Drug Content (μg/mL) | RSD% (Migration Time) | RSD% (Corrected Peak Area) | ||
|---|---|---|---|---|
| MET | PIO | MET | PIO | |
| Intra-day precision | ||||
| 20 | 0.74 | 0.49 | 5.79 | 5.03 |
| 40 | 1.42 | 2.23 | 5.45 | 0.81 |
| 60 | 1.36 | 1.98 | 3.36 | 4.31 |
| Inter-day precision | ||||
| 20 | 1.23 | 1.49 | 4.23 | 3.33 |
| 40 | 2.20 | 2.15 | 3.20 | 5.04 |
| 60 | 2.45 | 1.67 | 6.02 | 4.39 |
| Spiked Standard Mixture (μg/mL) | Recovery (%) | |
|---|---|---|
| MET | PIO | |
| 20 | 104.8 | 105.4 |
| 40 | 100.5 | 99.6 |
| 60 | 98.2 | 98.8 |
| Sample | tM a | PH b | PW c | Res d | PS e | N f | Rec g | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MET | PIO | MET | PIO | MET | PIO | MET | PIO | MET | PIO | MET | PIO | ||
| Glucophage® | 2.31 | - | 8917 | - | 0.09 | - | 7.18 | 1.00 | - | 397.0 | - | 99.2 | - |
| Actos® | 3.35 | - | 6387 | - | 0.10 | 1.98 | - | 1.50 | - | 425.0 | - | 98.4 | |
| Synthetic mixture | 2.30 | 3.44 | 11635 | 8065 | 0.10 | 0.09 | 1.64 | 1.00 | 1.00 | 394.9 | 449.7 | 98.8 | 98.4 |
| Actosmet® | 2.31 | 3.42 | 8917 | 8859 | 0.09 | 0.07 | 3.57 | 1.00 | 1.12 | 397.0 | 2417 | 100.5 | 99.6 |
| Analytical Feature | CZE | HPLC |
|---|---|---|
| Separation capillary/column | Fused-silica capillary column (40 × 37 cm, 52 μm) | RP-18e column (150 × 4.6 mm, 5.0 μm) a |
| Electrolyte/mobile phase composition | Sodium phosphate and 30% acetonitrile, pH 4 | Sodium phosphate:acetonitrile (55:45), pH 5 a |
| Consumed volumes of electrolyte/mobile phase (mL) | 7.85×10-4 | 8.00 |
| UV detection (nm) | PDA detector at 210 | PDA detector at 258 |
| Analysis time (min) | 17.00 | 8.00 |
| Waste production | In μLs | 8.02 mL |
| System stabilization time (min) | 12.00 | 3.00 |
| Sample frequency (samples/h) | 3 | 7 |
| Retention/migration time (min) for MET and PIO | 2.28 and 3.41 | 1.15 and 2.50 |
| Resolution | 10.37 between MET and IS; 1.64 between IS and PIO | 2.07 |
| Peak symmetry for MET and PIO | 1.00 and 1.30 | 1.00 and 1.00 |
| Theoretical plates for MET and PIO | 2409.8 and 5388.9 | 221.8 and 533.1 |
| Linear range (μg/mL) for MET and PIO | 10–80 and 10–100 | 5–100 and 10–100 |
| Correlation coefficient for MET and PIO | 0.998 and 0.998 | 0.997 and 0.990 |
| LOD (μg/mL) for MET and PIO | 0.09 and 0.10 | 0.09 and 0.25 |
| LOQ (μg/mL) for MET and PIO | 0.27 and 0.31 | 0.29 and 0.76 |
| Recovery (%) for MET and PIO | 98.8–100.5 and 98.4–99.6 | 91.1–91.9 and 98.9–100.2 |
| Intra-day precision (RSD%) for MET and PIO | 3.35–5.78 and 0.81–5.02 | 0.21 and 0.25 |
| Inter-day precision (RSD%) for MET and PIO | 3.12–4.23 and 1.38–3.32 | 0.52 and 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlThikrallah, M.K.I.; Idris, A.M.; Elbashir, A.A.; Elgorashe, R.E.E.; Buzid, A.; Alnajjar, A.O. Development of Capillary Zone Electrophoresis Method for the Simultaneous Separation and Quantification of Metformin and Pioglitazone in Dosage Forms; and Comparison with HPLC Method. Molecules 2023, 28, 1184. https://doi.org/10.3390/molecules28031184
AlThikrallah MKI, Idris AM, Elbashir AA, Elgorashe REE, Buzid A, Alnajjar AO. Development of Capillary Zone Electrophoresis Method for the Simultaneous Separation and Quantification of Metformin and Pioglitazone in Dosage Forms; and Comparison with HPLC Method. Molecules. 2023; 28(3):1184. https://doi.org/10.3390/molecules28031184
Chicago/Turabian StyleAlThikrallah, Maymonah K. I., Abubakr M. Idris, Abdalla Ahmed Elbashir, Rafea E. E. Elgorashe, Alyah Buzid, and Ahmed O. Alnajjar. 2023. "Development of Capillary Zone Electrophoresis Method for the Simultaneous Separation and Quantification of Metformin and Pioglitazone in Dosage Forms; and Comparison with HPLC Method" Molecules 28, no. 3: 1184. https://doi.org/10.3390/molecules28031184





