Characterization and Comparison of Extra Virgin Olive Oils of Turkish Olive Cultivars
Abstract
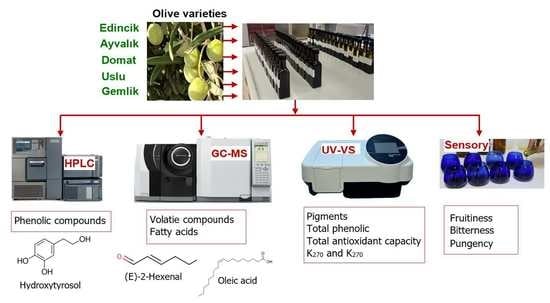
:1. Introduction
2. Results and Discussion
2.1. Quality Parameters
2.2. Pigment Contents
2.3. Fatty Acid Profile
2.4. Individual Phenolic Compounds and Total Phenolic Content (TPC)
2.5. Total Antioxidant Capacity (TAC)
2.6. Volatile Compounds (VCs)
2.7. Sensory Attributes
2.8. Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Samples
3.2. Chemicals
3.3. Quality Parameters Analysis
3.4. Extraction of Phenolic Compounds (PC)
3.5. Total Chlorophyll and Carotenoid Content Analysis
3.6. Total Phenolic Content (TPC) Analysis
3.7. Total Antioxidant Capacity (TAC) Analysis
3.8. Fatty Acid Composition Analysis
3.9. Phenolic Compounds (PC) Analysis
3.10. Volatile Compound (VC) Analysis
3.11. Sensory Analysis
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- European Commission (EC). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:01991R2568-20161204&from=EN (accessed on 15 November 2022).
- Marx, Í.M.G.; Casal, S.; Rodrigues, N.; Pinho, T.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Impact of the Malaxation Temperature on the Phenolic Profile of Cv. Cobrançosa Olive Oils and Assessment of the Related Health Claim. Food Chem. 2021, 337, 127726. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Reina, R.; Aparicio-Ruiz, R.; Morales, M.T.; García-González, D.L. Contribution of Specific Volatile Markers to Green and Ripe Fruity Attributes in Extra Virgin Olive Oils Studied with Three Analytical Methods. Food Chem. 2023, 399, 133942. [Google Scholar] [CrossRef]
- Žanetić, M.; Jukić Špika, M.; Ožić, M.M.; Brkić Bubola, K. Comparative Study of Volatile Compounds and Sensory Characteristics of Dalmatian Monovarietal Virgin Olive Oils. Plants 2021, 10, 1995. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Giambanelli, E.; Cane, A.; Zanoni, B.; Canuti, V.; Mulinacci, N.; Melani, F. Is the Volatile Compounds Profile a Suitable Tool for Authentication of Virgin Olive Oils (Olea Europaea L.) according to Cultivars? a Study by Using HS-SPME-GC-MS and Chemometrics. Food Control 2022, 139, 109092. [Google Scholar] [CrossRef]
- Spadafora, N.D.; Mascrez, S.; McGregor, L.; Purcaro, G. Exploring Multiple-Cumulative Trapping Solid-Phase Microextraction Coupled to Gas Chromatography-Mass Spectrometry for Quality and Authenticity Assessment of Olive Oil. Food Chem. 2022, 383, 132438. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Davalos, A.; López de las Hazas, M.; Crespo, M.C.; Tomé-Carneiro, J. An overview of the Pharmacology of Olive Oil and Its Active Ingredients. Br. J. Pharmacol. 2020, 177, 1316–1330. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Servili, M. Characterization of Phenolic and Volatile Composition of Extra Virgin Olive Oil Extracted from Six Italian Cultivars Using a Cooling Treatment of Olive Paste. LWT 2018, 87, 523–528. [Google Scholar] [CrossRef]
- European Commission (EC). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32012R0432&from=EN (accessed on 10 December 2022).
- European Food Safety Authority (EFSA). Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2012.2848 (accessed on 10 October 2022).
- Anna, R.; Russo, M.; Cacciola, F.; Salafia, F.İ.; Polkowska, Z.A.; Dugo, P.; Mondello, L. Concentration of Potentially Bioactive Compounds in Italian Extra Virgin Olive Oils from Various Sources by Using LC-MS and Multivariate Data Analysis. Foods 2020, 9, 1120. [Google Scholar] [CrossRef]
- Uylaşer, V.; Yildiz, G. The Historical Development and Nutritional Importance of Olive and Olive Oil Constituted an Important Part of the Mediterranean Diet. Crit. Rev. Food Sci. Nutr. 2014, 54, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council (IOC). The Olive Tree. Available online: https://www.internationaloliveoil.org/olive-world/olive-tree/ (accessed on 7 December 2022).
- International Olive Oil Council (IOC). Available online: http://www.internationaloliveoil.org/estaticos/view/131-world-olive-oil-figures (accessed on 6 December 2022).
- Turkish Statistical Institute (TUIK). Available online: https://biruni.tuik.gov.tr/medas/?kn=92&locale=tr (accessed on 8 July 2022).
- Diraman, H.; Saygi, H.; Hisil, Y. Relationship between Geographical Origin and Fatty Acid Composition of Turkish Virgin Olive Oils for Two Harvest Years. JAOCS 2010, 87, 781–789. [Google Scholar] [CrossRef]
- Ben Hmida, R.; Gargouri, B.; Chtourou, F.; Sevim, D.; Bouaziz, M. Fatty Acid and Triacyglycerid As Markers of Virgin Olive Oil from Mediterranean Region: Traceability and Chemometric Authentication. Eur. Food Res. Technol. 2022, 248, 1749–1764. [Google Scholar] [CrossRef]
- Topi, D.; Guclu, G.; Kelebek, H.; Selli, S. Comparative Elucidation of Phenolic Compounds in Albanian Olive Oils Using LC-DAD-ESI-MS/MS. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 203–212. [Google Scholar] [CrossRef]
- Ilyasoglu, H.; Ozcelik, B.; Van Hoed, V.; Verhe, R. Cultivar Characterization of Aegean Olive Oils with Respect to Their Volatile Compounds. Sci. Hortic. 2011, 129, 279–282. [Google Scholar] [CrossRef]
- Kaftan, A.; Elmaci, Y. Aroma Characterization of Virgin Olive Oil from Two Turkish Olive Varieties by SPME/GC/MS. Int. J. Food Prop. 2011, 14, 1160–1169. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Williams, M.; Chartzoulakis, K.; Harwood, J. Effect of Irrigation on Quality Attributes of Olive Oil. J. Agric. Food Chem. 2009, 57, 7048–7055. [Google Scholar] [CrossRef]
- Jolayemi, O.S.; Tokatli, F.; Ozen, B. Effects of Malaxation Temperature and Harvest Time on the Chemical Characteristics of Olive Oils. Food Chem. 2016, 211, 776–783. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Wu, G.; Jin, J.; Jin, Q.; Wang, X. Chemical and volatile characteristics of olive oils extracted from four varieties grown in southwest of China. Int. Food Res. J. 2021, 140, 109987. [Google Scholar] [CrossRef]
- European Commission (EC). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:295:0057:0077:en:PDF (accessed on 11 December 2022).
- International Olive Council (IOC). Available online: https://www.internationaloliveoil.org/wp-content/uploads/2021/11/COI-T15-NC3-REV-17_ENK.pdf (accessed on 8 October 2022).
- Uluata, S.; Altuntaş, U.; Özçelik, B. Characterization of Turkish Extra Virgin Olive Oils and Classification Based on Their Growth Regions Coupled with Multivariate Analysis. Food Anal. Methods 2021, 14, 1682–1694. [Google Scholar] [CrossRef]
- Kelebek, H.; Kesen, S.; Selli, S. Comparative Study of Bioactive Constituents in Turkish Olive Oils by LC-ESI/MS/MS. Int. J. Food Prop. 2015, 18, 2231–2245. [Google Scholar] [CrossRef]
- Rodrigues, N.; Peres, F.; Casal, S.; Santamaria-Echart, A.; Barreiro, F.; Peres, A.M. Alberto Pereira Geographical Discrimination of Olive Oils from Cv. ‘Galega Vulgar’. Food Chem. 2023, 398, 133945. [Google Scholar] [CrossRef]
- Antonini, E.; Farina, A.; Leone, A.; Mazzara, E.; Urbani, S.; Selvaggini, R.; Servili, M.; Ninfali, P. Phenolic Compounds and Quality Parameters of Family Farming Versus Protected Designation of Origin (PDO) Extra-Virgin Olive Oils. J. Food Compos. Anal. 2015, 43, 75–81. [Google Scholar] [CrossRef]
- Isabel Minguez-Mosquera, M.; Rejano-Navarro, L.; Gandul-Rojas, B.; SanchezGomez, A.H.; Garrido-Fernandez, J. Color-Pigment Correlation in Virgin Olive Oil. JAOCS 1991, 68, 332–336. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Morales, M.T.; Asuero, A.G. Characterization of Bioactive Compounds from Monovarietal Virgin Olive Oils: Relationship between Phenolic Compounds-Antioxidant Capacities. Int. J. Food Prop. 2015, 18, 348–358. [Google Scholar] [CrossRef]
- Jolayemi, O.S.; Tokatli, F.; Ozen, B. UV-Vis Spectroscopy for the Estimation of Variety and Chemical Parameters of Olive Oils. J. Food Meas. Charact. 2021, 15, 4138–4149. [Google Scholar] [CrossRef]
- Yang, Y.; Ferro, M.D.; Cavaco, I.; Liang, Y. Detection and Identification of Extra Virgin Olive Oil Adulteration by GC-MS Combined with Chemometrics. J. Agric. Food Chem. 2013, 61, 3693–3702. [Google Scholar] [CrossRef]
- Haddada, F.M.; Krichène, D.; Manai, H.; Oueslati, I.; Daoud, D.; Zarrouk, M. Analytical Evaluation of Six Monovarietal Virgin Olive Oils from Northern Tunisia. Eur. J. Lipid Sci. Technol 2008, 110, 905–913. [Google Scholar] [CrossRef]
- Chtourou, F.; Valli, E.; Ben Mansour, A.; Bendini, A.; Gallina Toschi, T.; Bouaziz, M. Characterization of Virgin Olive Oils Obtained from Minor Tunisian Varieties for Their Valorization. J. Food Meas. Charact. 2021, 15, 5060–5070. [Google Scholar] [CrossRef]
- Gargouri, B.; Ammar, S.; Zribi, A.; Mansour, A.B.; Bouaziz, M. Effect of Growing Region on Quality Characteristics and Phenolic Compounds of Chemlali Extra-Virgin Olive Oils. Acta Physiol. Plant. 2013, 35, 2801–2812. [Google Scholar] [CrossRef]
- Comlekcioglu, S.; Elgudayem, F.; Nogay, G.; Kafkas, N.E.; Ayed, R.B.; Ercisli, S.; Assouguem, A.; Almeer, R.; Najda, A. Biochemical Characterization of Six Traditional Olive Cultivars: A Comparative Study. Horticulture 2022, 8, 416. [Google Scholar] [CrossRef]
- Dorota, D.; Rupert, M.; Wołosiak, R.; Bzducha-Wróbel, A.; Ścibisz, I.; Matuszewska-Janica, A. Volatiles as Markers of Bioactive Components Found in Croatian Extra Virgin Olive Oils. LWT 2021, 139, 110532. [Google Scholar] [CrossRef]
- Kıvrak, Ş.; Kıvrak, İ. Ultrasonic-assisted Extraction Method of Phenolic Compounds in Extra-Virgin Olive Oils (EVOOs) by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC–MS/MS). Sep. Sci. Technol. 2021, 56, 322–329. [Google Scholar] [CrossRef]
- Negro, C.; Aprile, A.; Luvisi, A.; Nicolì, F.; Nutricati, E.; Vergine, M.; Miceli, A.; Blando, F.; Sabella, E.; De Bellis, L. Phenolic Profile and Antioxidant Activity of Italian Monovarietal Extra Virgin Olive Oils. Antioxidants 2019, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Taticchi, A.; Esposto, S.; Veneziani, G.; Urbani, S.; Selvaggini, R.; Servili, M. The Influence of the Malaxation Temperature on the Activity of Polyphenoloxidase and Peroxidase and on the Phenolic Composition of Virgin Olive Oil. Food Chem. 2013, 136, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.; Morozzi, G. Health and sensory Properties of Virgin Olive Oil Hydrophilic Phenols: Agronomic and Technological Aspects of Production That Affect Their Occurrence in The Oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Bayram, B.; Esatbeyoglu, T.; Schulze, N.; Ozcelik, B.; Frank, J.; Rimbach, G. Comprehensive Analysis of Polyphenols in 55 Extra Virgin Olive Oils by HPLC-ECD and Their Correlation with Antioxidant Activities. Plant Foods Hum. Nutr. 2012, 67, 326–336. [Google Scholar] [CrossRef]
- Klisović, D.; Novoselić, A.; Lukić, I.; Brkić Bubola, K. Extra Virgin Olive Oil under Simulated Consumption Conditions: Evaluation of Quality, Health, and Flavour Properties. J. Food Compos. Anal. 2022, 110, 104570. [Google Scholar] [CrossRef]
- Esposto, S.; Taticchi, A.; Servili, M.; Urbani, S.; Sordini, B.; Veneziani, G.; Daidone, L.; Selvaggini, R. Overall Quality Evolution of Extra Virgin Olive Oil Exposed to Light for 10 Months in Different Containers. Food Chem. 2021, 351, 129297. [Google Scholar] [CrossRef]
- Baccouri, B.; Rajhi, I.; Zarrouk, M. Bioactive compounds and Oxidative Stability of Feral Olive Oils from Tunisian Amazigh Mountains using LC-ESI-QTOF-MS Approach for the Development of Innovative Food Products. Eur. Food Res. Technol. 2022, 248, 2843–2855. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Williams, M.; Harwood, J. Changes in Virgin Olive Oil Characteristics during Different Storage Conditions. Eur. J. Lipid Sci. Technol. 2010, 112, 906–914. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic Molecules in Virgin Olive Oils: A Survey of Their Sensory Properties, Health Effects, Antioxidant Activity and Analytical Methods. An Overview of the Last Decade Alessandra. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [Green Version]
- Borges, T.H.; Serna, A.; López, L.C.; Lara, L.; Nieto, R.; Seiquer, I. Composition and Antioxidant Properties of Spanish Extra Virgin Olive Oil regarding Cultivar, Harvest Year and Crop Stage. Antioxidants 2019, 8, 217. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Carrasco-Pancorbo, A.; Simal-Gándara, J.; Giampieri, F.; et al. Characterization of Phenolic Extracts from Brava Extra Virgin Olive Oils and Their Cytotoxic Effects on MCF-7 Breast Cancer Cells. Food Chem. Toxicol. 2018, 119, 73–85. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive Oil Volatile Compounds, Flavour Development and Quality: A Critical Review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Urbani, S.; Di Maio, I.; Sordini, B.; Servili, M. Flash Thermal Conditioning of Olive Pastes during the Oil Mechanical Extraction Process: Cultivar Impact on the Phenolic and Volatile Composition of Virgin Olive Oil. J. Agric. Food Chem. 2015, 63, 6066–6074. [Google Scholar] [CrossRef]
- Tomé-Rodríguez, S.; Ledesma-Escobar, C.A.; Penco-Valenzuela, J.M.; Priego-Capote, F. Cultivar Influence on the Volatile Components of Olive Oil Formed in the Lipoxygenase Pathway. LWT 2021, 147, 111485. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Sacchi, R. Temporal Changes of Virgin Olive Oil Volatile Compounds in a Model System Simulating Domestic Consumption: The Role of Biophenols. Int. Food Res. J. 2015, 77, 670–674. [Google Scholar] [CrossRef]
- Marx, Í.M.G.; Casal, S.; Rodrigues, N.; Cruz, R.; Peres, F.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Impact of fresh Olive Leaves Addition during the Extraction of Arbequina Virgin Olive Oils on the Phenolic and Volatile Profiles. Food Chem. 2022, 393, 133327. [Google Scholar] [CrossRef]
- Ergönül, P.G.; Aydar, A.Y.; Göldeli, T.; Mentana, A.; Quinto, M. Changes in Volatile Compounds of Ayvalık (Edremit) and Uslu Olive Oils Depending on Conditions and Time of Storage. Ukr. Food J. 2021, 10, 717–735. [Google Scholar] [CrossRef]
- Kesen, S.; Kelebek, H.; Sen, K.; Ulas, M.; Selli, S. GC-MS-Olfactometric Characterization of the Key Aroma Compounds in Turkish Olive Oils by Application of the Aroma Extract Dilution Analysis. Int. Food Res. J. 2013, 54, 1987–1994. [Google Scholar] [CrossRef]
- Cecchi, T.; Alfei, B. Volatile Profiles of Italian Monovarietal Extra Virgin Olive Oils Via HS-SPME-GC-MS: Newly Identified Compounds, Flavors Molecular Markers, and Terpenic Profile. Food Chem. 2013, 141, 2025–2035. [Google Scholar] [CrossRef]
- Karagoz, S.G.; Yilmazer, M.; Ozkan, G.; Carbonell-Barrachina, Á.A.; Kiralan, M.; Ramadan, M.F. Effect of Cultivar and Harvest Time on C6 and C5 Volatile Compounds of Turkish Olive Oils. Eur. Food Res. Technol. 2017, 243, 1193–1200. [Google Scholar] [CrossRef]
- International Olive Council (IOC). Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-T20-Doc.-15-REV-10-2018-Eng.pdf (accessed on 15 October 2022).
- Caporale, G.; Policastro, S.; Monteleone, E. Bitterness Enhancement Induced by Cut Grass Odorant (cis-3-hexen-1-ol) in a Model Olive Oil. Food Qual. Prefer. 2004, 15, 219–227. [Google Scholar] [CrossRef]
- Republic of Turkey Ministry of Agriculture and Forestry. Turkey Olive Varieties Catalog; Olive Research Institute: İzmir, Turkey, 2015; pp. 5–56. [Google Scholar]
- Republic of Turkey Ministry of Agriculture and Forestry. Available online: https://www.tarimorman.gov.tr/BUGEM/kumelenme/Belgeler/Budama/Zeytinde%20%C3%87e%C5%9Fit%20Tan%C4%B1lama.pdf (accessed on 28 January 2023).
- International Olive Council (IOC). Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-OH-Doc.-1-2011-Eng.pdf (accessed on 27 October 2021).
- Turkish Official Gazette. Available online: https://www.resmigazete.gov.tr/eskiler/2014/11/20141120-21.htm (accessed on 20 October 2021).
- International Olive Council (IOC). Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/06/Doc.-No-29-REV-2_ENK.pdf (accessed on 15 December 2021).
- Rodrigues, N.; Casal, S.; Pinho, T.; Peres, A.M.; Bento, A.; Baptista, P.; Pereira, J.A. Ancient Olive Trees as a Source of Olive Oils Rich in Phenolic Compounds. Food Chem. 2019, 276, 231–239. [Google Scholar] [CrossRef]
- Capanoglu, E.; de Vos, R.C.H.; Hall, R.D.; Boyacioglu, D.; Beekwilder, J. Changes in polyphenol Content during Production of Grape Juice Concentrate. Food Chem. 2013, 139, 521–526. [Google Scholar] [CrossRef]
- Osei, J.B.D.; Amiri, A.; Wang, J.; Tavares, M.T.; Kiatkittipong, W.; Najdanovic-Visak, V. Recovery of oils and antioxidants from olive stones. Biomass Bioenergy 2022, 166, 106623. [Google Scholar] [CrossRef]
- Korkmaz, A.; Atasoy, A.F.; Hayaloglu, A.A. Changes in volatile Compounds, Sugars and Organic Acids of Different Spices of Peppers (Capsicum annuum L.) during Storage. Food Chem. 2020, 311, 125910. [Google Scholar] [CrossRef]





| Parameter | Edincik | Ayvalık | Domat | Uslu | Gemlik |
|---|---|---|---|---|---|
| FFA (% oleic acid) | 0.09 ± 0.01 b | 0.12 ± 0.01 a | 0.06 ± 0.00 c | 0.06 ± 0.01 c | 0.05 ± 0.00 c |
| PV (meq O2·kg−1) | 5.23 ± 0.79 ab | 6.24 ± 0.23 a | 5.45 ± 0.16 ab | 3.50 ± 0.27 c | 6.33 ± 0.72 a |
| K232 | 1.78 ± 0.04 c | 1.89 ± 0.01 b | 1.99 ± 0.02 a | 1.74 ± 0.02 c | 1.64 ± 0.01 d |
| K270 | 0.18 ± 0.02 a | 0.16 ± 0.01 b | 0.16 ± 0.01 b | 0.18 ± 0.01 a | 0.13 ± 0.00 c |
| Chlorophylls (mg·kg−1) | 3.60 ± 0.12 c | 5.52 ± 0.01 a | 1.92 ± 0.16 d | 4.53 ± 0.11 b | 1.84 ± 0.12 d |
| Carotenoids (mg·kg−1) | 1.99 ± 0.04 b | 4.39 ± 0.01 a | 1.59 ± 0.06 c | 4.47 ± 0.02 a | 1.67 ± 0.05 c |
| Total pigments (mg·kg−1) | 5.58 ± 0.16 c | 9.90 ± 0.02 a | 3.51 ± 0.22 d | 9.00 ± 0.13 a | 3.51 ± 0.18 d |
| Fatty Acid | Edincik | Ayvalık | Domat | Uslu | Gemlik |
|---|---|---|---|---|---|
| Oleic acid (C18:1) | 69.73 ± 0.04 d | 70.21 ± 0.02 b | 70.08 ± 0.03 c | 67.87 ± 0.05 e | 74.13 ± 0.01 a |
| Palmitic acid (C16:0) | 12.88 ± 0.01 e | 14.58 ± 0.01 a | 14.10 ± 0.02 c | 14.51 ± 0.02 b | 13.84 ± 0.01 d |
| Linoleic Acid (C18:2) | 10.80 ± 0.06 b | 9.47 ± 0.02 d | 10.10 ± 0.02 c | 11.11 ± 0.02 a | 5.43 ± 0.02 e |
| Stearic acid (C18:0) | 2.98 ± 0.01 a | 2.26 ± 0.00 d | 1.96 ± 0.01 e | 2.53 ± 0.01 c | 2.74 ± 0.01 b |
| Linolenic acid (C18:3) | 0.94 ± 0.01 b | 0.68 ± 0.01 b | 0.94 ± 0.01 a | 0.95 ± 0.02 a | 0.68 ± 0.01 a |
| 9-Hexadecenoic acid (C16:1) | 0.74 ± 0.01 d | 0.88 ± 0.00 c | 1.10 ± 0.00 a | 0.93 ± 0.03 b | 0.96 ± 0.01 b |
| Heptadecanoic acid (C17:0) | 0.20 ± 0.01 a | 0.15 ± 0.02 b | 0.12 ± 0.01 b | 0.15 ± 0.01 b | 0.15 ± 0.01 b |
| 10-Heptadecanoic acid (C17:1) | 0.26 ± 0.01 a | 0.25 ± 0.00 a | 0.24 ± 0.01 b | 0.25 ± 0.01 ab | 0.23 ± 0.00 b |
| Eicosanoic acid (C20:0) | 0.40 ± 0.06 ab | 0.44 ± 0.01 a | 0.34 ± 0.00 b | 0.44 ± 0.01 a | 0.46 ± 0.01 a |
| 11-Eicosenoic acid (C20:1) | 0.30 ± 0.04 a | 0.32 ± 0.00 a | 0.29 ± 0.01 a | 0.28 ± 0.00 a | 0.28 ± 0.01 a |
| Docosanoic acid (C22:0) | 0.12 ± 0.00 a | 0.13 ± 0.00 a | 0.10 ± 0.00 b | 0.12 ± 0.00 a | 0.13 ± 0.01 a |
| Tricosanoic acid (C23:0) | 0.62 ± 0.01 c | 0.52 ± 0.01 e | 0.56 ± 0.01 d | 0.76 ± 0.01 b | 0.82 ± 0.01 a |
| SFAs | 17.27 ± 0.05 c | 18.15 ± 0.03 b | 17.25 ± 0.01 c | 18.57 ± 0.01 a | 18.23 ± 0.03 b |
| PUFAs | 11.73 ± 0.05 b | 10.15 ± 0.03 d | 11.03 ± 0.01 c | 12.05 ± 0.03 a | 6.10 ± 0.02 e |
| MUFAs | 71.02 ± 0.01 c | 71.67 ± 0.02 b | 71.71 ± 0.02 b | 69.34 ± 0.02 d | 75.61 ± 0.03 a |
| MUFAs/PUFAs | 6.06 ± 0.025 d | 7.07 ± 0.015 b | 6.51 ± 0.005 c | 5.76 ± 0.015 e | 12.4 ± 0.03 a |
| Phenolic Compound | Edincik | Ayvalık | Domat | Uslu | Gemlik |
|---|---|---|---|---|---|
| Hydroxytyrosol | 48.022 ± 1.186 a | 11.621 ± 0.420 c | 11.646 ± 0.229 c | 15.679 ± 1.735 b | 18.165 ± 0.592 b |
| Oleuropein | 10.351 ± 1.139 b | 2.317 ± 0.235 c | 10.604 ± 0.642 b | 13.722 ± 0.416 a | 2.415 ± 0.239 c |
| Pinoresinol | 6.899 ± 0.366 d | 15.247 ± 0.524 a | 7.265 ± 0.355 d | 13.263 ± 0.376 b | 10.499 ± 0.837 c |
| Apigenin | 2.409 ± 0.030 e | 6.521 ± 0.212 c | 11.034 ± 0.204 b | 4.679 ± 0.348 d | 12.824 ± 0.010 a |
| Luteolin | 1.640 ± 0.089 c | 2.658 ± 0.080 a | 2.358 ± 0.050 b | 1.833 ± 0.107 c | 1.850 ± 0.043 c |
| Tyrosol | 1.403 ± 0.023 d | 3.500 ± 0.109 a | 1.887 ± 0.066 c | 2.017 ± 0.020 c | 2.477 ± 0.111 b |
| Vanillic acid | 1.991 ± 0.031 a | 1.703 ± 0.045 ab | 1.550 ± 0.037 b | 1.445 ± 0.220 b | 1.617 ± 0.024 ab |
| p-Qumaric acid | 0.079 ± 0.006 b | 0.715 ± 0.005 a | 0.644 ± 0.011 a | 0.797 ± 0.152 a | 0.233 ± 0.005 b |
| t-Ferrulic acid | 0.095 ± 0.020 e | 0.944 ± 0.016 a | 0.194 ± 0.006 d | 0.537 ± 0.028 b | 0.342 ± 0.034 c |
| Catechin | 0.440 ± 0.020 d | 0.294 ± 0.025 a | 0.623 ± 0.033 c | 0.644 ± 0.036 b | 0.086 ± 0.005 c |
| Caffeic acid | 0.009 ± 0.001 d | 0.184 ± 0.002 a | 0.033 ± 0.001 c | 0.129 ± 0.001 b | 0.034 ± 0.001 c |
| TPC | 294.090 ± 13.810 c | 234.71 ± 1.490 d | 350.6 ± 8.770 a | 291.145 ± 5.485 c | 321.905 ± 6.185 b |
| No | Volatile Compound | RI 1 | Edincik | Ayvalık | Domat | Uslu | Gemlik |
|---|---|---|---|---|---|---|---|
| Terpenoids | 7.584 a | 3.8905 b | 3.218 b | 3.765 b | 3.994 b | ||
| 1 | (Z)- β-Ocimene | 1245 | 1.642 a | 0.183 b | 1.534 a | 1.707 a | 0.129 b |
| 2 | α-Bergamotene | 1596 | nd | 1.029 a | nd | nd | nd |
| 3 | β-Sesquiphellandrene | 1730 | nd | 0.462 a | nd | nd | nd |
| 4 | α-Muurolene | 1741 | 0.692 a | 0.259 b | nd | 0.174 b | nd |
| 5 | α-Curcumene | 1788 | 0.264 ab | 0.627 a | nd | nd | 0.214 ab |
| 6 | α -Copaene | 1506 | 3.056 a | 0.391 c | 0.628 c | 1.024 b | 0.713 bc |
| 7 | Cyclosativene | 1499 | 0.887 a | 0.440 b | nd | nd | nd |
| 8 | (E)-4,8-Dimethyl-1,3,7-nonatriene | 1322 | 1.042 b | 0.499 a | 1.056 b | 0.860 bc | 2.938 a |
| Alcohols | 7.455 c | 14.391 b | 11.713 bc | 15.995 b | 23.793 a | ||
| 9 | Ethanol | 993 | nd | nd | 0.238 a | 0.096 bc | 0.175 ab |
| 10 | 1-Penten-3-ol | 1179 | nd | 3.930 c | 4.162 c | 8.797 b | 15.681 a |
| 11 | 1-Pentanol | 1243 | 2.351 b | 3.278 ab | 2.416 ab | 4.182 ab | 4.933 a |
| 12 | (Z)-2-Penten-1-ol | 1331 | 0.450 c | 1.013 ab | 0.681 bc | 0.858 ab | 1.259 a |
| 13 | 1-Hexanol | 1361 | 0.530 c | 1.466 a | 0.792 b | 0.438 c | 0.250 d |
| 14 | (Z)-3-Hexen-1-ol | 1392 | 3.061 b | 3.920 a | 1.574 c | 1.625 c | 1.495 c |
| 15 | (E)-2-Hexen-1-ol | 1413 | 1.062 a | nd | 0.517 b | nd | nd |
| 16 | 2-Ethylhexanol | 1492 | nd | 0.383 b | 0.582 a | nd | nd |
| 17 | Phenylmethanol | 1884 | nd | nd | 0.484 a | nd | nd |
| 18 | Phenylethanol | 1922 | nd | 0.402 a | 0.267 b | nd | nd |
| Aldehydes | 67.029 ab | 25.788 c | 68.165 a | 61.976 ab | 55.620 b | ||
| 19 | Pentanal | 1030 | nd | nd | nd | 0.401 a | nd |
| 20 | Hexanal | 1080 | 3.939 ab | 3.218 bc | 2.864 c | 4.180 a | 2.362 c |
| 21 | (E)-2-Pentenal | 1130 | 0.220 a | 0.343 a | 0.265 a | 0.343 a | 0.313 a |
| 22 | 3-Hexenal | 1132 | 11.026 b | 7.248 c | 6.609 c | 9.152 bc | 17.237 a |
| 23 | (E)-2-Hexenal | 1228 | 48.964 b | 9.558 d | 54.485 a | 44.874 b | 33.647 c |
| 24 | (E)-2-Heptenal | 1341 | nd | nd | 0.402 a | nd | nd |
| 25 | Nonanal | 1408 | 0.540 a | 0.386 a | 0.350 a | nd | nd |
| 26 | 2,4-Hexanedienal | 1413 | 2.339 ab | 4.160 a | 2.958 ab | 3.025 ab | 1.703 b |
| 27 | Benzaldehyde | 1541 | nd | 0.382 a | 0.232 b | nd | nd |
| 28 | Pyran aldehyde | 1611 | nd | 0.493 a | nd | nd | nd |
| 29 | (E)-2-Decenal | 1658 | nd | nd | nd | nd | 0.358 a |
| Esters | 0.388 b | 7.3185 a | nd | 0.378 b | 0.357 b | ||
| 30 | Hexyl acetate | 1291 | nd | 1.282 a | nd | nd | 0.356 b |
| 31 | (E)-3-Hexen-1-ol acetate | 1334 | 0.388 b | 6.036 a | nd | 0.378 b | nd |
| Acids | 0.144 a | 0.188 a | nd | nd | nd | ||
| 32 | Octanoic acid | 2094 | nd | 0.188 a | nd | nd | nd |
| 33 | Decanoic acid | 2228 | 0.144 a | nd | nd | nd | nd |
| Miscellaneous | 1.156 b | 0.794 b | 1.981 b | 5.872 a | 1.281 b | ||
| 34 | Methylbenzene | nd | nd | nd | 0.536 a | 0 | |
| 35 | 3-Ethyl-1,5-octadiene | 1053 | 0.715 ab | 0.400 bc | 0.770 a | 0.278 c | 0.652 ab |
| 36 | 1,2-Dimethyl benzene | 1183 | 0.181 b | nd | 0.641 b | 4.805 a | 0.629 b |
| 37 | Pyridine | 1194 | nd | nd | 0.200 a | nd | nd |
| 38 | 5-Ethyl-2(5H)-furanone | 1610 | 0.260 a | 0.394 a | 0.370 a | 0.252 a | nd |
| Total | 83.754 a | 52.368 b | 85.077 a | 87.987 a | 85.044 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korkmaz, A. Characterization and Comparison of Extra Virgin Olive Oils of Turkish Olive Cultivars. Molecules 2023, 28, 1483. https://doi.org/10.3390/molecules28031483
Korkmaz A. Characterization and Comparison of Extra Virgin Olive Oils of Turkish Olive Cultivars. Molecules. 2023; 28(3):1483. https://doi.org/10.3390/molecules28031483
Chicago/Turabian StyleKorkmaz, Aziz. 2023. "Characterization and Comparison of Extra Virgin Olive Oils of Turkish Olive Cultivars" Molecules 28, no. 3: 1483. https://doi.org/10.3390/molecules28031483