A New Route of Valorization of Petrochemical Wastewater: Recovery of 1,3,5-Tris (4-tert-butyl-3-hydroxy-2,6-dimethyl benzyl)–1,3,5-triazine-2,4,6-(1H,3H,5H)-trione (Cyanox 1790) and Its Subsequent Application in a PP Matrix to Improve Its Thermal Stability
Abstract
:1. Introduction
2. Analysis and Discussion
2.1. Identification, Quantification, Repeatability, Reproducibility, and Linearity Analysis of Multiple Cyanox 1790 Standards
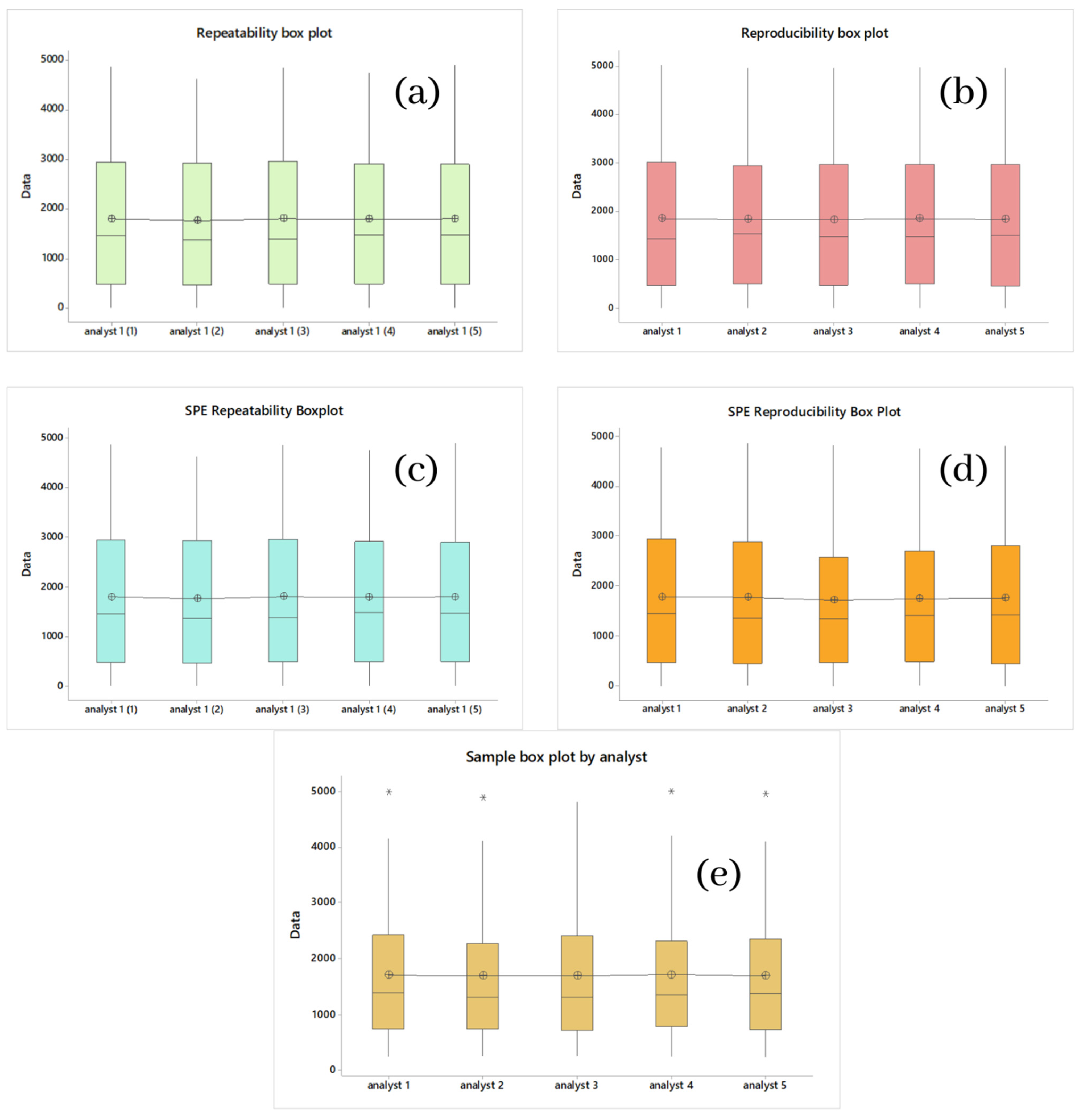
2.1.1. Multistandard Repeatability Analysis of Cyanox 1790 in CH2Cl2 and Multistandard per SPE in CH2CN
2.1.2. Multistandard Reproducibility Analysis of Cyanox 1790 in CH2Cl2 and Multistandard by SPE in CH2CN
2.1.3. Repeatability Linearity and Reproducibility of Cyanox 1790 Multistandards
2.2. Identification, Quantification, Repeatability, Reproducibility, and Linearity Analysis of Cyanox 1790 in Industrial Wastewater Samples
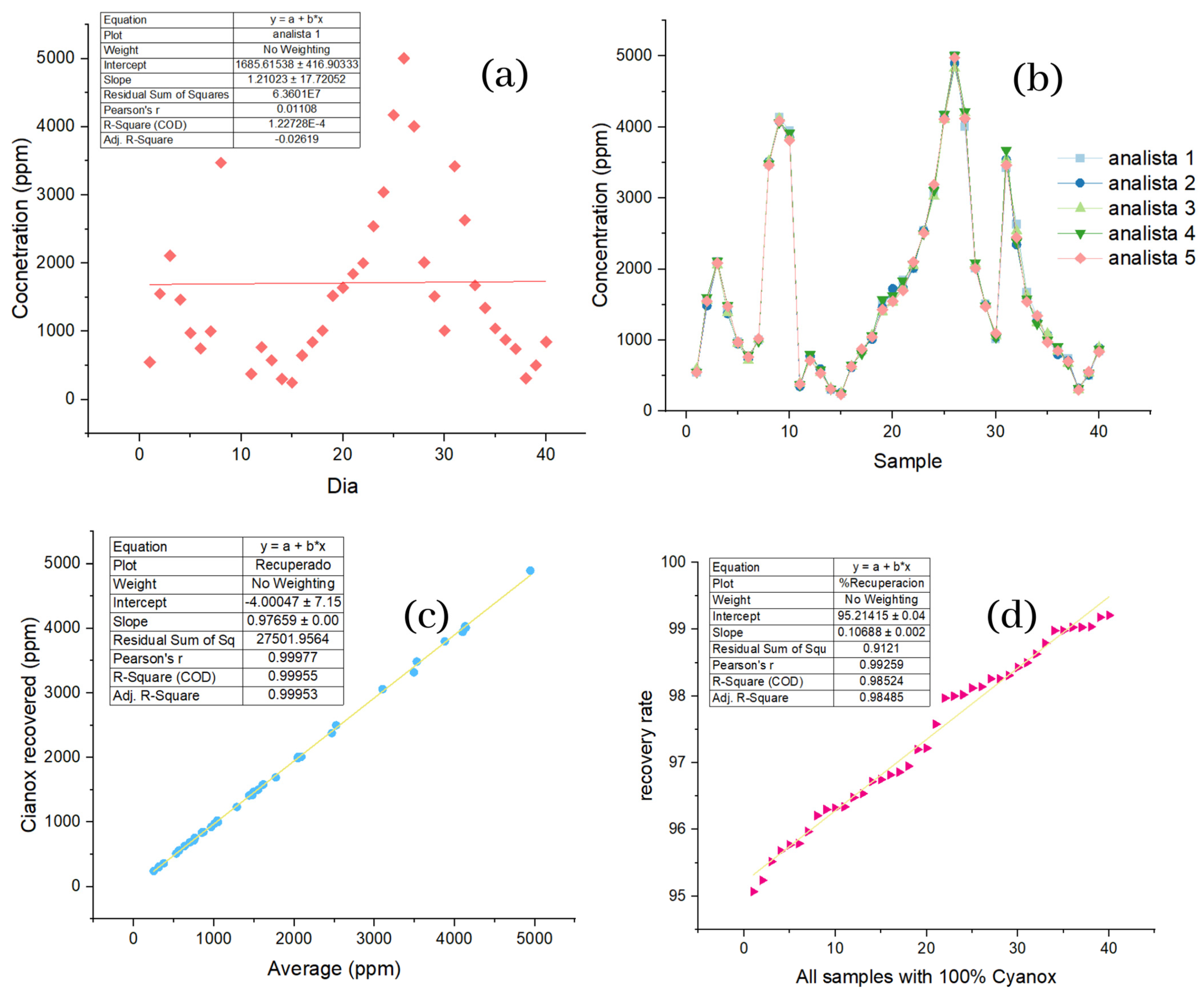
2.2.1. Reproducibility Analysis Industrial Wastewater Samples
2.2.2. Linearity and Distribution of Industrial Wastewater Sample Data
2.3. Characterization of Recovered Dust and Comparison with a Pattern
2.3.1. Thermogravimetry Analysis (TGA) of Pure Standard and Recovered Dust
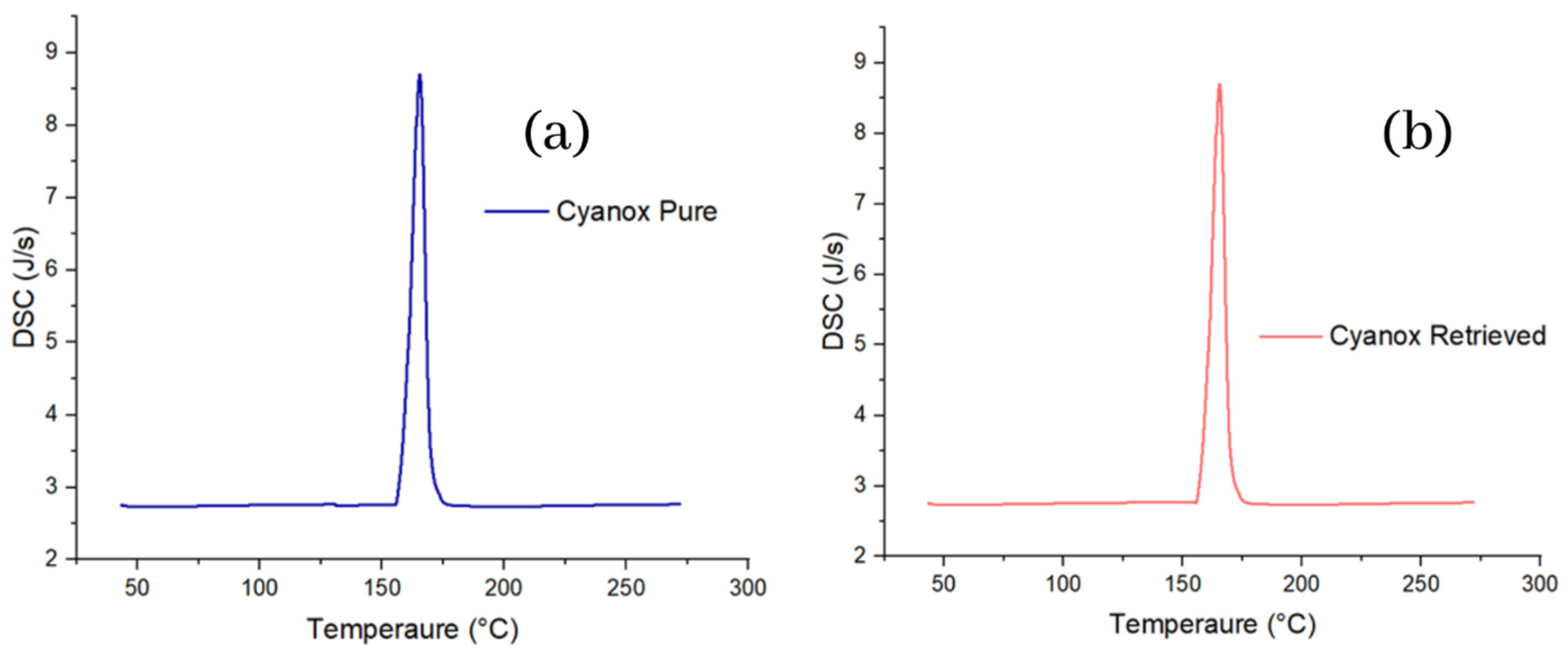
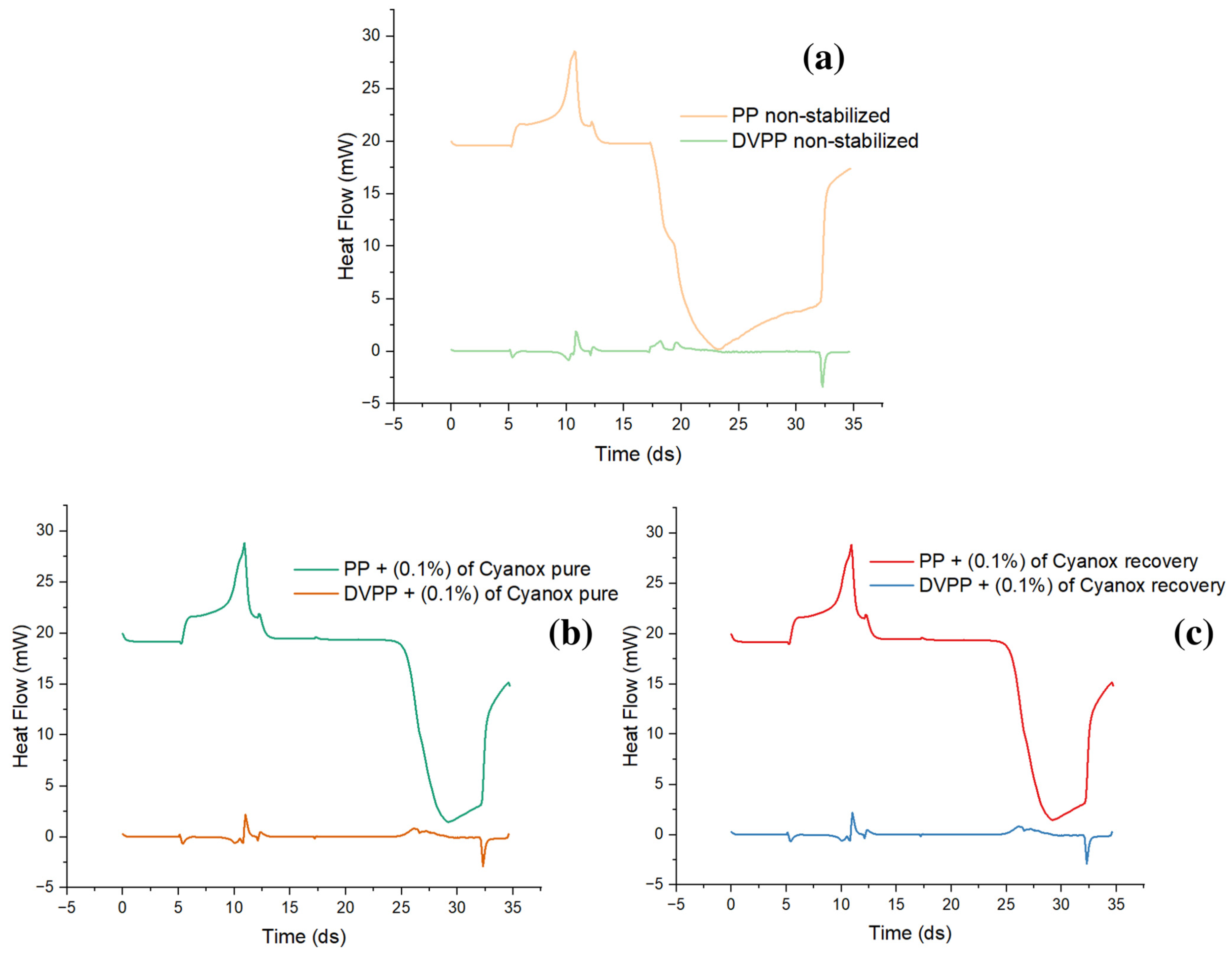
2.3.2. Pure Standard DSC Analysis and Recovered Dust
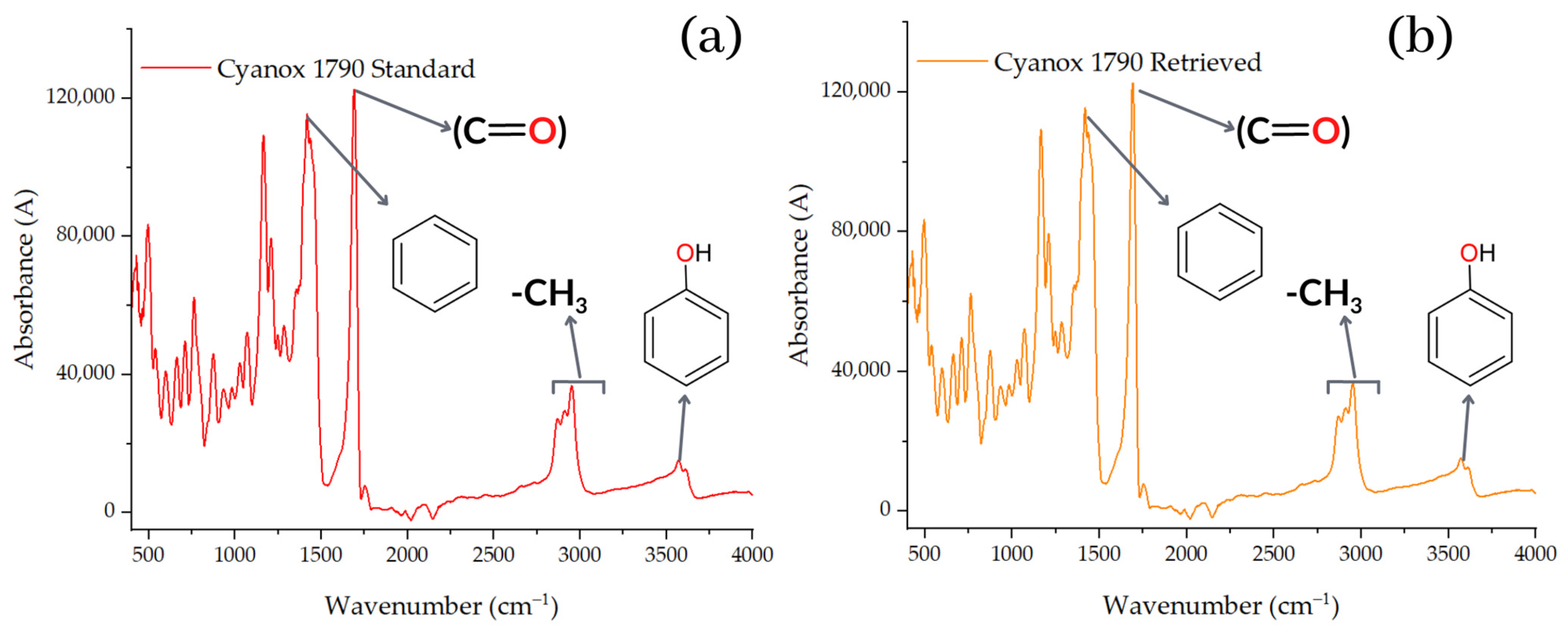
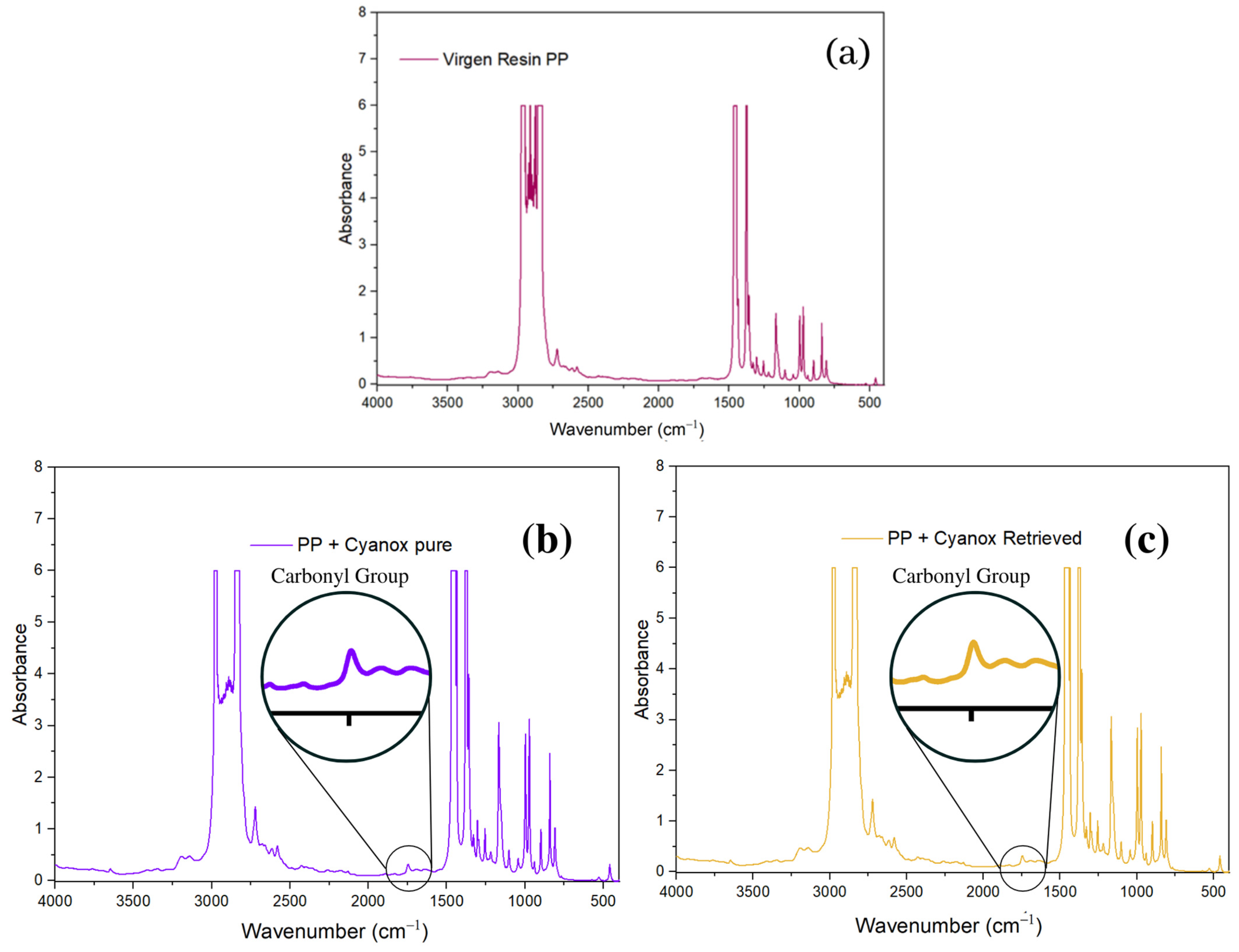
2.3.3. Pure Standard FTIR Analysis and Recovered Dust
2.3.4. Use of Recovered Dust and Application to Polypropylene Resins to Evaluate Their Efficiency
2.3.5. OIT PP, (PP + Cyanox Pure), and (PP + Cyanox Recovered)
3. Materials and Methods
3.1. Sampling
3.2. Extraction Solid Phases (SPE)
3.3. HPLC-DAD/MS/MS (High-Performance Liquid Chromatography with Diode Array Detector and Mass Spectrometry)
3.4. The Recovered Additive Is Added to the PP Matrix
3.4.1. Preparation of PP Sampling
3.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.3. Differential Scanning Calorimeter (DSC)
3.4.4. Analysis by Thermogravimetry (TGA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Marquès, M.; Domingo, J.L.; Nadal, M.; Schuhmacher, M. Health risks for the population living near petrochemical industrial complexes. 2. Adverse health outcomes other than cancer. Sci. Total. Environ. 2020, 730, 139122. [Google Scholar] [CrossRef]
- Nyashina, G.; Kuznetsov, G.; Strizhak, P. Effects of plant additives on the concentration of sulfur and nitrogen oxides in the combustion products of coal-water slurries containing petrochemicals. Environ. Pollut. 2019, 258, 113682. [Google Scholar] [CrossRef]
- Fernández, J.H.; Rincón, D.; López-Martínez, J. Development and validation of a prototype for the on-line simultaneous analysis of quality caprolactam synthesized on an industrial scale. Methodsx 2022, 10, 101952. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Guerra, Y.; Espinosa, E. Development and Application of a Principal Component Analysis Model to Quantify the Green Ethylene Content in Virgin Impact Copolymer Resins During Their Synthesis on an Industrial Scale. J. Polym. Environ. 2022, 30, 4800–4808. [Google Scholar] [CrossRef]
- Kayyal-Tarabeia, I.; Blank, M.; Zick, A.; Agay-Shay, K. Residence near industrial complex and cancer incidence: A registry-based cohort of 1,022,637 participants with a follow-up of 21 years, Israel. Environ. Res. 2023, 216, 114471. [Google Scholar] [CrossRef]
- Lin, X.-Y.; Liu, Y.-X.; Zhang, Y.-J.; Shen, H.-M.; Guo, Y. Polycyclic aromatic hydrocarbon exposure and DNA oxidative damage of workers in workshops of a petrochemical group. Chemosphere 2022, 303, 135076. [Google Scholar] [CrossRef]
- Radelyuk, I.; Tussupova, K.; Klemeš, J.J.; Persson, K.M. Oil refinery and water pollution in the context of sustainable development: Developing and developed countries. J. Clean. Prod. 2021, 302, 126987. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Guerra, Y.; Puello-Polo, E.; Marquez, E. Effects of Different Concentrations of Arsine on the Synthesis and Final Properties of Polypropylene. Polymers 2022, 14, 3123. [Google Scholar] [CrossRef]
- Fernández, J.H.; Cano, H.; Guerra, Y.; Polo, E.P.; Ríos-Rojas, J.F.; Vivas-Reyes, R.; Oviedo, J. Identification and Quantification of Microplastics in Effluents of Wastewater Treatment Plant by Differential Scanning Calorimetry (DSC). Sustainability 2022, 14, 4920. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Cano-Cuadro, H.; Puello-Polo, E. Emission of Bisphenol A and Four New Analogs from Industrial Wastewater Treatment Plants in the Production Processes of Polypropylene and Polyethylene Terephthalate in South America. Sustainability 2022, 14, 10919. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Cano, H.; Rodríguez-Couto, S. Quantification and Removal of Volatile Sulfur Compounds (VSCs) in Atmospheric Emissions in Large (Petro) Chemical Complexes in Different Countries of America and Europe. Sustainability 2022, 14, 11402. [Google Scholar] [CrossRef]
- Barrington, D.; Prior, A.; Ho, G. The role of water auditing in achieving water conservation in the process industry. J. Clean. Prod. 2013, 52, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Hu, Y.; Lei, Y.; Wu, H.; Yang, G.; Wang, Y.; Wei, G. Vitrification of petrochemical sludge for rapid, facile, and sustainable fixation of heavy metals. J. Environ. Chem. Eng. 2022, 10, 108812. [Google Scholar] [CrossRef]
- Wang, S.; Kalkhajeh, Y.K.; Qin, Z.; Jiao, W. Spatial distribution and assessment of the human health risks of heavy metals in a retired petrochemical industrial area, south China. Environ. Res. 2020, 188, 109661. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, Z.; Cao, H.; Xu, M.; Xu, L. Concentrations, speciation, and ecological risk of heavy metals in the sediment of the Songhua River in an urban area with petrochemical industries. Chemosphere 2018, 219, 538–545. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Castro-Suarez, J.R.; Toloza, C.A.T. Iron Oxide Powder as Responsible for the Generation of Industrial Polypropylene Waste and as a Co-Catalyst for the Pyrolysis of Non-Additive Resins. Int. J. Mol. Sci. 2022, 23, 11708. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Vivas-Reyes, R.; Toloza, C.A.T. Experimental Study of the Impact of Trace Amounts of Acetylene and Methylacetylene on the Synthesis, Mechanical and Thermal Properties of Polypropylene. Int. J. Mol. Sci. 2022, 23, 12148. [Google Scholar] [CrossRef]
- Mesa, S.L.; Orjuela, J.; Ortega, A.; Sandoval, J.A. Revisión del panorama actual del manejo de agua de producción en la industria petrolera colombiana Review of the current state of wastewater management in the Colombian oil industry. Gestión Ambiente 2018, 21, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Kanu, I.; Achi, O. Industrial Effluents and Their Impact on Water Quality of Receiving Rivers in Nigeria Medical Microbiology View Project Fermented Food Development View Project. 2011. Available online: https://www.researchgate.net/publication/287104597 (accessed on 15 December 2022).
- Ghosh, P.; Samanta, A.N.; Ray, S. COD reduction of petrochemical industry wastewater using Fenton’s oxidation. Can. J. Chem. Eng. 2010, 88, 1021–1026. [Google Scholar] [CrossRef]
- Lee, W.-J.; Liow, M.-C.; Tsai, P.-J.; Hsieh, L.-T. Emission of polycyclic aromatic hydrocarbons from medical waste incinerators. Atmos. Environ. 2002, 36, 781–790. [Google Scholar] [CrossRef]
- Bonachela, S.; López, J.C.; Granados, M.R.; Magán, J.J.; Hernández, J.; Baille, A. Effects of gravel mulch on surface energy balance and soil thermal regime in an unheated plastic greenhouse. Biosyst. Eng. 2020, 192, 1–13. [Google Scholar] [CrossRef]
- Joaquin, H.-F.; Juan, L. Quantification of poisons for Ziegler Natta catalysts and effects on the production of polypropylene by gas chromatographic with simultaneous detection: Pulsed discharge helium ionization, mass spectrometry and flame ionization. J. Chromatogr. A 2019, 1614, 460736. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Puello-Polo, E.; Trilleras, J. Characterization of Microplastics in Total Atmospheric Deposition Sampling from Areas Surrounding Industrial Complexes in Northwestern Colombia. Sustainability 2022, 14, 13613. [Google Scholar] [CrossRef]
- Farzadkia, M.; Shahamat, Y.D.; Nasseri, S.; Mahvi, A.H.; Gholami, M.; Shahryari, A. Catalytic Ozonation of Phenolic Wastewater: Identification and Toxicity of Intermediates. J. Eng. 2014, 2014, 520929. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Fernandez, J.; Rodríguez, E. Determination of phenolic antioxidants additives in industrial wastewater from polypropylene production using solid phase extraction with high-performance liquid chromatography. J. Chromatogr. A 2019, 1607, 460442. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Lopez-Martinez, J.; Barceló, D. Quantification and elimination of substituted synthetic phenols and volatile organic compounds in the wastewater treatment plant during the production of industrial scale polypropylene. Chemosphere 2020, 263, 128027. [Google Scholar] [CrossRef]
- Hernández-Fernández, J. Quantification of oxygenates, sulphides, thiols and permanent gases in propylene. A multiple linear regression model to predict the loss of efficiency in polypropylene production on an industrial scale. J. Chromatogr. A 2020, 1628, 461478. [Google Scholar] [CrossRef]
- Wiles, D.M.; Scott, G. Polyolefins with controlled environmental degradability. Polym. Degrad. Stab. 2006, 91, 1581–1592. [Google Scholar] [CrossRef]
- Al-Malaika, S. Perspectives in Stabilisation of Polyolefins. In Long Term Properties of Polyolefins; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2004; pp. 121–150. [Google Scholar] [CrossRef]
- Pospíšil, J. Mechanistic Action of Phenolic Antioxidants in Polymers—A Review. Polym. Degrad. Stab. 1988, 20, 181–202. [Google Scholar] [CrossRef]
- Dương, T.-B.; Dwivedi, R.; Bain, L.J. 2,4-di-tert-butylphenol exposure impairs osteogenic differentiation. Toxicol. Appl. Pharmacol. 2023, 461, 116386. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Synthetic Phenolic Antioxidants: A Review of Environmental Occurrence, Fate, Human Exposure, and Toxicity. Environ. Sci. Technol. 2020, 54, 11706–11719. [Google Scholar] [CrossRef]
- Liu, R.; Mabury, S.A. Synthetic phenolic antioxidants and transformation products in dust from different indoor environments in Toronto, Canada. Sci. Total. Environ. 2019, 672, 23–29. [Google Scholar] [CrossRef]
- Makahleh, A.; Saad, B.; Bari, M. Synthetic phenolics as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 51–78. [Google Scholar] [CrossRef]
- Gonçalves-Filho, D.; De Souza, D. Detection of Synthetic Antioxidants: What Factors Affect the Efficiency in the Chromatographic Analysis and in the Electrochemical Analysis? Molecules 2022, 27, 7137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Q.; Zhang, Q.; Zuo, C.; Shi, H. An Overview of Chemical Additives on (Micro)Plastic Fibers: Occurrence, Release, and Health Risks. Rev. Environ. Contam. Toxicol. 2022, 22, 260. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef]
- Wang, W.; Asimakopoulos, A.G.; Abualnaja, K.O.; Covaci, A.; Gevao, B.; Johnson-Restrepo, B.; Kumosani, T.A.; Malarvannan, G.; Minh, T.B.; Moon, H.-B.; et al. Synthetic Phenolic Antioxidants and Their Metabolites in Indoor Dust from Homes and Microenvironments. Environ. Sci. Technol. 2015, 50, 428–434. [Google Scholar] [CrossRef]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Cui, S.; Yu, Y.; Zhan, T.; Gao, Y.; Zhang, J.; Zhang, L.; Ge, Z.; Liu, W.; Zhang, C.; Zhuang, S. Carcinogenic Risk of 2,6-Di-tert-Butylphenol and Its Quinone Metabolite 2,6-DTBQ Through Their Interruption of RARβ: In Vivo, In Vitro, and In Silico Investigations. Environ. Sci. Technol. 2021, 56, 480–490. [Google Scholar] [CrossRef]
- Aggelis, G.; Iconomou, D.; Christou, M.; Bokas, D.; Kotzailias, S.; Christou, G.; Tsagou, V.; Papanikolaou, S. Phenolic removal in a model olive oil mill wastewater using Pleurotus ostreatus in bioreactor cultures and biological evaluation of the process. Water Res. 2003, 37, 3897–3904. [Google Scholar] [CrossRef]
- Lee, Y.G.; Hwang, S.H.; Kim, S.D. Predicting the Toxicity of Substituted Phenols to Aquatic Species and Its Changes in the Stream and Effluent Waters. Arch. Environ. Contam. Toxicol. 2006, 50, 213–219. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Yavuz, Y.; Koparal, A.S.; Öğütveren, Ü.B. Treatment of petroleum refinery wastewater by electrochemical methods. Desalination 2010, 258, 201–205. [Google Scholar] [CrossRef]
- Universidad Iberoamericana. Estudios con Reconocimiento de Validez Oficial por Decreto Presidencial Del 3 de Abril de 1981 DORIAN PRATO GARCÍA; Universidad Iberoamericana: Mexico City, Mexico, 2007. [Google Scholar]
- Serna, I.; Torres, J. Recuperación de fenol de aguas residuales industriales por tratamiento con NaCl. Dyna 2003, 70, 25–34. [Google Scholar]
- Ma, J.; Xiao, R.; Li, J.; Yu, J.; Zhang, Y.; Chen, L. Determination of 16 polycyclic aromatic hydrocarbons in environmental water samples by solid-phase extraction using multi-walled carbon nanotubes as adsorbent coupled with gas chromatography–mass spectrometry. J. Chromatogr. A 2010, 1217, 5462–5469. [Google Scholar] [CrossRef]
- Galán-Cano, F.; Bernabé-Zafón, V.; Lucena, R.; Cárdenas, S.; Herrero-Martínez, J.M.; Ramis-Ramos, G.; Valcárcel, M. Sensitive determination of polycyclic aromatic hydrocarbons in water samples using monolithic capillary solid-phase extraction and on-line thermal desorption prior to gas chromatography–mass spectrometry. J. Chromatogr. A 2011, 1218, 1802–1807. [Google Scholar] [CrossRef]
- Gosetti, F.; Chiuminatto, U.; Mazzucco, E.; Robotti, E.; Calabrese, G.; Gennaro, M.C.; Marengo, E. Simultaneous determination of thirteen polycyclic aromatic hydrocarbons and twelve aldehydes in cooked food by an automated on-line solid phase extraction ultra high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 6308–6318. [Google Scholar] [CrossRef]
- Gao, Y.; Gu, Y.; Wei, Y. Determination of Polymer Additives–Antioxidants and Ultraviolet (UV) Absorbers by High-Performance Liquid Chromatography Coupled with UV Photodiode Array Detection in Food Simulants. J. Agric. Food Chem. 2011, 59, 12982–12989. [Google Scholar] [CrossRef]
- Arias, M.; Penichet, I.; Ysambertt, F.; Bauza, R.; Zougagh, M.; Ríos, Á. Fast supercritical fluid extraction of low- and high-density polyethylene additives: Comparison with conventional reflux and automatic Soxhlet extraction. J. Supercrit. Fluids 2009, 50, 22–28. [Google Scholar] [CrossRef]
- Burman, L.; Albertsson, A.-C.; Höglund, A. Solid-phase microextraction for qualitative and quantitative determination of migrated degradation products of antioxidants in an organic aqueous solution. J. Chromatogr. A 2005, 1080, 107–116. [Google Scholar] [CrossRef]
- Dopico, S.; Vilariño, J.M.L.; Rodríguez, M.V.G. Determination of antioxidants by solid-phase extraction method in aqueous food simulants. Talanta 2005, 66, 1103–1107. [Google Scholar] [CrossRef]
- Joaquin, H.-F.; Juan, L.-M. Autocatalytic influence of different levels of arsine on the thermal stability and pyrolysis of polypropylene. J. Anal. Appl. Pyrolysis 2021, 161, 105385. [Google Scholar] [CrossRef]
- Pavón, C.; Aldas, M.; Hernández-Fernández, J.; López-Martínez, J. Comparative characterization of gum rosins for their use as sustainable additives in polymeric matrices. J. Appl. Polym. Sci. 2022, 139, 51734. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Rayón, E.; López, J.; Arrieta, M.P. Enhancing the Thermal Stability of Polypropylene by Blending with Low Amounts of Natural Antioxidants. Macromol. Mater. Eng. 2019, 304, 1900379. [Google Scholar] [CrossRef]
- Thörnblom, K.; Palmlöf, M.; Hjertberg, T. The extractability of phenolic antioxidants into water and organic solvents from polyethylene pipe materials—Part I. Polym. Degrad. Stab. 2011, 96, 1751–1760. [Google Scholar] [CrossRef]
- Alsabri, A.; Tahir, F.; Al-Ghamdi, S.G. Environmental impacts of polypropylene (PP) production and prospects of its recycling in the GCC region. Mater. Today Proc. 2021, 56, 2245–2251. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; López-Martínez, J. Experimental Study of the Auto-Catalytic Effect of Triethylaluminum and TiCl4 Residuals at the Onset of Non-Additive Polypropylene Degradation and Their Impact on Thermo-Oxidative Degradation and Pyrolysis. J. Anal. Appl. Pyrolysis. 2021, 155, 105052. [Google Scholar] [CrossRef]
- Yu, W.; Reitberger, T.; Hjertberg, T.; Oderkerk, J.; Costa, F.; Englund, V.; Gedde, U. Chlorine dioxide resistance of different phenolic antioxidants in polyethylene. Polym. Degrad. Stab. 2014, 111, 1–6. [Google Scholar] [CrossRef]
- Reingruber, E.; Himmelsbach, M.; Sauer, C.; Buchberger, W. Identification of degradation products of antioxidants in polyolefins by liquid chromatography combined with atmospheric pressure photoionisation mass spectrometry. Polym. Degrad. Stab. 2010, 95, 740–745. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Chung, D. Antioxidant-Based Phase-Change Thermal Interface Materials with High Thermal Stability. J. Electron. Mater. 2008, 37, 448–461. [Google Scholar] [CrossRef] [Green Version]
- Aoyagi, Y.; Chung, D.D.L. Effects of antioxidants and the solid component on the thermal stability of polyol-ester-based thermal pastes. J. Mater. Sci. 2007, 42, 2358–2375. [Google Scholar] [CrossRef]
- Gómez-Contreras, P.; Figueroa-Lopez, K.J.; Hernández-Fernández, J.; Rodríguez, M.C.; Ortega-Toro, R. Effect of Different Essential Oils on the Properties of Edible Coatings Based on Yam (Dioscorea rotundata L.) Starch and Its Application in Strawberry (Fragaria vesca L.) Preservation. Appl. Sci. 2021, 11, 11057. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; López-Martínez, J.; Hernández-Fernández, J.; Arrieta, M. Films Based on Thermoplastic Starch Blended with Pine Resin Derivatives for Food Packaging. Foods 2021, 10, 1171. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Puello-Polo, E.; Castro-Suarez, J.R. Characterization of the Morphological and Chemical Profile of Different Families of Microplastics in Samples of Breathable Air. Molecules 2023, 28, 1042. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.H.; Guerra, Y.; Cano, H. Detection of Bisphenol A and Four Analogues in Atmospheric Emissions in Petrochemical Complexes Producing Polypropylene in South America. Molecules 2022, 27, 4832. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, H.E.; Yagoubi, N.; Ferrier, D. Extraction of Polypropylene Additives and Their Analysis by HPLC. Chromatographia 1998, 48, 491–496. [Google Scholar] [CrossRef]
- Jordan, S.L.; Taylor, L.T. HPLC Separation with Solvent Elimination FTIR Detection of Polymer Additives. J. Chromatogr. Sci. 1997, 35, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Francenia Santos Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant Compounds and Their Antioxidant Mechanism. In Antioxidants; Shalaby, E., Ed.; Intechopen: London, UK, 2019; pp. 1–28. [Google Scholar]
- Chacon, H.; Cano, H.; Fernández, J.H.; Guerra, Y.; Puello-Polo, E.; Ríos-Rojas, J.F.; Ruiz, Y. Effect of Addition of Polyurea as an Aggregate in Mortars: Analysis of Microstructure and Strength. Polymers 2022, 14, 1753. [Google Scholar] [CrossRef]
- Alladio, E.; Amante, E.; Bozzolino, C.; Seganti, F.; Salomone, A.; Vincenti, M.; Desharnais, B. Effective validation of chromatographic analytical methods: The illustrative case of androgenic steroids. Talanta 2020, 215, 120867. [Google Scholar] [CrossRef]
- Coutinho, F.M.B.; da Costa, M.; Simão, R.; Nicolini, L.F. Estudo da interação entre as fases da mistura poliestireno/elastômeros butadiênicos: Aspectos morfológicos e térmicos. In Proceedings of the 10° Congresso Brasileiro de Polímeros, Foz do Iguaçu, Brazil, 13–17 October 2009. [Google Scholar]
- Spectrabase. Cyanox 1790—FTIR—Spectrum—SpectraBase, 22 August 2022. CYANOX 1790 ANTIOXIDANT. Available online: https://spectrabase.com/spectrum/I3g5xqi3f4q (accessed on 18 January 2023).
- McMurry, J.; Mondragón, C.H.; Pozo, V.G. Química Orgánica, 8th ed.; Cengage Learning: Mexico City, Mexico, 2008. [Google Scholar]




| Cyanox Calibration Curve with Dichloromethane on the HPLC-MS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intraday Test (Same Day) | ||||||||||
| Theoretical | STDA | Analyst 1 | Analyst 1 | Analyst 1 | Analyst 1 | Analyst 1 | Average | Deviation | RSD | Error |
| 0 | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0 |
| 500 | 2 | 495.0 | 490.0 | 492.0 | 501.0 | 510.0 | 497.6 | 8.1 | 1.6 | 0.5 |
| 1000 | 3 | 942.0 | 972.0 | 981.0 | 1010.0 | 967.0 | 974.4 | 24.6 | 2.5 | 2.6 |
| 1500 | 4 | 1450.0 | 1514.0 | 1487.0 | 1491.0 | 1511.0 | 1490.6 | 25.6 | 1.7 | 0.6 |
| 2000 | 5 | 2012.0 | 1963.0 | 1976.0 | 1988.0 | 2013.0 | 1990.4 | 22.0 | 1.1 | 0.5 |
| 3000 | 6 | 2975.0 | 2989.0 | 3016.0 | 2991.0 | 2985.0 | 2991.2 | 15.2 | 0.5 | 0.3 |
| 5000 | 7 | 5021.0 | 4997.0 | 4987.0 | 5011.0 | 4991.0 | 5001.4 | 14.2 | 0.3 | 0.0 |
| Interday Test (Different Days) | ||||||||||
| Theoretical | STDA | Analyst 1 | Analyst 2 | Analyst 3 | Analyst 4 | Analyst 5 | Average | Deviation | RSD | Error |
| 0 | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0 |
| 500 | 2 | 475.0 | 501.0 | 476.0 | 501.0 | 453.0 | 481.2 | 20.3 | 4.2 | 3.8 |
| 1000 | 3 | 1015.0 | 947.0 | 958.0 | 1024.0 | 974.0 | 983.6 | 34.3 | 3.5 | 1.6 |
| 1500 | 4 | 1435.0 | 1542.0 | 1485.0 | 1476.0 | 1511.0 | 1489.8 | 40.0 | 2.7 | 0.7 |
| 2000 | 5 | 2024.0 | 1945.0 | 1975.0 | 2015.0 | 1986.0 | 1989.0 | 31.8 | 1.6 | 0.6 |
| 3000 | 6 | 3021.0 | 2946.0 | 2978.0 | 2976.0 | 2976.0 | 2979.4 | 26.8 | 0.9 | 0.7 |
| 5000 | 7 | 5032.0 | 4978.0 | 4967.0 | 4987.0 | 4969.0 | 4986.6 | 26.6 | 0.5 | 0.3 |
| SPE with Acetonitrile (HPLC-MS Results) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Intraday Test (Same Day) | |||||||||||
| Theoretical | STDA | Analyst 1 | Analyst 1 | Analyst 1 | Analyst 1 | Analyst 1 | Average | Deviation | RSD | Error | %Recovery |
| 0 | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0 | 0 |
| 500 | 2 | 478.0 | 462.0 | 486.0 | 498.0 | 490.0 | 482.8 | 13.7 | 2.8 | 3.4 | 97 |
| 1000 | 3 | 942.0 | 946.0 | 976.0 | 948.0 | 927.0 | 947.8 | 17.8 | 1.9 | 5.2 | 95 |
| 1500 | 4 | 1465.0 | 1375.0 | 1389.0 | 1486.0 | 1475.0 | 1438.0 | 51.9 | 3.6 | 4.1 | 96 |
| 2000 | 5 | 1929.0 | 1988.0 | 1976.0 | 1946.0 | 1900.0 | 1947.8 | 35.5 | 1.8 | 2.6 | 97 |
| 3000 | 6 | 2941.0 | 2937.0 | 2955.0 | 2913.0 | 2900.0 | 2929.2 | 22.3 | 0.8 | 2.4 | 98 |
| 5000 | 7 | 4876.0 | 4642.0 | 4863.0 | 4772.0 | 4912.0 | 4813.0 | 108.6 | 2.3 | 3.7 | 96 |
| Interday Test (Different Days) | |||||||||||
| Theoretical | STDA | Analyst 1 | Analyst 2 | Analyst 3 | Analyst 4 | Analyst 5 | Average | Deviation | RSD | Error | %Recovery |
| 0 | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0 | 0 |
| 500 | 2 | 468.0 | 452.0 | 472.0 | 488.0 | 451.0 | 466.2 | 15.4 | 3.3 | 6.8 | 93 |
| 1000 | 3 | 951.0 | 934.0 | 956.0 | 937.0 | 918.0 | 939.2 | 15.0 | 1.6 | 6.1 | 94 |
| 1500 | 4 | 1455.0 | 1356.0 | 1345.0 | 1425.0 | 1436.0 | 1403.4 | 49.6 | 3.5 | 6.4 | 94 |
| 2000 | 5 | 1941.0 | 1942.0 | 1900.0 | 1922.0 | 1942.0 | 1929.4 | 18.5 | 1.0 | 3.5 | 96 |
| 3000 | 6 | 2945.0 | 2901.0 | 2586.0 | 2712.0 | 2815.0 | 2791.8 | 145.5 | 5.2 | 6.9 | 93 |
| 5000 | 7 | 4786.0 | 4875.0 | 4825.0 | 4772.0 | 4821.0 | 4815.8 | 40.1 | 0.8 | 3.7 | 96 |
| Repeatability of Standards with CH2Cl2 | |||
|---|---|---|---|
| Factor | N | Average | Grouping |
| Analyst 1–3 | 7 | 1806 | A |
| Analyst 1–1 | 7 | 1804 | A |
| Analyst 1–5 | 7 | 1801 | A |
| Analyst 1–4 | 7 | 1795 | A |
| Analyst 1–2 | 7 | 1764 | A |
| Reproducibility of standards with CH2Cl2 | |||
| Analyst 1 | 7 | 1857 | A |
| Analyst 4 | 7 | 1854 | A |
| Analyst 5 | 7 | 1838 | A |
| Analyst 2 | 7 | 1837 | A |
| Analyst 3 | 7 | 1834 | A |
| Repeatability of standards with CH2CN in SPE | |||
| Analyst 1–3 | 7 | 1806 | A |
| Analyst 1–1 | 7 | 1804 | A |
| Analyst 1–5 | 7 | 1801 | A |
| Analyst 1–4 | 7 | 1795 | A |
| Analyst 1–2 | 7 | 1764 | A |
| Reproducibility of standards with CH2CN in SPE | |||
| Analyst 1 | 7 | 1792 | A |
| Analyst 4 | 7 | 1780 | A |
| Analyst 5 | 7 | 1769 | A |
| Analyst 2 | 7 | 1751 | A |
| Analyst 3 | 7 | 1726 | A |
| Reproducibility of samples with CH2CN in SPE | |||
| Analyst 1 | 40 | 1718 | A |
| Analyst 4 | 40 | 1710 | A |
| Analyst 5 | 40 | 1699 | A |
| Analyst 2 | 40 | 1696 | A |
| Analyst 3 | 40 | 1695 | A |
| Analysis of Final Samples with SPE with Acetonitrile (HPLC-MS Results) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | Sample | Analyst 1 | Analyst 2 | Analyst 3 | Analyst 4 | Analyst 5 | Average | Deviation | RSD | Error | %Recovery |
| 1 | 1 | 550 | 557 | 596 | 550 | 549 | 560.4 | 20.2 | 3.6 | 555 | 99.04 |
| 2 | 2 | 1552 | 1485 | 1561 | 1600 | 1548 | 1549.2 | 41.4 | 2.7 | 1500 | 96.82 |
| 3 | 3 | 2110 | 2078 | 2059 | 2115 | 2085 | 2089.4 | 23.2 | 1.1 | 2012 | 96.3 |
| 4 | 4 | 1465 | 1375 | 1389 | 1486 | 1475 | 1438 | 51.9 | 3.6 | 1413 | 98.26 |
| 5 | 5 | 978 | 948 | 956 | 967 | 975 | 964.8 | 12.7 | 1.3 | 924 | 95.77 |
| 6 | 6 | 748 | 733 | 715 | 791 | 765 | 750.4 | 29.3 | 3.9 | 718 | 95.68 |
| 7 | 7 | 1000 | 1012 | 1014 | 986 | 1021 | 1006.6 | 13.8 | 1.4 | 975 | 96.86 |
| 8 | 8 | 3474 | 3514 | 3520 | 3483 | 3465 | 3491.2 | 24.5 | 0.7 | 3319 | 95.07 |
| 9 | 9 | 4142 | 4085 | 4123 | 4065 | 4086 | 4100.2 | 31.4 | 0.8 | 3945 | 96.21 |
| 10 | 10 | 3945 | 3845 | 3865 | 3921 | 3811 | 3877.4 | 55 | 1.4 | 3800 | 98 |
| 11 | 11 | 375 | 348 | 396 | 373 | 382 | 374.8 | 17.5 | 4.7 | 361 | 96.32 |
| 12 | 12 | 768 | 777 | 745 | 800 | 715 | 761 | 32.4 | 4.3 | 755 | 99.21 |
| 13 | 13 | 575 | 596 | 542 | 585 | 536 | 566.8 | 26.5 | 4.7 | 561 | 98.98 |
| 14 | 14 | 300 | 312 | 324 | 317 | 313 | 313.2 | 8.8 | 2.8 | 310 | 98.98 |
| 15 | 15 | 245 | 263 | 260 | 246 | 235 | 249.8 | 11.6 | 4.6 | 241 | 96.48 |
| 16 | 16 | 645 | 615 | 635 | 650 | 631 | 635.2 | 13.6 | 2.1 | 630 | 99.18 |
| 17 | 17 | 842 | 865 | 849 | 812 | 876 | 848.8 | 24.5 | 2.9 | 834 | 98.26 |
| 18 | 18 | 1012 | 1015 | 1073 | 1062 | 1046 | 1041.6 | 27.4 | 2.6 | 1024 | 98.31 |
| 19 | 19 | 1522 | 1463 | 1400 | 1572 | 1429 | 1477.2 | 69.8 | 4.7 | 1415 | 95.79 |
| 20 | 20 | 1642 | 1725 | 1534 | 1629 | 1549 | 1615.8 | 77.4 | 4.8 | 1583 | 97.97 |
| 21 | 21 | 1842 | 1736 | 1742 | 1832 | 1700 | 1770.4 | 63 | 3.6 | 1691 | 95.52 |
| 22 | 22 | 2000 | 2015 | 2053 | 2075 | 2100 | 2048.6 | 41.4 | 2 | 2008 | 98.02 |
| 23 | 23 | 2541 | 2542 | 2530 | 2500 | 2510 | 2524.6 | 18.8 | 0.7 | 2500 | 99.03 |
| 24 | 24 | 3041 | 3145 | 3024 | 3108 | 3190 | 3101.6 | 69.7 | 2.2 | 3059 | 98.63 |
| 25 | 25 | 4174 | 4125 | 4108 | 4180 | 4110 | 4139.4 | 35 | 0.8 | 4013 | 96.95 |
| 26 | 26 | 5004 | 4900 | 4829 | 5010 | 4976 | 4943.8 | 77.7 | 1.6 | 4896 | 99.03 |
| 27 | 27 | 4005 | 4120 | 4200 | 4215 | 4119 | 4131.8 | 83.6 | 2 | 4032 | 97.58 |
| 28 | 28 | 2010 | 2042 | 2075 | 2090 | 2014 | 2046.2 | 35.8 | 1.7 | 1989 | 97.2 |
| 29 | 29 | 1512 | 1500 | 1498 | 1482 | 1475 | 1493.4 | 14.8 | 1 | 1470 | 98.43 |
| 30 | 30 | 1012 | 1042 | 1058 | 1043 | 1095 | 1050 | 30.2 | 2.9 | 1000 | 95.24 |
| 31 | 31 | 3421 | 3542 | 3541 | 3674 | 3465 | 3528.6 | 96.3 | 2.7 | 3486 | 98.79 |
| 32 | 32 | 2631 | 2345 | 2541 | 2384 | 2446 | 2469.4 | 116.8 | 4.7 | 2379 | 96.34 |
| 33 | 33 | 1674 | 1600 | 1631 | 1583 | 1543 | 1606.2 | 49.5 | 3.1 | 1576 | 98.12 |
| 34 | 34 | 1342 | 1263 | 1245 | 1234 | 1345 | 1285.8 | 53.7 | 4.2 | 1234 | 95.97 |
| 35 | 35 | 1042 | 1075 | 1093 | 1000 | 975 | 1037 | 49.5 | 4.8 | 1003 | 96.72 |
| 36 | 36 | 875 | 800 | 843 | 912 | 849 | 855.8 | 41.4 | 4.8 | 843 | 98.5 |
| 37 | 37 | 742 | 715 | 675 | 668 | 700 | 700 | 30.2 | 4.3 | 687 | 98.14 |
| 38 | 38 | 312 | 326 | 300 | 321 | 300 | 311.8 | 11.9 | 3.8 | 301 | 96.54 |
| 39 | 39 | 501 | 512 | 536 | 542 | 555 | 529.2 | 22.2 | 4.2 | 512 | 96.75 |
| 40 | 40 | 846 | 894 | 900 | 875 | 836 | 870.2 | 28.4 | 3.3 | 846 | 97.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Fernández, J.; Ortega-Toro, R.; López-Martinez, J. A New Route of Valorization of Petrochemical Wastewater: Recovery of 1,3,5-Tris (4-tert-butyl-3-hydroxy-2,6-dimethyl benzyl)–1,3,5-triazine-2,4,6-(1H,3H,5H)-trione (Cyanox 1790) and Its Subsequent Application in a PP Matrix to Improve Its Thermal Stability. Molecules 2023, 28, 2003. https://doi.org/10.3390/molecules28052003
Hernández-Fernández J, Ortega-Toro R, López-Martinez J. A New Route of Valorization of Petrochemical Wastewater: Recovery of 1,3,5-Tris (4-tert-butyl-3-hydroxy-2,6-dimethyl benzyl)–1,3,5-triazine-2,4,6-(1H,3H,5H)-trione (Cyanox 1790) and Its Subsequent Application in a PP Matrix to Improve Its Thermal Stability. Molecules. 2023; 28(5):2003. https://doi.org/10.3390/molecules28052003
Chicago/Turabian StyleHernández-Fernández, Joaquín, Rodrigo Ortega-Toro, and Juan López-Martinez. 2023. "A New Route of Valorization of Petrochemical Wastewater: Recovery of 1,3,5-Tris (4-tert-butyl-3-hydroxy-2,6-dimethyl benzyl)–1,3,5-triazine-2,4,6-(1H,3H,5H)-trione (Cyanox 1790) and Its Subsequent Application in a PP Matrix to Improve Its Thermal Stability" Molecules 28, no. 5: 2003. https://doi.org/10.3390/molecules28052003









