Influence of High-Pressure Homogenization on the Physicochemical Properties and Betalain Pigments of Red Beetroot (Beta vulgaris L.) Juice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Temperature Changes during the HPH Processing
2.2. Total Soluble Solids (TSS), pH and Titratable Acidity (TA)
2.3. Direct Turbidity and Serum Cloudiness
2.4. Influence of HPH on Juice Color and Viscosity
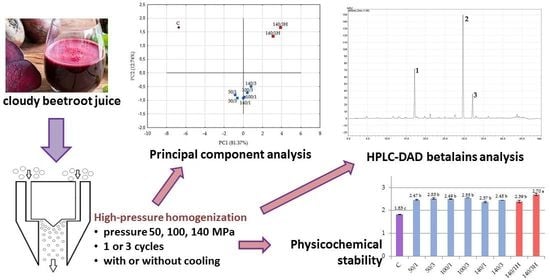
2.5. Qualitative and Quantitative Determinations of Betalains
2.6. Comprehensive Overwiew of All Samples—PCA Analysis Results
3. Materials and Methods
3.1. Juice Preparation and HPH Treatment
3.2. Analysis of Total Soluble Solids, pH and Titratable Acidity
3.3. Direct Turbidity and Serum Cloudiness
3.4. Viscosity Measurement
3.5. Color Parameters
3.6. Chromatographic Determination of Betalains
3.7. Spectrophotometric Quantification of Betalains
3.8. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Wang, Y.; Do, T.; Marshall, L.J.; Boesch, C. Effect of Two-Week Red Beetroot Juice Consumption on Modulation of Gut Microbiota in Healthy Human Volunteers—A Pilot Study. Food Chem. 2023, 406, 134989. [Google Scholar] [CrossRef] [PubMed]
- Aliahmadi, M.; Amiri, F.; Bahrami, L.S.; Hosseini, A.F.; Abiri, B.; Vafa, M. Effects of Raw Red Beetroot Consumption on Metabolic Markers and Cognitive Function in Type 2 Diabetes Patients. J. Diabetes Metab. Disord. 2021, 20, 673–682. [Google Scholar] [CrossRef] [PubMed]
- EUROSTAT Database. Available online: https://Ec.Europa.Eu/Eurostat/Web/Main/Data/Database (accessed on 27 December 2022).
- Clifford, T.; Howatson, G.; West, D.; Stevenson, E. The Potential Benefits of Red Beetroot Supplementation in Health and Disease. Nutrients 2015, 7, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological Effects of Red Beetroot and Betalains: A Review. Phytother. Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef] [PubMed]
- Ceclu, L.; Nistor, O.-V. Red Beetroot: Composition and Health Effects—A Review. J. Nutr. Med. Diet Care 2020, 6, 1–9. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Ryan, L. A Beetroot Juice Shot Is a Significant and Convenient Source of Bioaccessible Antioxidants. J. Funct. Foods 2011, 3, 329–334. [Google Scholar] [CrossRef]
- Fidelis, M.; Santos, J.S.; Coelho, A.L.K.; Rodionova, O.Y.; Pomerantsev, A.; Granato, D. Authentication of Juices from Antioxidant and Chemical Perspectives: A Feasibility Quality Control Study Using Chemometrics. Food Control 2017, 73, 796–805. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Sharma, P.; Gat, Y.; Panghal, A. Bioactive Compounds of Beetroot and Utilization in Food Processing Industry: A Critical Review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef]
- Naseer, S.; Hussain, S.; Abid, A. Betalain as a Food Colorant: Its Sources, Chemistry and Health Benefits. Proc. Pak. Acad. Sci. B (Life Environ. Sci.) 2019, 56, 31–37. [Google Scholar]
- Szopińska, A.A.; Gawęda, M. Comparison of Yield and Quality of Red Beet Roots Cultivated Using Conventional, Integrated and Organic Method. J. Hortic. Res. 2013, 21, 107–114. [Google Scholar] [CrossRef]
- Masih, D.; Singh, N.; Singh, A. Red Beetroot: A Source of Natural Colourant and Antioxidants: A Review. J. Pharmacogn. Phytochem. 2019, 8, 162–166. [Google Scholar]
- Roobab, U.; Shabbir, M.A.; Khan, A.W.; Arshad, R.N.; Bekhit, A.E.-D.; Zeng, X.-A.; Inam-Ur-Raheem, M.; Aadil, R.M. High-Pressure Treatments for Better Quality Clean-Label Juices and Beverages: Overview and Advances. LWT 2021, 149, 111828. [Google Scholar] [CrossRef]
- Fei, Y.; Yang, Z.; Niazi, S.; Chen, G.; Nasir, M.A.; Khan, I.M.; Rehman, A.; Aadil, R.M.; Trif, M.; Coşier, V. Proteolysis of β-Lactoglobulin Assisted by High Hydrostatic Pressure Treatment for Development of Polysaccharides-Peptides Based Coatings and Films. Coatings 2022, 12, 1577. [Google Scholar] [CrossRef]
- Dumay, E.; Chevalier-Lucia, D.; Picart-Palmade, L.; Benzaria, A.; Gràcia-Julià, A.; Blayo, C. Technological Aspects and Potential Applications of (Ultra) High-Pressure Homogenisation. Trends Food Sci. Technol. 2013, 31, 13–26. [Google Scholar] [CrossRef]
- Mesa, J.; Hinestroza-Córdoba, L.I.; Barrera, C.; Seguí, L.; Betoret, E.; Betoret, N. High Homogenization Pressures to Improve Food Quality, Functionality and Sustainability. Molecules 2020, 25, 3305. [Google Scholar] [CrossRef]
- Benjamin, O.; Gamrasni, D. Microbial, Nutritional, and Organoleptic Quality of Pomegranate Juice Following High-pressure Homogenization and Low-temperature Pasteurization. J. Food Sci. 2020, 85, 592–599. [Google Scholar] [CrossRef]
- Kruszewski, B.; Zawada, K.; Karpiński, P. Impact of High-Pressure Homogenization Parameters on Physicochemical Characteristics, Bioactive Compounds Content, and Antioxidant Capacity of Blackcurrant Juice. Molecules 2021, 26, 1802. [Google Scholar] [CrossRef]
- Szczepańska, J.; Skąpska, S.; Połaska, M.; Marszałek, K. High Pressure Homogenization with a Cooling Circulating System: The Effect on Physiochemical and Rheological Properties, Enzymes, and Carotenoid Profile of Carrot Juice. Food Chem. 2022, 370, 131023. [Google Scholar] [CrossRef]
- Moscovici Joubran, A.; Katz, I.H.; Okun, Z.; Davidovich-Pinhas, M.; Shpigelman, A. The Effect of Pressure Level and Cycling in High-Pressure Homogenization on Physicochemical, Structural and Functional Properties of Filtered and Non-Filtered Strawberry Nectar. Innov. Food Sci. Emerg. 2019, 57, 102203. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Wang, W.; Ge, Z.; Zhang, L.; Li, C.; Zhang, B.; Zong, W. Comparison of the Effects of Dynamic High-pressure Microfluidization and Conventional Homogenization on the Quality of Peach Juice. J. Sci. Food Agric. 2019, 99, 5994–6000. [Google Scholar] [CrossRef]
- Levy, R.; Okun, Z.; Shpigelman, A. High-Pressure Homogenization: Principles and Applications Beyond Microbial Inactivation. Food Eng. Rev. 2021, 13, 490–508. [Google Scholar] [CrossRef]
- Liu, J.; Bi, J.; Liu, X.; Liu, D.; Verkerk, R.; Dekker, M.; Lyu, J.; Wu, X. Modelling and Optimization of High-Pressure Homogenization of Not-from-Concentrate Juice: Achieving Better Juice Quality Using Sustainable Production. Food Chem. 2022, 370, 131058. [Google Scholar] [CrossRef] [PubMed]
- Velázquez-Estrada, R.; Hernández-Herrero, M.; Guamis-López, B.; Roig-Saguès, A. Influence of Ultra-high Pressure Homogenisation on Physicochemical and Sensorial Properties of Orange Juice in Comparison with Conventional Thermal Processing. Int. J. Food Sci. Technol. 2019, 54, 1858–1864. [Google Scholar] [CrossRef]
- Suárez-Jacobo, Á.; Rüfer, C.E.; Gervilla, R.; Guamis, B.; Roig-Sagués, A.X.; Saldo, J. Influence of Ultra-High Pressure Homogenisation on Antioxidant Capacity, Polyphenol and Vitamin Content of Clear Apple Juice. Food Chem. 2011, 127, 447–454. [Google Scholar] [CrossRef]
- Karacam, C.H.; Sahin, S.; Oztop, M.H. Effect of High Pressure Homogenization (Microfluidization) on the Quality of Ottoman Strawberry (F. Ananassa) Juice. LWT 2015, 64, 932–937. [Google Scholar] [CrossRef]
- Silva, V.M.; Sato, A.C.K.; Barbosa, G.; Dacanal, G.; Ciro-Velásquez, H.J.; Cunha, R.L. The Effect of Homogenisation on the Stability of Pineapple Pulp: Homogenisation of Pineapple Pulp. Int. J. Food Sci. Technol. 2010, 45, 2127–2133. [Google Scholar] [CrossRef]
- de Oliveira Ribeiro, L.; Almeida, A.C.S.; de Carvalho, C.W.P.; Borguini, R.G.; Ferreira, J.C.S.; Freitas, S.P.; da Matta, V.M. Effect of Processing on Bioactive Compounds, Physicochemical and Rheological Characteristics of Juçara, Banana and Strawberry Smoothie. Plant Foods Hum. Nutr. 2018, 73, 222–227. [Google Scholar] [CrossRef]
- Szczepańska, J.; Skąpska, S.; Marszałek, K. Continuous High-Pressure Cooling-Assisted Homogenization Process for Stabilization of Apple Juice. Food Bioprocess Technol. 2021, 14, 1101–1117. [Google Scholar] [CrossRef]
- Zhou, L.; Guan, Y.; Bi, J.; Liu, X.; Yi, J.; Chen, Q.; Wu, X.; Zhou, M. Change of the Rheological Properties of Mango Juice by High Pressure Homogenization. LWT—Food Sci. Technol. 2017, 82, 121–130. [Google Scholar] [CrossRef]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of Processing of Red Beet on Betalain Content and Antioxidant Activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain Stability and Degradation -Structural and Chromatic Aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Skalicky, M.; Kubes, J.; Shokoofeh, H.; Tahjib-Ul-Arif, M.; Vachova, P.; Hejnak, V. Betacyanins and Betaxanthins in Cultivated Varieties of Beta Vulgaris L. Compared to Weed Beets. Molecules 2020, 25, 5395. [Google Scholar] [CrossRef] [PubMed]
- Aztatzi-Rugerio, L.; Granados-Balbuena, S.Y.; Zainos-Cuapio, Y.; Ocaranza-Sánchez, E.; Rojas-López, M. Analysis of the Degradation of Betanin Obtained from Beetroot Using Fourier Transform Infrared Spectroscopy. J. Food Sci. Technol. 2019, 56, 3677–3686. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Pünsch, M.; Venir, E. Effect of Processing and Storage on the Quality of Beetroot and Apple Mixed Juice. Foods 2021, 10, 1052. [Google Scholar] [CrossRef]
- Wang, T.; Liu, L.; Rakhmanova, A.; Wang, X.; Shan, Y.; Yi, Y.; Liu, B.; Zhou, Y.; Lü, X. Stability of Bioactive Compounds and in Vitro Gastrointestinal Digestion of Red Beetroot Jam: Effect of Processing and Storage. Food Biosci. 2020, 38, 100788. [Google Scholar] [CrossRef]
- Kujala, T.; Vienola, M.; Klika, K.; Loponen, J.; Pihlaja, K. Betalain and Phenolic Compositions of Four Beetroot (Beta vulgaris) Cultivars. Eur. Food Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R. Evaluation of Colour Properties and Chemical Quality Parameters of Cactus Juices. Eur. Food Res. Technol. 2003, 216, 303–311. [Google Scholar] [CrossRef]
- Raczyk, M.; Kruszewski, B.; Zachariasz, E. Effect of Tomato, Beetroot and Carrot Juice Addition on Physicochemical, Antioxidant and Texture Properties of Wheat Bread. Antioxidants 2022, 11, 2178. [Google Scholar] [CrossRef]



| Pressure | Cooling after Each Cycle | Inlet Temperature [°C] | Maximum Temperature Monitored Directly after the Valve Head [°C] |
|---|---|---|---|
| 50 MPa/1 and 3 cycles | yes | 20.7 ± 0.5 | 28.1 ± 1.1 |
| 100 MPa/1 and 3 cycles | yes | 20.6 ± 0.5 | 35.0 ± 1.2 |
| 140 MPa/1 and 3 cycles | yes | 20.5 ± 0.5 | 41.6 ± 1.0 |
| 140 MPa/1 cycle H | no | 20.6 ± 0.5 | 41.8 ± 1.2 |
| 140 MPa/3 cycles H | no | 20.7 ± 0.5 | 57.6 ± 2.0 |
| Sample | pH | TA (g of Citric Acid/100 mL) | TSS (°Brix) |
|---|---|---|---|
| C | 6.01 ± 0.02 a | 0.12 ± 0.01 a | 9.3 ± 0.1 a |
| 50 MPa/1 cycle | 6.14 ± 0.01 b | 0.11 ± 0.01 a | 9.3 ± 0.1 a |
| 50 MPa/3 cycles | 6.13 ± 0.01 b | 0.11 ± 0.01 a | 9.3 ± 0.1 a |
| 100 MPa/1 cycle | 6.13 ± 0.01 b | 0.11 ± 0.01 a | 9.3 ± 0.1 a |
| 100 MPa/3 cycles | 6.15 ± 0.00 b | 0.11 ± 0.01 a | 9.3 ± 0.1 a |
| 140 MPa/1 cycle | 6.14 ± 0.00 b | 0.11 ± 0.01 a | 9.3 ± 0.1 a |
| 140 MPa/3 cycles | 6.14 ± 0.00 b | 0.11 ± 0.01 a | 9.3 ± 0.1 a |
| 140 MPa/1 cycle H | 6.16 ± 0.01 b | 0.11 ± 0.01 a | 8.9 ± 0.1 b |
| 140 MPa/3 cycles H | 6.14 ± 0.00 b | 0.11 ± 0.01 a | 8.9 ± 0.1 b |
| Sample | ΔE* | Viscosity (mPa s) |
|---|---|---|
| C | - | 5.51 ± 0.02 b |
| 50 MPa/1 cycle | 0.13 ± 0.04 a | 3.76 ± 0.04 a |
| 50 MPa/3 cycles | 0.29 ± 0.01 b | 3.76 ± 0.05 a |
| 100 MPa/1 cycle | 0.13 ± 0.04 a | 4.01 ± 0.05 a |
| 100 MPa/3 cycles | 0.38 ± 0.02 c | 4.01 ± 0.07 a |
| 140 MPa/1 cycle | 0.17 ± 0.03 a | 3.82 ± 0.06 a |
| 140 MPa/3 cycles | 0.43 ± 0.03 c | 3.95 ± 0.04 a |
| 140 MPa/1 cycle H | 0.69 ± 0.04 d | 3.76 ± 0.08 a |
| 140 MPa/3 cycles H | 0.94 ± 0.06 e | 3.82 ± 0.05 a |
| Samples | Qualitative (% Relative Peak Area) | Quantitative (mg/100 mL) | |||
|---|---|---|---|---|---|
| Betanin | Isobetanin | Vulgaxanthin I and II | Betacyanins | Betaxanthins | |
| Control—raw juice | 100 | 100 | 100 | 75.3 ± 2.8 c | 24.8 ± 0.6 c |
| Homogenization: | |||||
| 50 MPa/1 cycle | 87.2 ± 0.3 c | 85.5 ± 0.2 d | 92.6 ± 0.1 c | 68.9 ± 1.5 bc | 22.5 ± 0.6 ab |
| 50 MPa/3 cycles | 87.5 ± 0.8 c | 86.2 ± 0.9 d | 93.0 ± 1.2 c | 67.1 ± 1.4 b | 23.2 ± 0.3 b |
| 100 MPa/1 cycle | 85.3 ± 1.0 b | 82.0 ± 1.1 c | 88.9 ± 1.8 b | 66.6 ± 1.3 ab | 21.9 ± 0.1 ab |
| 100 MPa/3 cycles | 84.4 ± 0.6 b | 81.6 ± 0.4 c | 89.6 ± 0.6 b | 66.4 ± 0.2 ab | 22.3 ± 0.1 ab |
| 140 MPa/1 cycle | 83.7 ± 1.2 b | 81.4 ± 1.2 c | 89.1 ± 1.0 b | 67.3 ± 0.3 b | 22.8 ± 0.3 ab |
| 140 MPa/3 cycles | 83.9 ± 0.2 b | 79.1 ± 0.3 c | 87.2 ± 0.4 b | 65.3 ± 1.3 ab | 22.1 ± 0.2 ab |
| 140 MPa/1 cycle H | 75.4 ± 0.7 a | 70.9 ± 0.8 b | 83.8 ± 1.3 a | 62.6 ± 2.2 ab | 21.1 ± 0.3 a |
| 140 MPa/3 cycles H | 74.4 ± 1.1 a | 66.0 ± 1.2 a | 83.7 ± 1.3 a | 60.1 ± 2.6 a | 21.2 ± 0.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruszewski, B.; Domian, E.; Nowacka, M. Influence of High-Pressure Homogenization on the Physicochemical Properties and Betalain Pigments of Red Beetroot (Beta vulgaris L.) Juice. Molecules 2023, 28, 2018. https://doi.org/10.3390/molecules28052018
Kruszewski B, Domian E, Nowacka M. Influence of High-Pressure Homogenization on the Physicochemical Properties and Betalain Pigments of Red Beetroot (Beta vulgaris L.) Juice. Molecules. 2023; 28(5):2018. https://doi.org/10.3390/molecules28052018
Chicago/Turabian StyleKruszewski, Bartosz, Ewa Domian, and Małgorzata Nowacka. 2023. "Influence of High-Pressure Homogenization on the Physicochemical Properties and Betalain Pigments of Red Beetroot (Beta vulgaris L.) Juice" Molecules 28, no. 5: 2018. https://doi.org/10.3390/molecules28052018