Determination of Anthraquinone-Tagged Amines Using High-Performance Liquid Chromatography with Online UV Irradiation and Luminol Chemiluminescence Detection
Abstract
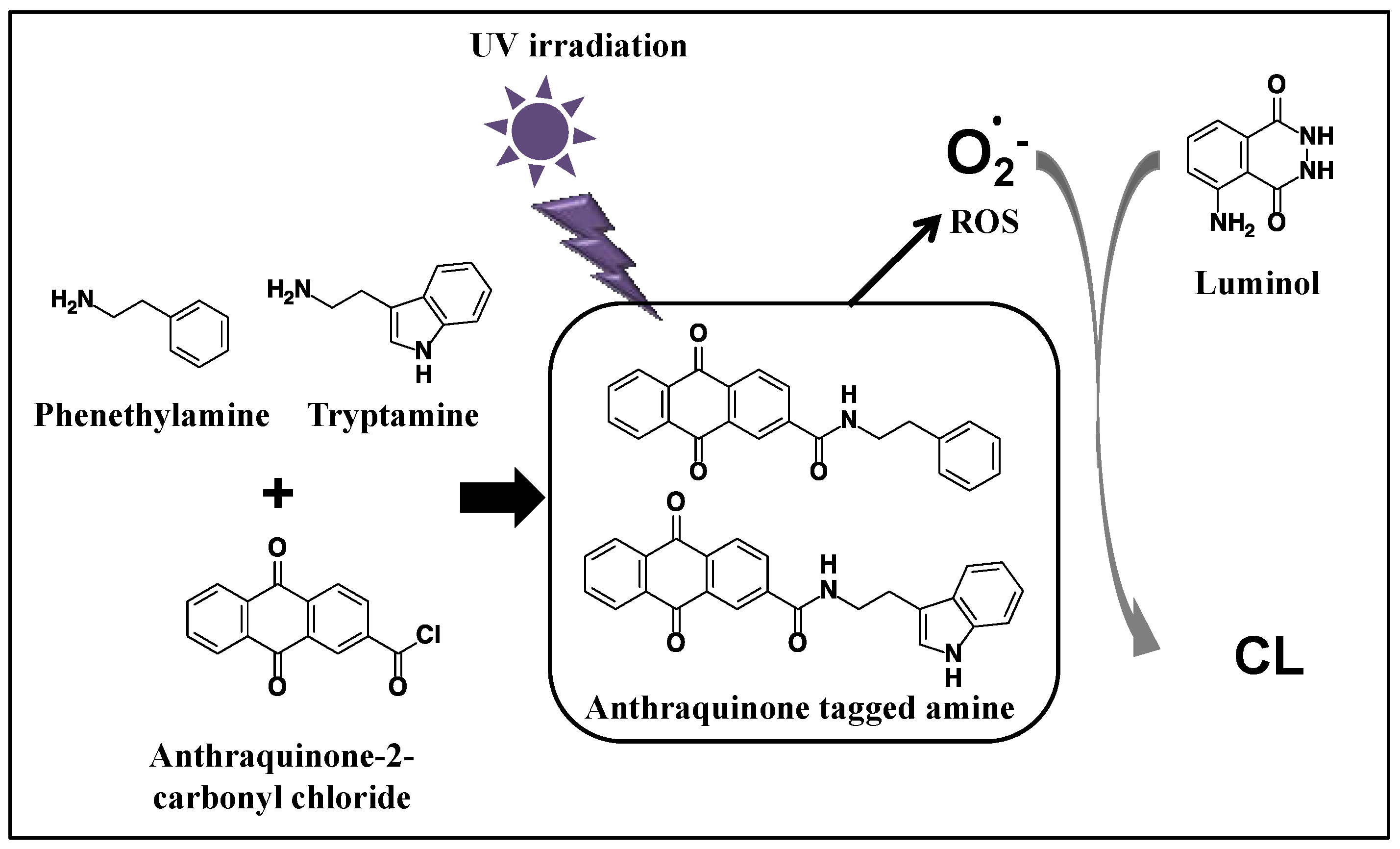
:1. Introduction
2. Result and Discussion
2.1. Chromatogram of Amines after the Reaction with Anthraquinone-2-Carbonyl Chloride
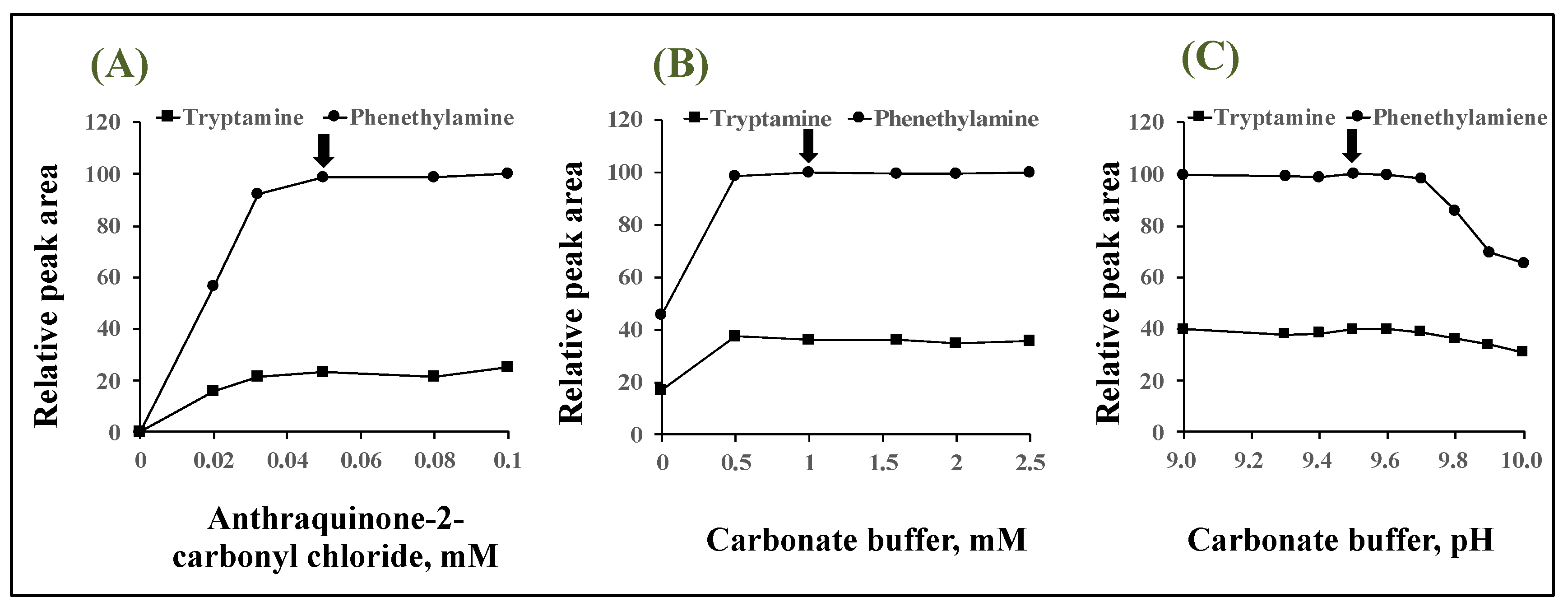
2.2. Optimization of Derivatization Reaction
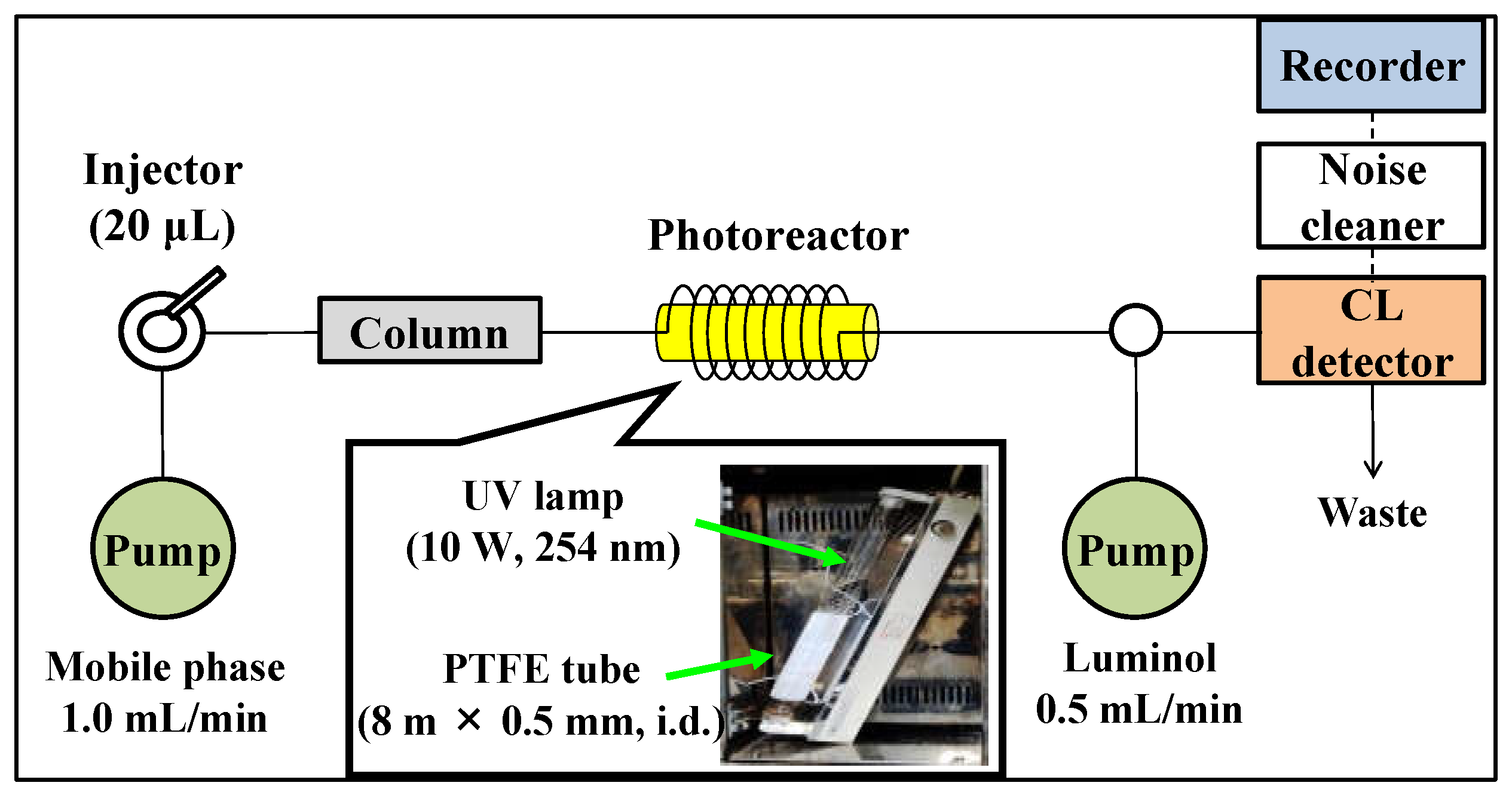
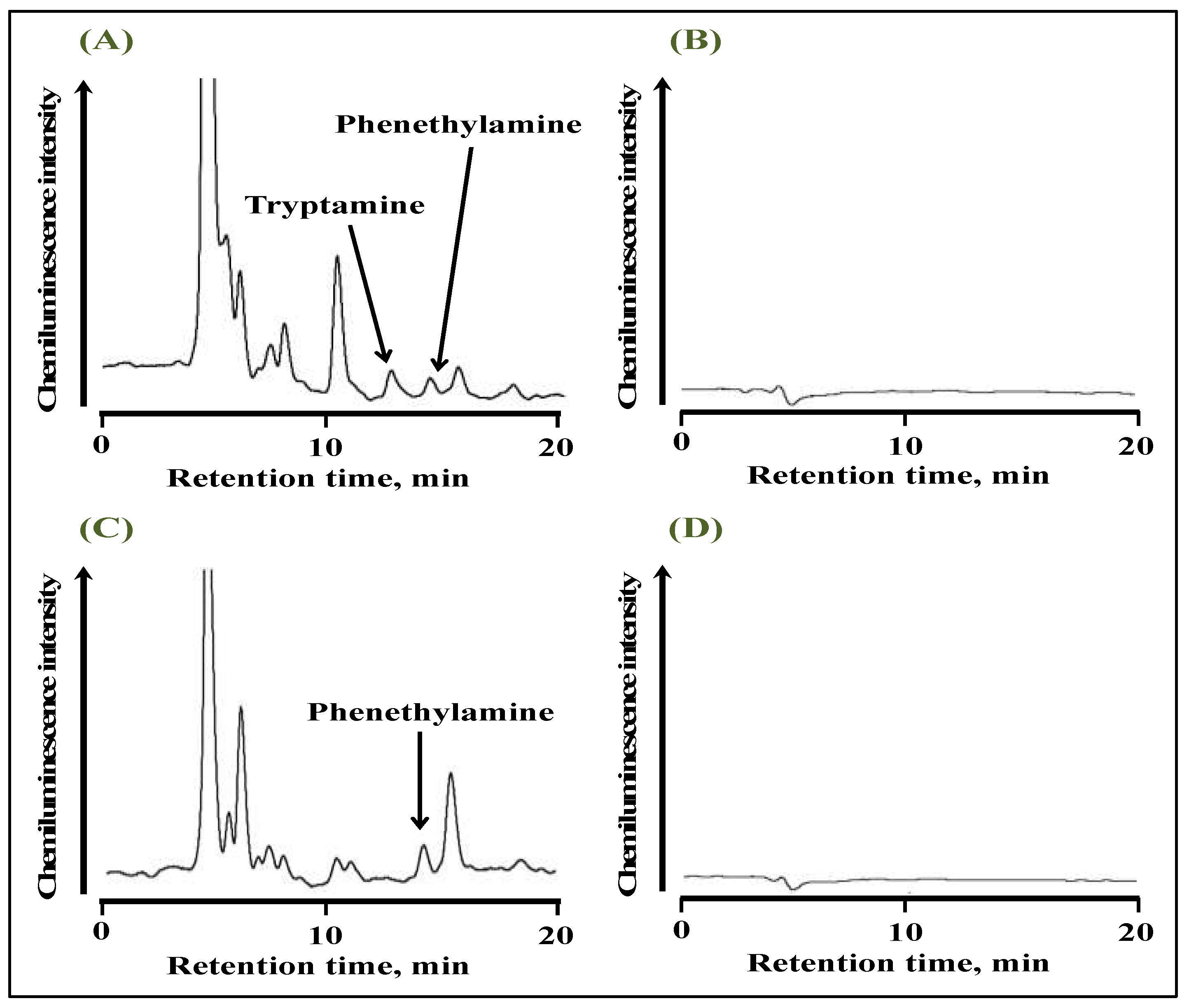
2.3. Optimization of UV Irradiation and Chemiluminescence Conditions
2.4. Calibration Curve, the Limit of Detection, and Precision Study
2.5. Application for the Determination of Amines in Wine Samples
3. Experimental Section
3.1. Material and Reagents
3.2. HPLC System and Conditions
3.3. Derivatization of Amines with Anthraquinone-2-Carbonyl Chloride
3.4. Assay Procedure for Amines in Wine Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Núñez, O.; Lucci, P. Application of Liquid Chromatography in Food Analysis. Foods 2020, 9, 1277. [Google Scholar] [CrossRef]
- Muguruma, Y.; Nunome, M.; Inoue, K. A Review on the Foodomics Based on Liquid Chromatography Mass Spectrometry. Chem. Pharm. Bull. 2022, 70, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kishikawa, N. Foreword to the Special Issue: Liquid Chromatographic Techniques in Food Sciences. Chem. Pharm. Bull. 2022, 70, 10–11. [Google Scholar] [CrossRef]
- Kishikawa, N.; El-Maghrabey, M.H.; Kuroda, N. Chromatographic Methods and Sample Pretreatment Techniques for Aldehydes Determination in Biological, Food, and Environmental Samples. J. Pharm. Biomed. Anal. 2019, 175, 112782. [Google Scholar] [CrossRef]
- Kishikawa, N. Derivatization Techniques for Chromatographic Analysis. Anal. Sci. 2018, 34, 1109–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Maghrabey, M.; Mine, M.; Kishikawa, N.; Ohyama, K.; Kuroda, N. A Novel Dual Labeling Approach Enables Converting Fluorescence Labeling Reagents into Fluorogenic Ones via Introduction of Purification Tags. Application to Determination of Glyoxylic Acid in Serum. Talanta 2018, 180, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Kishikawa, N.; Yamanouchi, A.; El-Maghrabey, M.H.; Ohyama, K.; Kuroda, N. Determination of Tanshinones in Danshen (Salvia Miltiorrhiza) by High-Performance Liquid Chromatography with Fluorescence Detection after Pre-Column Derivatisation. Phytochem. Anal. 2018, 29, 112–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashed, N.; Zayed, S.; Fouad, F.; Abdelazeem, A. Sensitive and Fast Determination of Ceftiofur in Honey and Veterinary Formulation by HPLC-UV Method with Pre-Column Derivatization. J. Chromatogr. Sci. 2021, 59, 15–22. [Google Scholar] [CrossRef]
- Walash, M.I.; Belal, F.F.; El-Enany, N.M.; El-Maghrabey, M.H. Utility of Certain Nucleophilic Aromatic Substitution Reactions for the Assay of Pregabalin in Capsules. Chem. Cent. J. 2011, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- García-Villar, N.; Saurina, J.; Hernández-Cassou, S. High-Performance Liquid Chromatographic Determination of Biogenic Amines in Wines with an Experimental Design Optimization Procedure. Anal. Chim. Acta 2006, 575, 97–105. [Google Scholar] [CrossRef]
- Simpson, J.T.; Torok, D.S.; Worman, R.; Quearry, B.J.; Markey, S.P.; Ziffer, H. Characterization of Anthraquinone-2-Carbonyl Chloride as an Alcohol Derivatization Reagent for Negative Ion Chemical Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 1995, 6, 148–151. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Feng, F.; Ma, M.; Cui, S.; Xie, D.; Xu, S. Pharmacokinetic Study of Three Cardiovascular Drugs by High-Performance Liquid Chromatography Using Pre-Column Derivatization with 9,10-Anthraquinone-2-Sulfonyl Chloride. J. Chromatogr. B 2007, 858, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Uno, B.; Goto, M.; Zhang, Z.; An, D. Evaluation of a New Reagent: Anthraquinone-2-Sulfonyl Chloride for the Determination of Phenol in Water by Liquid Chromatography Using Precolumn Phase-Transfer Catalyzed Derivatization. J. Chromatogr. Sci. 2003, 41, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Maghrabey, M.H.; Kishikawa, N.; Ohyama, K.; Kuroda, N. Analytical Method for Lipoperoxidation Relevant Reactive Aldehydes in Human Sera by High-Performance Liquid Chromatography-Fluorescence Detection. Anal. Biochem. 2014, 464, 36–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Maghrabey, M.; Kishikawa, N.; Kuroda, N. 9,10-Phenanthrenequinone as a Mass-Tagging Reagent for Ultra-Sensitive Liquid Chromatography-Tandem Mass Spectrometry Assay of Aliphatic Aldehydes in Human Serum. J. Chromatogr. A 2016, 1462, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Kishikawa, N.; Ohkubo, N.; Ohyama, K.; Nakashima, K.; Kuroda, N. Chemiluminescence Assay for Quinones Based on Generation of Reactive Oxygen Species through the Redox Cycle of Quinone. Anal. Bioanal. Chem. 2009, 393, 1337–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Maghrabey, M.; Kishikawa, N.; Harada, S.; Ohyama, K.; Kuroda, N. Quinone-Based Antibody Labeling Reagent for Enzyme-Free Chemiluminescent Immunoassays. Application to Avidin and Biotinylated Anti-Rabbit IgG Labeling. Biosens. Bioelectron. 2020, 160, 112215. [Google Scholar] [CrossRef]
- Fukuda, M.; Kishikawa, N.; Samemoto, T.; Ohta, K.; Ohyama, K.; El-Maghrabey, M.H.; Ikemoto, K.; Kuroda, N. Determination Method for Pyrroloquinoline Quinone in Food Products by HPLC-UV Detection Using a Redox-Based Colorimetric Reaction. Chem. Pharm. Bull. 2022, 70, 32–36. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Sato, Y.; Kaladari, F.; Kishikawa, N.; Kuroda, N. Development of Quinone Linked Immunosorbent Assay (QuLISA) Based on Using Folin’s Reagent as a Non-Enzymatic Tag: Application to Analysis of Food Allergens. Sens. Actuators B Chem. 2022, 368, 132167. [Google Scholar] [CrossRef]
- Elgawish, M.S.; Kishikawa, N.; Kuroda, N. Quinones as Novel Chemiluminescent Probes for the Sensitive and Selective Determination of Biothiols in Biological Fluids. Analyst 2015, 140, 8148–8156. [Google Scholar] [CrossRef]
- Elgawish, M.S.; Kishikawa, N.; Kuroda, N. Redox-Based Chemiluminescence Assay of Aminothiols in Human Urine: A Fundamental Study. Talanta 2017, 164, 116–120. [Google Scholar] [CrossRef]
- Ahmed, S.; Fujii, S.; Kishikawa, N.; Ohba, Y.; Nakashima, K.; Kuroda, N. Selective Determination of Quinones by High-Performance Liquid Chromatography with online Post Column Ultraviolet Irradiation and Peroxyoxalate Chemiluminescence Detection. J. Chromatogr. A 2006, 1133, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Elgawish, M.S.; Shimomai, C.; Kishikawa, N.; Ohyama, K.; Nakashima, K.; Kuroda, N. Microplate Analytical Method for Quinones by Pulse Photo-Irradiation and Chemiluminescence Detection. Analyst 2012, 137, 4802. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Kishikawa, N.; Ohyama, K.; Imazato, T.; Ueki, Y.; Kuroda, N. Selective Chemiluminescence Method for Monitoring of Vitamin K Homologues in Rheumatoid Arthritis Patients. Talanta 2011, 85, 230–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.; Kishikawa, N.; Ohyama, K.; Maki, T.; Kurosaki, H.; Nakashima, K.; Kuroda, N. An Ultrasensitive and Highly Selective Determination Method for Quinones by High-Performance Liquid Chromatography with Photochemically Initiated Luminol Chemiluminescence. J. Chromatogr. A 2009, 1216, 3977–3984. [Google Scholar] [CrossRef] [Green Version]
- Erdag, D.; Merhan, O.; Yildiz, B. Biochemical and Pharmacological Properties of Biogenic Amines. In Biogenic Amines; Proestos, C., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Santos, M.H.S. Biogenic Amines: Their Importance in Foods. Int. J. Food Microbiol. 1996, 29, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Özdestan, Ö.; Üren, A. A Method for Benzoyl Chloride Derivatization of Biogenic Amines for High Performance Liquid Chromatography. Talanta 2009, 78, 1321–1326. [Google Scholar] [CrossRef]
- He, L.; Ren, J.; Shi, Z.; Xu, Z. Separation of Key Biogenic Amines by Capillary Electrophoresis and Determination of Possible Indicators of Sport Fatigue in Athlete’s Urine. J. Chromatogr. Sci. 2016, 54, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Ying, L.-Y.; Zhou, S.-C.; Ying, M.; Shen, W.; Qiu, D.-H. Chromatographic Determination of Biogenic Amines in Wines after Treatment with Ionic Liquids as Novel Media. J. Sep. Sci. 2011, 34, 1055–1062. [Google Scholar] [CrossRef]
- Jia, S.; Kang, Y.P.; Park, J.H.; Lee, J.; Kwon, S.W. Simultaneous Determination of 23 Amino Acids and 7 Biogenic Amines in Fermented Food Samples by Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry. J. Chromatogr. A 2011, 1218, 9174–9182. [Google Scholar] [CrossRef]
- Jia, S.; Ryu, Y.; Kwon, S.W.; Lee, J. An In Situ Benzoylation-Dispersive Liquid–Liquid Microextraction Method Based on Solidification of Floating Organic Droplets for Determination of Biogenic Amines by Liquid Chromatography–Ultraviolet Analysis. J. Chromatogr. A 2013, 1282, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, X.-E.; Wang, R.; Wei, N.; Sun, J.; Dang, J.; Chen, G.; Liu, Z.; Zhu, S.; You, J. Simultaneous Determination of Food-Related Biogenic Amines and Precursor Amino Acids Using in Situ Derivatization Ultrasound-Assisted Dispersive Liquid–Liquid Microextraction by Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 8225–8234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, F.; Liu, Y.; Wang, W. Determination of Biogenic Amines in Wine Using Modified Liquid-Liquid Extraction with High Performance Liquid Chromatography-Fluorescence Detector. Food Anal. Methods 2020, 13, 911–922. [Google Scholar] [CrossRef]
- Lorenzo, C.; Bordiga, M.; Pérez-Álvarez, E.P.; Travaglia, F.; Arlorio, M.; Salinas, M.R.; Coïsson, J.D.; Garde-Cerdán, T. The Impacts of Temperature, Alcoholic Degree and Amino Acids Content on Biogenic Amines and Their Precursor Amino Acids Content in Red Wine. Food Res. Int. 2017, 99, 328–335. [Google Scholar] [CrossRef]
- Manetta, A.C.; Di Giuseppe, L.; Tofalo, R.; Martuscelli, M.; Schirone, M.; Giammarco, M.; Suzzi, G. Evaluation of Biogenic Amines in Wine: Determination by an Improved HPLC-PDA Method. Food Control 2016, 62, 351–356. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Suzuki, H.; Kishikawa, N.; Kuroda, N. A Sensitive Chemiluminescence Detection Approach for Determination of 2,4-Dinitrophenylhydrazine Derivatized Aldehydes Using Online UV Irradiation—Luminol CL Reaction. Application to the HPLC Analysis of Aldehydes in Oil Samples. Talanta 2021, 233, 122522. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Kishikawa, N.; Kamimura, S.; Ohyama, K.; Kuroda, N. Design of a Dual Functionalized Chemiluminescence Ultrasensitive Probe for Quinones Based on Their Redox Cycle. Application to the Determination of Doxorubicin in Lyophilized Powder and Human Serum. Sens. Actuators B Chem. 2021, 329, 129226. [Google Scholar] [CrossRef]
- Guo, Y.-Y.; Yang, Y.-P.; Peng, Q.; Han, Y. Biogenic Amines in Wine: A Review. Int. J. Food Sci. Technol. 2015, 50, 1523–1532. [Google Scholar] [CrossRef]
- Jastrzębska, A.; Piasta, A.; Kowalska, S.; Krzemiński, M.; Szłyk, E. A New Derivatization Reagent for Determination of Biogenic Amines in Wines. J. Food Compos. Anal. 2016, 48, 111–119. [Google Scholar] [CrossRef]
- Piasta, A.M.; Jastrzębska, A.; Krzemiński, M.P.; Muzioł, T.M.; Szłyk, E. New Procedure of Selected Biogenic Amines Determination in Wine Samples by HPLC. Anal. Chim. Acta 2014, 834, 58–66. [Google Scholar] [CrossRef]
- Francisco, K.C.A.; Brandão, P.F.; Ramos, R.M.; Gonçalves, L.M.; Cardoso, A.A.; Rodrigues, J.A. Salting-out Assisted Liquid–Liquid Extraction with Dansyl Chloride for the Determination of Biogenic Amines in Food. Int. J. Food Sci. Technol. 2020, 55, 248–258. [Google Scholar] [CrossRef]


| Linear Range, μM | Calibration Equation * | Correlation Coefficient (r) | LOD **, nM | |
|---|---|---|---|---|
| Tryptamine | 0.2–20 | Y = 2.8 × 105 X + 8.6 × 103 | 0.998 | 124 |
| Phenethylamine | 0.1–10 | Y = 1.1 × 106 X + 1.2 × 105 | 0.999 | 84 |
| Method | Derivatizing Agent | LOD | Sample | Ref | |
|---|---|---|---|---|---|
| Tryptamine | Phenethylamine | ||||
| HPLC-UV | Benzoyl chloride | 1248 | 4126 | Wine samples | [28] |
| CZE-UV | None | 300 | 600 | Urine | [29] |
| HPLC-FL | Dansyl chloride | 625 | 825 | Wine | [30] |
| LC-MS/MS | Dansyl chloride | 125 | 41 | Food samples | [31] |
| LC-MS/MS | 4’-Carbonyl chloride rosamine | 2.5 | 2.5 | Food samples | [32] |
| HPLC-UV | Benzoyl chloride | 63 | 83 | Wine | [33] |
| HPLC-CL | Anthraquinone-2-carbonyl chloride | 124 | 84 | Wine | This work |
| Concentration, µM | Precision (RSD, %) | ||
|---|---|---|---|
| Intra-Day (n = 5) | Inter-Day (n = 5) | ||
| Tryptamine | 0.5 | 3.3 | 9.4 |
| 2.5 | 4.7 | 7.4 | |
| 10 | 6.1 | 9.8 | |
| Phenethylamine | 0.2 | 4.3 | 8.5 |
| 2.5 | 5.7 | 6.3 | |
| 10 | 5.2 | 9.9 | |
| Sample | Concentration, µM | |
|---|---|---|
| Tryptamine | Phenethylamine | |
| Red wine 1 | 1.7 | 2.3 |
| Red wine 2 | 3.3 | 4.2 |
| White wine 1 | n.d.* | 4.6 |
| White wine 2 | n.d.* | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishikawa, N.; El-Maghrabey, M.; Kawamoto, A.; Ohyama, K.; Kuroda, N. Determination of Anthraquinone-Tagged Amines Using High-Performance Liquid Chromatography with Online UV Irradiation and Luminol Chemiluminescence Detection. Molecules 2023, 28, 2146. https://doi.org/10.3390/molecules28052146
Kishikawa N, El-Maghrabey M, Kawamoto A, Ohyama K, Kuroda N. Determination of Anthraquinone-Tagged Amines Using High-Performance Liquid Chromatography with Online UV Irradiation and Luminol Chemiluminescence Detection. Molecules. 2023; 28(5):2146. https://doi.org/10.3390/molecules28052146
Chicago/Turabian StyleKishikawa, Naoya, Mahmoud El-Maghrabey, Ayaka Kawamoto, Kaname Ohyama, and Naotaka Kuroda. 2023. "Determination of Anthraquinone-Tagged Amines Using High-Performance Liquid Chromatography with Online UV Irradiation and Luminol Chemiluminescence Detection" Molecules 28, no. 5: 2146. https://doi.org/10.3390/molecules28052146





