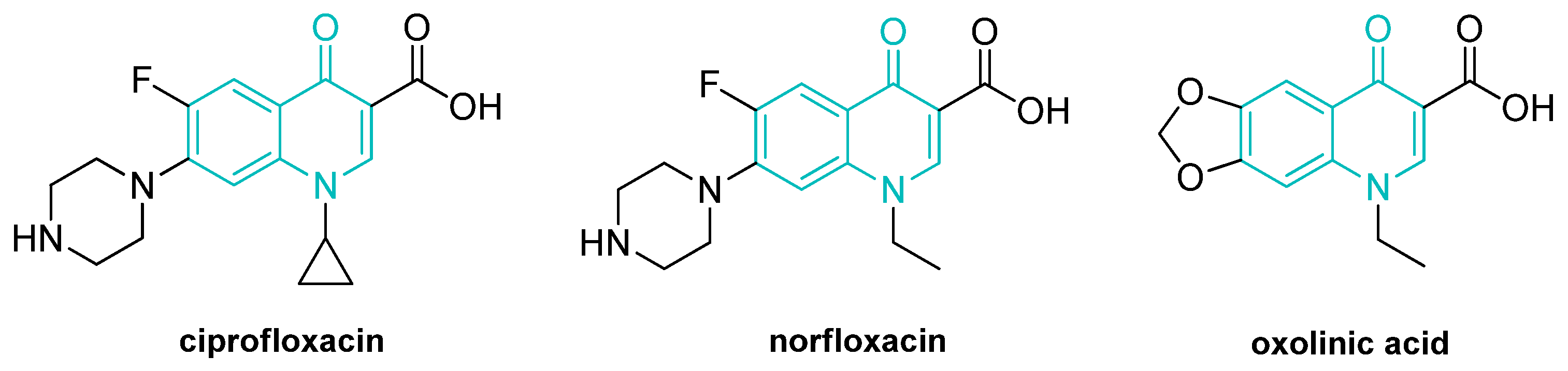
Hydrogen Bond Assisted Three-Component Tandem Reactions to Access N-Alkyl-4-Quinolones
Abstract
:1. Introduction
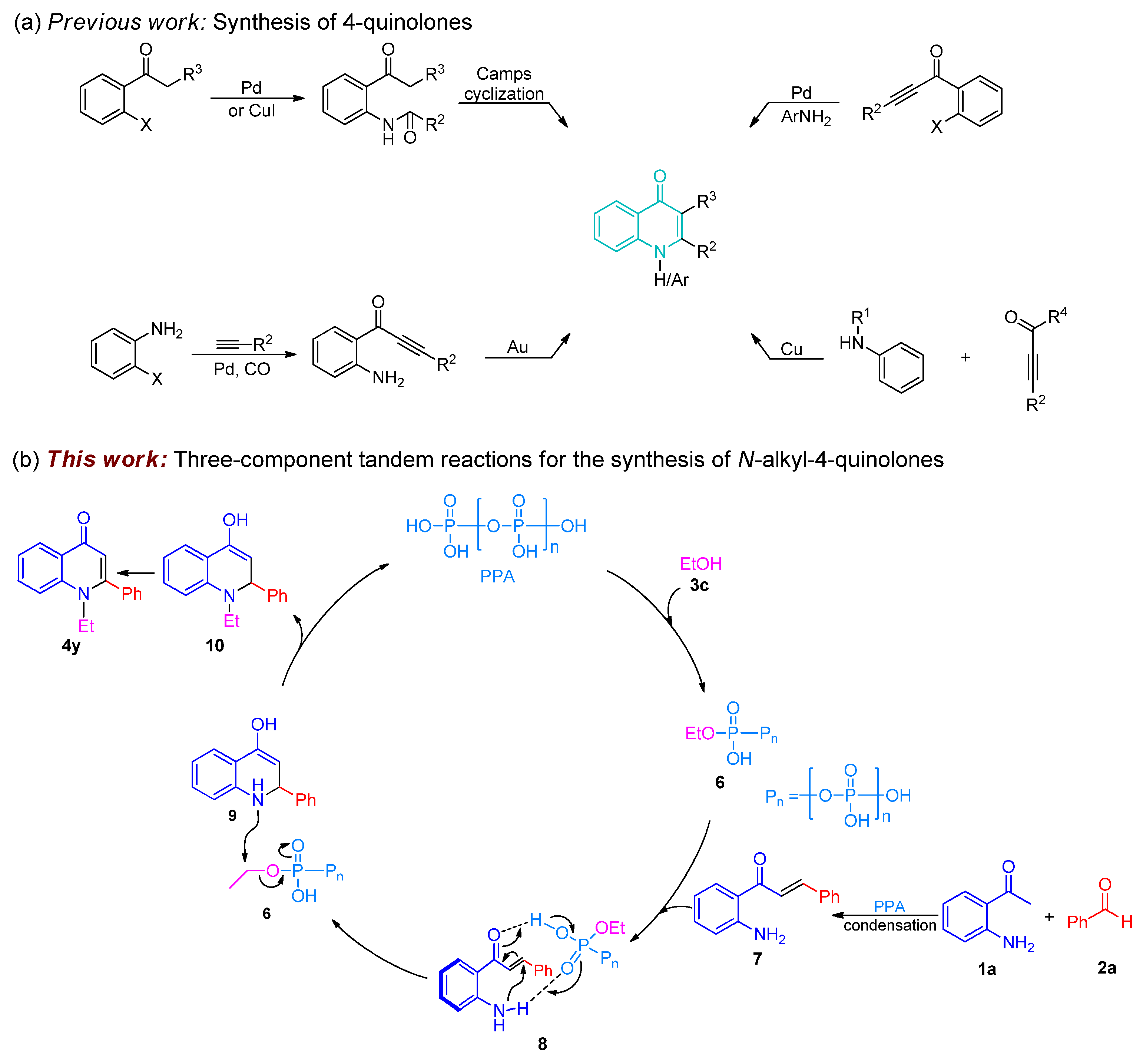
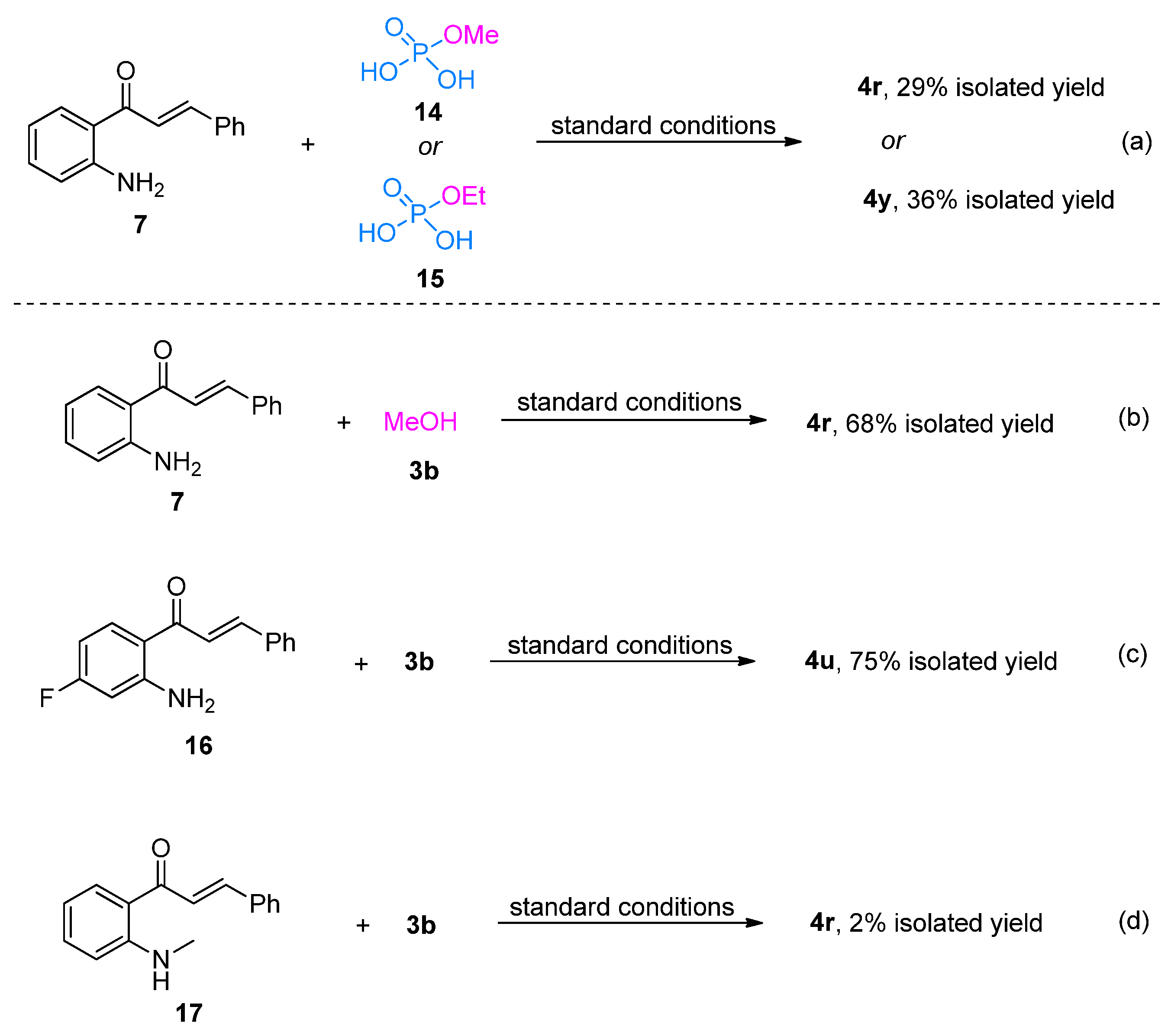
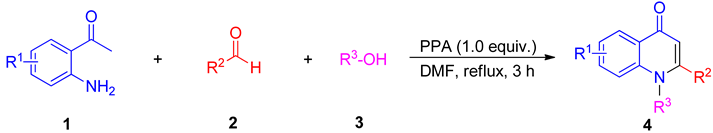
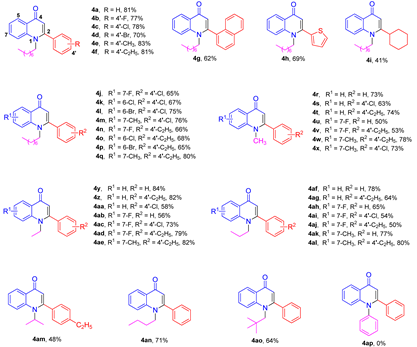
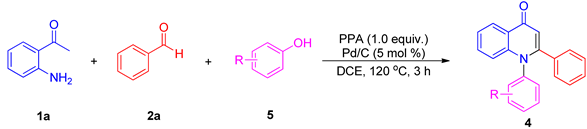
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Compounds 4a–4ao
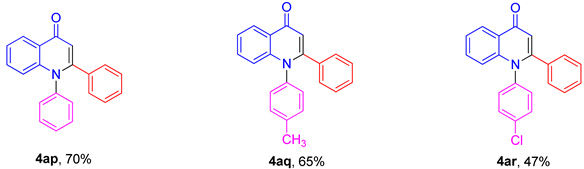
3.3. Synthesis of Compounds 4ap–4ar
3.4. Biological Evaluation
3.4.1. Cell Culture Conditions
3.4.2. Protective Effects of 4-Quinolones 4a-4ar against NMDA-induced Injury in PC12 Cells
3.4.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fort, D.M.; Litvak, J.; Chen, J.L.; Lu, Q.; Phuan, P.W.; Cooper, R.; Bierer, D.E. Isolation and unambiguous synthesis of cryptolepinone: An oxidation artifact of cryptolepine. J. Nat. Prod. 1998, 61, 1528–1530. [Google Scholar] [CrossRef] [PubMed]
- Koyama, J.; Toyokuni, I.; Tagahara, K. Synthesis of 2-arylquinoline and 2-aryl-4-quinolone alkaloids via Diels-Alder reaction of 1,2,3-benzotriazine with enamines. Chem. Pharm. Bull. 1999, 47, 1029–1038. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurthy, M.; Gooch, B.D.; Beal, P.A. Peptide quinoline conjugates: A new class of RNA-binding molecules. Org. Lett. 2004, 6, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.; Tan, J.; Farina, V. Convergent synthesis of the quinolone substructure of biln 2061 via carbonylative sonogashira coupling/cyclization. J. Org. Chem. 2006, 71, 5031–5034. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, M.; Simon, K.; Orendt, A.M.; Beal, P.A. Macrocyclic helix-threading peptides for targeting RNA. Angew. Chem. Int. Ed. 2007, 46, 7044–7047. [Google Scholar] [CrossRef] [PubMed]
- Hadjeri, M.; Mariotte, A.M.; Boumendjel, A. Alkylation of 2-phenyl-4-quinolones: Synthetic and structural studies. Chem. Pharm. Bull. 2001, 49, 1352–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Y.; Yang, Z.Y.; Xia, P.; Hackl, T.; Hamel, E.; Mauger, A.; Wu, J.H.; Lee, K.H. Antitumor agents. 211. fluorinated 2-phenyl-4-quinolone derivatives as antimitotic antitumor agents. J. Med. Chem. 2001, 44, 3932–3936. [Google Scholar] [CrossRef]
- Hadjeri, M.; Peiller, E.L.; Beney, C.; Deka, N.; Lawson, M.A.; Dumontet, C.; Boumendjel, A. Antimitotic activity of 5-hydroxy-7-methoxy-2-phenyl-4-quinolones. J. Med. Chem. 2004, 47, 4964–4970. [Google Scholar] [CrossRef]
- Lucero, B.A.; Gomes, C.R.B.; Frugulhetti, I.C.D.P.P.; Faro, L.V.; Alvarenga, L.; Souza, M.C.B.V.; de Souza, T.M.L.; Ferreira, V.F. Synthesis and anti-HSV-1 activity of quinolonic acyclovir analogues. Bioorg. Med. Chem. Lett. 2006, 16, 1010–1013. [Google Scholar] [CrossRef]
- Santo, R.D.; Costi, R.; Roux, A.; Artico, M.; Lavecchia, A.; Marinelli, L.; Novellino, E.; Palmisano, L.; Andreotti, M.; Amici, R.; et al. Novel bifunctional quinolonyl diketo acid derivatives as HIV-1 integrase inhibitors: Design, synthesis, biological activities, and mechanism of action. J. Med. Chem. 2006, 49, 1939–1945. [Google Scholar] [CrossRef] [Green Version]
- Kuo, S.; Lee, H.; Juang, J.; Lin, Y.; Wu, T.; Chang, J.; Lednicer, D.; Paull, K.D.; Lin, C.; Hamel, E.; et al. Synthesis and cytotoxicity of 1,6,7,8-substituted 2-(4′-substituted phenyl)-4-quinolones and related compounds: Identification as antimitotic agents interacting with tubulin. J. Med. Chem. 1993, 36, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Llinas-Brunet, M.; Bailey, M.D.; Ghiro, E.; Gorys, V.; Halmos, T.; Poirier, M.; Rancourt, J.; Goudreau, N. A systematic approach to the optimization of substrate-based inhibitors of the hepatitis C virus NS3 protease: Discovery of potent and specific tripeptide inhibitors. J. Med. Chem. 2004, 47, 6584–6594. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kozuka, M.; Bastow, K.F.; Tokuda, H.; Nishino, H.; Suzuki, M.; Tatsuzaki, J.; Natschke, S.L.M.; Kuo, S.C.; Lee, K.H. Cancer preventive agents, part 2: Synthesis and evaluation of 2-phenyl-4-quinolone and 9-oxo-9,10-dihydroacridine derivatives as novel antitumor promoters. Bioorg. Med. Chem. 2005, 13, 4396–4401. [Google Scholar] [CrossRef] [PubMed]
- Reitsema, R.H. The chemistry of 4-hydroxyquinolines. Chem. Rev. 1948, 43, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Brouet, J.C.; Gu, S.; Peet, N.P.; Williams, J.D. Survey of solvents for the conrad-limpach synthesis of 4-hydroxyquinolones. Synthetic Commun. 2009, 39, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Romek, A.; Opatz, T. Microwave-assisted synthesis of polysubstituted 4-quinolones from deprotonated α-aminonitriles. Eur. J. Org. Chem. 2010, 30, 5841–5849. [Google Scholar] [CrossRef]
- Zewge, D.; Chen, C.Y.; Deer, C.; Dormer, P.G.; Hughes, D.L. A mild and efficient synthesis of 4-quinolones and quinolone heterocycles. J. Org. Chem. 2007, 72, 4276–4279. [Google Scholar] [CrossRef]
- Niementowski, S. Synthesen der chinolinderivate. Ber. Dtsch. Chem. Ges. 1894, 27, 1394–1403. [Google Scholar] [CrossRef] [Green Version]
- Fuson, R.C.; Burness, D.M. A new synthesis of 2-aryl-4-hydroxyquinolines. J. Am. Chem. Soc. 1946, 68, 1270–1272. [Google Scholar] [CrossRef]
- Ogata, Y.; Kawasaki, A.; Tsujimura, K. Kinetics and mechanism of the formation of 4-hydroxyquinoline from methyl anthranilate. Tetrahedron 1971, 27, 2765–2770. [Google Scholar] [CrossRef]
- Son, J.K.; Kim, S.I.; Jahng, Y. A modified niementowski reaction for the synthesis of 4-hydroxyquinoline and its related compounds. Heterocycles 2001, 55, 1981–1986. [Google Scholar]
- Camps, R. Synthese von α-und γ-oxychinolinen. Arch. Pharm. 1899, 237, 659–691. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.P.; Anderson, K.W.; Buchwald, S.L. Sequential Cu-catalyzed amidation-base-mediated camps cyclization: A two-step synthesis of 2-aryl-4-quinolones from o-halophenones. J. Org. Chem. 2007, 72, 7968–7973. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, Y.; King, A.O.; Dilmeghani, M.; Larsen, R.D.; Faul, M.M. A mild, one-pot synthesis of 4-quinolones via sequential Pd-catalyzed amidation and base-promoted cyclization. Org. Lett. 2008, 10, 2609–2612. [Google Scholar] [CrossRef]
- Deng, Y.; Gong, W.; He, J.; Yu, J.Q. Ligand-enabled triple C-H activation reactions: One-pot synthesis of diverse 4-aryl-2-quinolinones from propionamides. Angew. Chem. Int. Ed. 2014, 53, 6810–6813. [Google Scholar] [CrossRef]
- Torii, S.; Okumoto, H.; Xu, L.H. Palladium-catalyzed carbonylation to form 2-substituted 1, 4-dihydro-4-oxo-quinoline. Tetrahedron Lett. 1991, 32, 237–240. [Google Scholar] [CrossRef]
- Seppanen, O.; Muuronen, M.; Helaja, J. Gold-catalyzed conversion of aryl- and alkyl-substituted 1-(o-aminophenyl)-2-propyn-1-ones to the corresponding 2-substituted 4-quinolones. Eur. J. Org. Chem. 2014, 2014, 4044–4052. [Google Scholar] [CrossRef]
- Zhao, T.K.; Xu, B. Palladium-catalyzed tandem amination reaction for the synthesis of 4-quinolones. Org. Lett. 2010, 12, 212–215. [Google Scholar] [CrossRef]
- Åkerbladh, L.; Nordeman, P.; Wejdemar, M.; Odell, L.R.; Larhed, M. Synthesis of 4-quinolones via a carbonylative sonogashira cross-coupling using molybdenum hexacarbonyl as a CO source. J. Org. Chem. 2015, 80, 1464–1471. [Google Scholar] [CrossRef]
- Fei, X.; Zhou, Z.; Li, W.; Zhu, Y.; Shen, J. Buchwald-hartwig coupling/michael addition reactions: One-pot synthesis of 1,2-disubstituted 4-quinolones from chalcones and primary amines. Eur. J. Org. Chem. 2012, 2012, 3001–3008. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X. Direct synthesis of 4-quinolones via copper-catalyzed anilines and alkynes. Org. Lett. 2017, 19, 4984–4987. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Huang, X.M.; Hong, X.H.; Liu, B.X.; Xu, B. Synthesis of N-akyl-substituted 4-quinolones via tandem alkenyl and aryl C-N bond formation. Synthesis 2012, 44, 1798–1805. [Google Scholar]
- Iaroshenko, V.O.; Mkrtchyan, S.; Villinger, A. Efficient [5+1] synthesis of 4-quinolones by domino amination and conjugate addition reactions of 1-(2-fluorophenyl)prop-2-yn-1-ones with amines. Synthesis 2013, 45, 205–218. [Google Scholar] [CrossRef]
- Okamoto, N.; Takeda, K.; Ishikura, M.; Yanada, R. One-pot approach to 2,3-disubstituted-2,3-dihydro-4-quinolones from 2-alkynylbenzamides. J. Org. Chem. 2011, 76, 9139–9143. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lin, J.; Song, L.; Long, Y. Direct synthesis of 2-aryl-4-quinolones via transition-metal-free intramolecular oxidative C (sp3)-H/C (sp3)-H coupling. Org. Lett. 2015, 17, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Guo, C.; Zhan, Z.; Lu, G.; Zhang, Y.; Luo, X.; Huang, G. Transition-metal-free oxidative intermolecular cyclization reaction: Synthesis of 2-aryl-4-quinolones. New J. Chem. 2017, 41, 5280–5283. [Google Scholar] [CrossRef]
- Doyle, A.G.; Jacobsen, E.N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 2007, 107, 5713–5743. [Google Scholar] [CrossRef]
- Gondi, V.B.; Gravel, M.; Rawal, V.H. Hydrogen bond catalyzed enantioselective vinylogous mukaiyama aldol reaction. Org. Lett. 2015, 7, 5657–5660. [Google Scholar] [CrossRef] [Green Version]
- Grayson, M.N.; Yang, Z.; Houk, K.N. Chronology of CH…O hydrogen bonding from molecular dynamics studies of the phosphoric acid-catalyzed allylboration of benzaldehyde. J. Am. Chem. Soc. 2017, 139, 7717–7720. [Google Scholar] [CrossRef] [Green Version]
- Connon, S.J. Organocatalysis mediated by (thio) urea derivatives. Chem. Eur. J. 2006, 12, 5418–5427. [Google Scholar] [CrossRef]
- Taylor, M.S.; Jacobsen, E.N. Asymmetric catalysis by chiral hydrogen-bond donors. Angew. Chem. Int. Ed. 2006, 45, 1043–1520. [Google Scholar] [CrossRef] [PubMed]
- Connon, S.J. Chiral phosphoric acids: Powerful organocatalysts for asymmetric addition reactions to imines. Angew. Chem. Int. Ed. 2006, 45, 3909–3912. [Google Scholar] [CrossRef] [PubMed]
- Marccelli, T.; van Maarseveen, J.H.; Hiemstra, H. Cupreines and cupreidines: An emerging class of bifunctional cinchona organocatalysts. Angew. Chem. Int. Ed. 2006, 45, 7496–7504. [Google Scholar] [CrossRef] [PubMed]
- Sigman, M.S.; Jacobsen, E.N. Schiff base catalysts for the asymmetric Strecker reaction identified and optimized from parallel synthetic libraries. J. Am. Chem. Soc. 1998, 120, 4901–4902. [Google Scholar] [CrossRef]
- Vachal, P.; Jacobsen, E.N. Structure-based analysis and optimization of a highly enantioselective catalyst for the Strecker reaction. J. Am. Chem. Soc. 2002, 124, 10012–10014. [Google Scholar] [CrossRef] [PubMed]
- List, B.; Lerner, R.A.; Barbas, C.F. Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 2000, 122, 2395–2396. [Google Scholar] [CrossRef]
- Tang, Z.; Jiang, F.; Yu, L.T.; Cui, X.; Gong, L.Z.; Mi, A.Q.; Jiang, Y.Z.; Wu, Y.D. Novel small organic molecules for a highly enantioselective direct aldol reaction. J. Am. Chem. Soc. 2003, 125, 5262–5263. [Google Scholar] [CrossRef]
- Rajaram, S.; Sigman, M.S. Design of hydrogen bond catalysts based on a modular oxazoline template: Application to an enantioselective hetero Diels-Alder reaction. Org. Lett. 2005, 7, 5375–5473. [Google Scholar] [CrossRef]
- Yang, K.; Lou, Y.; Wang, C.; Qi, L.W.; Fang, T.; Zhang, F.; Song, Q. Chiral brønsted acid from chiral phosphoric acid boron complex and water: Asymmetric reduction of indoles. Angew. Chem. 2020, 132, 3320–3325. [Google Scholar] [CrossRef]
- Rajkumar, S.; Tang, M.; Yang, X. Chiral phosphoric acid catalyzed kinetic resolution of 2-amido benzyl alcohols: Asymmetric synthesis of 4H-3,1-benzoxazines. Angew. Chem. 2020, 132, 2353–2357. [Google Scholar] [CrossRef]
- Ermanis, K.; Colgan, A.C.; Proctor, R.S.; Hadrys, B.W.; Phipps, R.J.; Goodman, J.M. A computational and experimental investigation of the origin of selectivity in the chiral phosphoric acid catalyzed enantioselective minisci reaction. J. Am. Chem. Soc. 2020, 142, 21091–21101. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Huang, J.; Mochizuki, T.; Hatano, M.; Ishihara, K. Enantio- and diastereoselective carbonyl-ene Cyclization-acetalization tandem reaction catalyzed by tris(pentafluorophenyl) borane-assisted chiral phosphoric acids. ACS Catal. 2021, 11, 6121–6127. [Google Scholar] [CrossRef]
- Mukherjee, S.; List, B. Chiral counteranions in asymmetric transition-metal catalysis: Highly enantioselective Pd/Brønsted acid-catalyzed direct α-allylation of aldehydes. J. Am. Chem. Soc. 2007, 129, 11336–11337. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Nimmagadda, S.K.; Liu, M.; Engle, K.M. Recent applications of chiral phosphoric acids in palladium catalysis. Org. Biomol. Chem. 2020, 18, 618–637. [Google Scholar] [CrossRef]
- Zhou, F.; Yamamoto, H. A powerful chiral phosphoric acid catalyst for enantioselective Mukaiyama-Mannich reactions. Angew. Chem. 2016, 128, 9116–9120. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, B.; Chen, Z.; Hu, J.; Zeng, X.; Zhong, G. Chiral phosphoric acid catalyzed enantioselective N-alkylation of indoles with in situ generated cyclic N-acyl ketimines. Chem. Commun. 2018, 54, 9230–9233. [Google Scholar] [CrossRef]
- Zhu, Y.; He, W.; Wang, W.; Pitsch, P.E.; Wang, X.; Wang, X. Enantioselective tandem cyclization of alkyne-tethered indoles using cooperative silver (I)/chiral phosphoric acid catalysis. Angew. Chem. 2017, 129, 12374–12377. [Google Scholar] [CrossRef]
- Kanaoka, Y.; Miyashita, K.; Yonemttsu, O. 3H-Indoles-II: Synthesis of 3-alkyl-3H-indoles by the alkylation of 2,3-disubstituted indoles with polyphosphate ester and some reactions of the 3H-indole system. Terahedron 1969, 25, 2757–2766. [Google Scholar] [CrossRef]
- Yang, L.; Wang, E.; Fan, Y.; Yang, J.; Luo, Z.; Wang, Y.; Peng, M.; Deng, T.; Yang, X. One-pot synthesis of (E)-3-benzylideneflavanones from 2-hydroxyacetophenones and aromatic aldehydes. Tetrahedron Lett. 2020, 61, 151180–151181. [Google Scholar] [CrossRef]
- Wang, E.; Yang, L.; Yang, Q.; Yang, F.; Luo, J.; Gan, M.; Wang, X.; Song, S.; Lei, Y.; Yang, X. Polyphosphoric acid-promoted one-pot synthesis and neuroprotective effects of flavanones against NMDA-induced injury in PC12 cells. RSC Adv. 2022, 12, 28098–28103. [Google Scholar] [CrossRef]
- Gaunt, M.J.; Spencer, J.B. Derailing the wacker oxidation: Development of a palladium-catalyzed amidation reaction. Org. Lett. 2000, 3, 25–28. [Google Scholar] [CrossRef]
- Tan, B.; Lu, Y.; Zeng, X.; Chua, P.J.; Zhong, G. Facile domino access to chiral bicyclo[3.2.1]octanes and discovery of a new catalytic activation mode. Org. Lett. 2010, 12, 2682–2685. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, W.; Richmond, L.; Han, Z.; Yang, W.; Tan, D.; Huang, K.W.; Jiang, Z. Direct asymmetric vinylogous aldol reaction of allyl ketones with isatins: Divergent synthesis of 3-hydroxy-2-oxindole derivatives. Angew. Chem. Int. Ed. 2013, 125, 6798–6802. [Google Scholar] [CrossRef]
- Cordi, A.A.; Desos, P.; Randle, J.C.R.; Lepagnol, J. Structure-activity relationships in a series of 3-sulfonylamino-2-(1H)-quinolones as new AMPA/kainate and glycine antagonists. Bioorg. Med. Chem. 1995, 3, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Desos, P.; Lepagnol, J.M.; Morain, P.; Lestage, P.; Cordi, A.A. Structure-activity relationships in a series of 2(1H)-quinolones bearing different acidic function in the 3-position: 6,7-dichloro-2(1H)-oxoquinoline-3-phosphonic acid, a new potent and selective AMPA/kainate antagonist with neuroprotective properties. J. Med. Chem. 1996, 39, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Choi, J.S.; Lee, J.I. An efficient synthesis of 1-alkyl-2-phenyl-4-quinolones from 2-halobenzoic acids. Bull. Korean Chem. Soc. 2013, 34, 3117–3120. [Google Scholar] [CrossRef] [Green Version]
- Klier, L.; Bresser, T.; Nigst, T.A.; Karaghiosoff, K.; Knochel, P. Lewis acid-triggered selective zincation of chromones, quinolones, and thiochromones: Application to the preparation of natural flavones and isoflavones. J. Am. Chem. Soc. 2012, 134, 13584–13587. [Google Scholar] [CrossRef]
- Wang, D.; Sun, P.; Jia, P.; Peng, J.; Yue, Y.; Chen, C. Transition-metal-free one-pot tandem synthesis of 4-quinolone and 4H-thiochromen-4-one derivatives through sequential nucleophilic addition-elimination-SNAr reaction. Synthesis 2017, 49, 4309–4320. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, ex-cited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Account. 2008, 120, 215–241. [Google Scholar]
- Ochterski, J.W.; Petersson, G.A.; Montgomery, J.A., Jr. A complete basis set model chemistry. V. Extensions to six or more heavy atoms. J. Chem. Phys. 1996, 104, 2598–2619. [Google Scholar]
- Grisafi, A.; Wilkins, D.M.; Csányi, G.; Ceriotti, M. Symmetry-adapted machine learning for tensorial properties of atomistic systems. Phys. Rev. Lett. 2018, 120, 036002. [Google Scholar]
- Lu, T.; Chen, Q. van der Waals potential: An important complement to molecular electrostatic potential in studying intermolecular interactions. J. Mol. Model. 2020, 26, 315. [Google Scholar]
- Peterson, K.I.; Pullman, D.P. Determining the structure of oxalate anion using infrared and Raman spectroscopy coupled with Gaussian calculations. J. Chem. Educ. 2016, 93, 1130–1133. [Google Scholar]
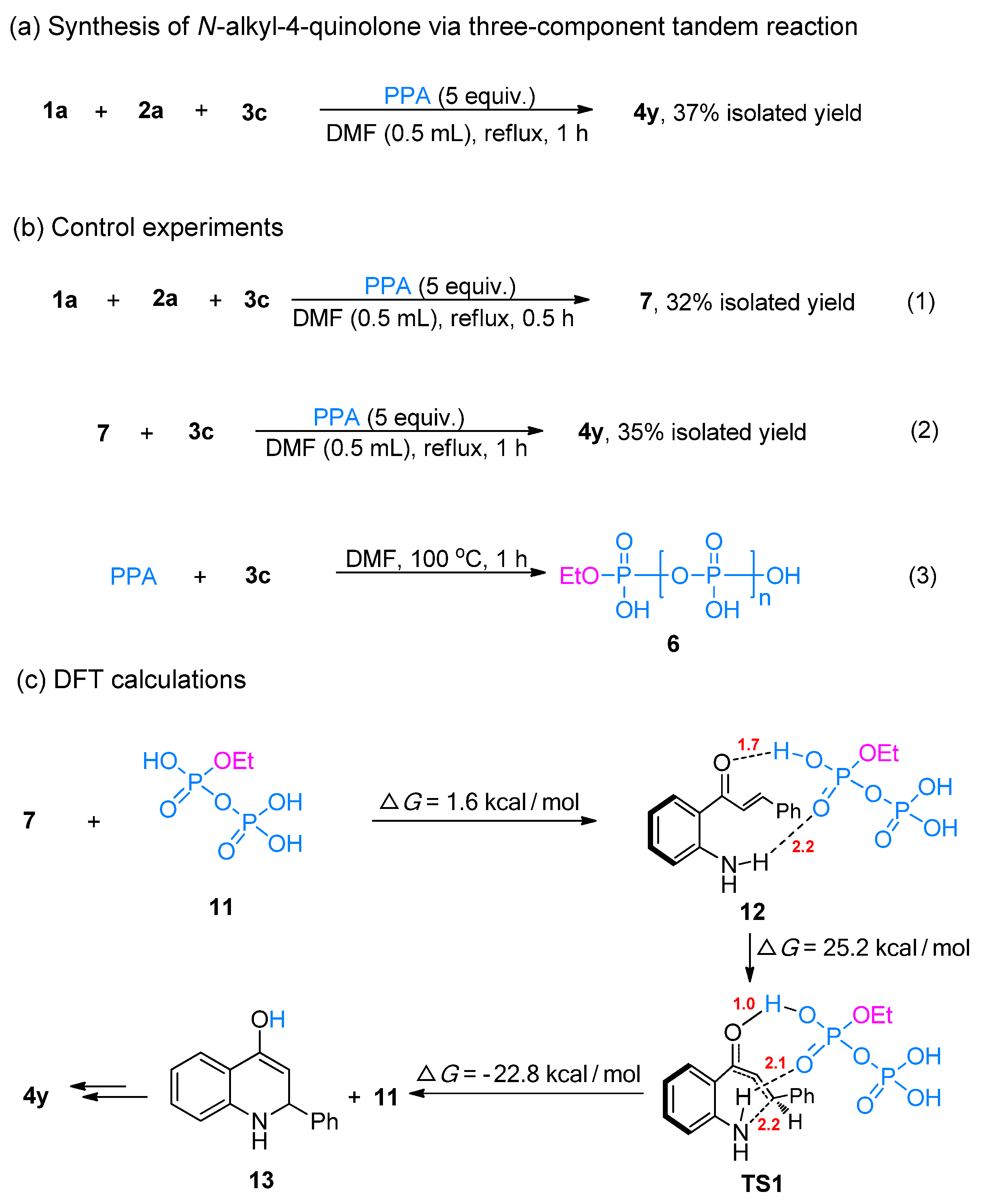

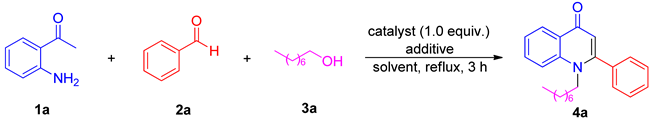 | ||||
|---|---|---|---|---|
| Entry | Additive | Solvent | 4a (%) b | |
| 1 | P2O5 | DMF | 69 | |
| 2 c | PPA/P2O5 | DMF | 58 | |
| 3 | PPA | DMA | 50 | |
| 4 | PPA | acetone | 24 | |
| 5 | PPA | CH3CN | 63 | |
| 6 | PPA | DCM | 56 | |
| 7 | PPA | toluene | 29 | |
| 8 | PPA | ether | ND | |
| 9 | PPA | 4 Å MS | DMF | 78 |
| 10 d | PPA | DMF | 70 | |
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Liu, H.; Wang, E.; Li, L.; Luo, Z.; Cao, J.; Chen, J.; Yang, L.; Yang, X. Hydrogen Bond Assisted Three-Component Tandem Reactions to Access N-Alkyl-4-Quinolones. Molecules 2023, 28, 2304. https://doi.org/10.3390/molecules28052304
Liu H, Liu H, Wang E, Li L, Luo Z, Cao J, Chen J, Yang L, Yang X. Hydrogen Bond Assisted Three-Component Tandem Reactions to Access N-Alkyl-4-Quinolones. Molecules. 2023; 28(5):2304. https://doi.org/10.3390/molecules28052304
Chicago/Turabian StyleLiu, Huanhuan, Huadan Liu, Enhua Wang, Liangqun Li, Zhongsheng Luo, Jiafu Cao, Jialin Chen, Lishou Yang, and Xiaosheng Yang. 2023. "Hydrogen Bond Assisted Three-Component Tandem Reactions to Access N-Alkyl-4-Quinolones" Molecules 28, no. 5: 2304. https://doi.org/10.3390/molecules28052304






