Adsorption Performance of Methylene Blue by KOH/FeCl3 Modified Biochar/Alginate Composite Beads Derived from Agricultural Waste
Abstract
:1. Introduction
2. Results and Discussion
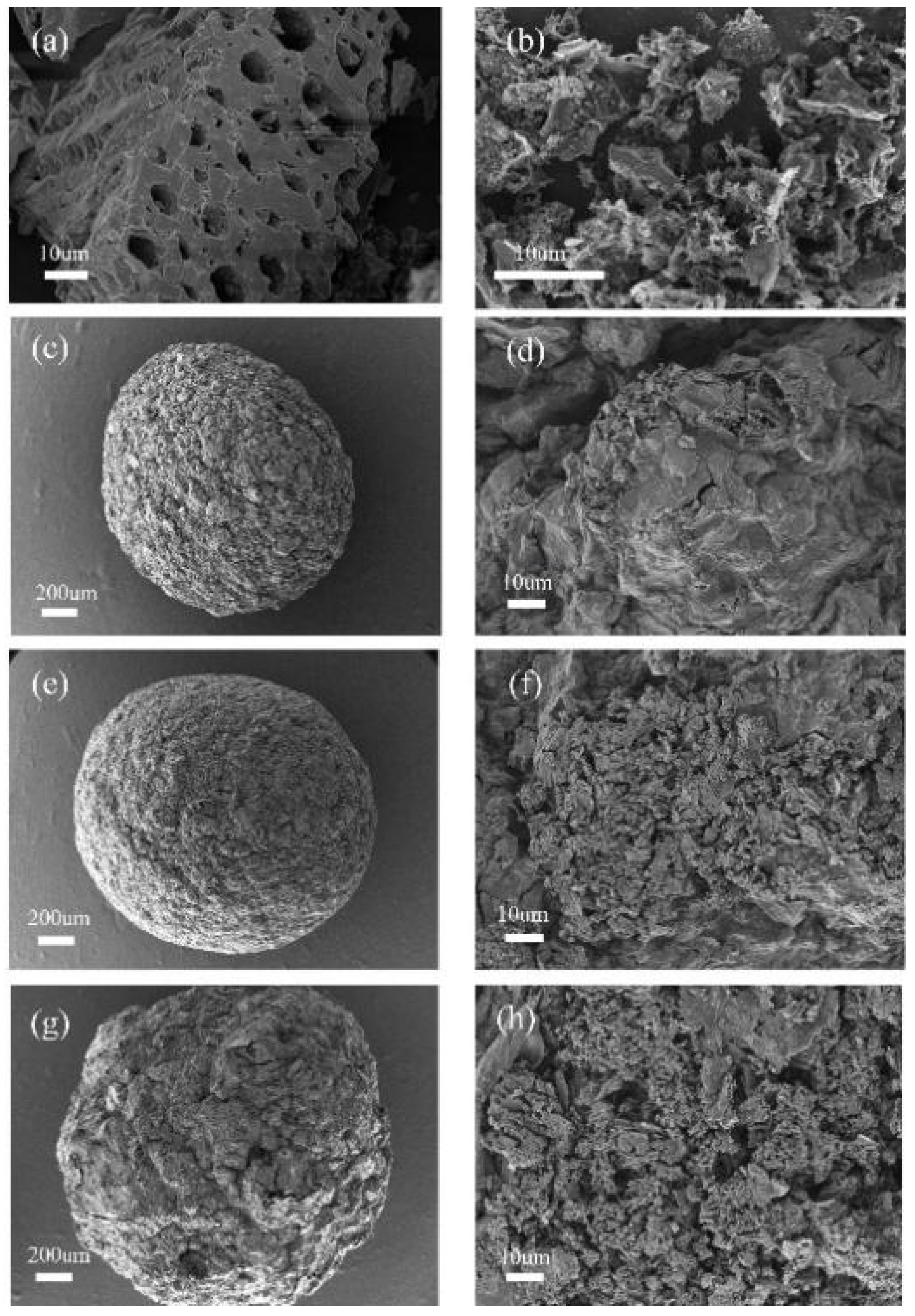
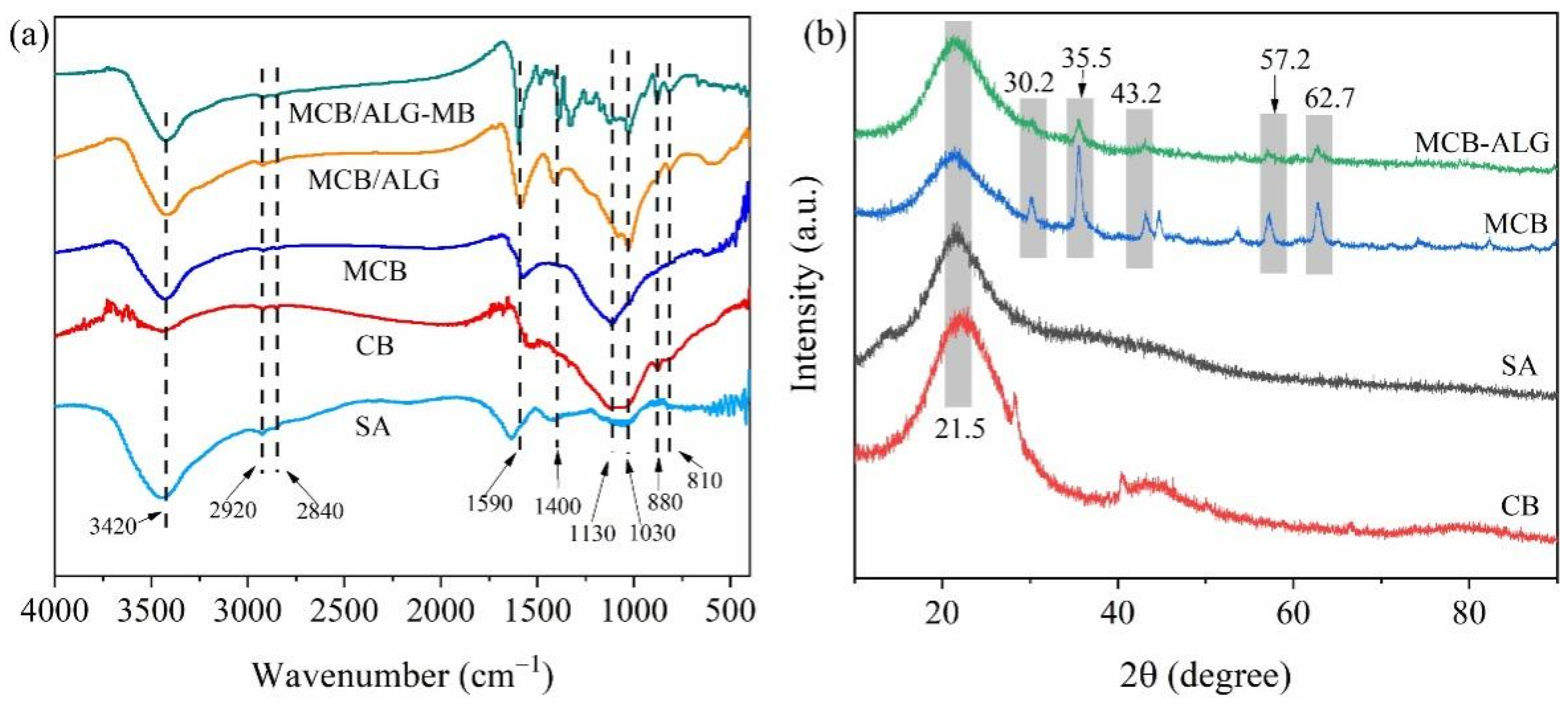
2.1. Characterization
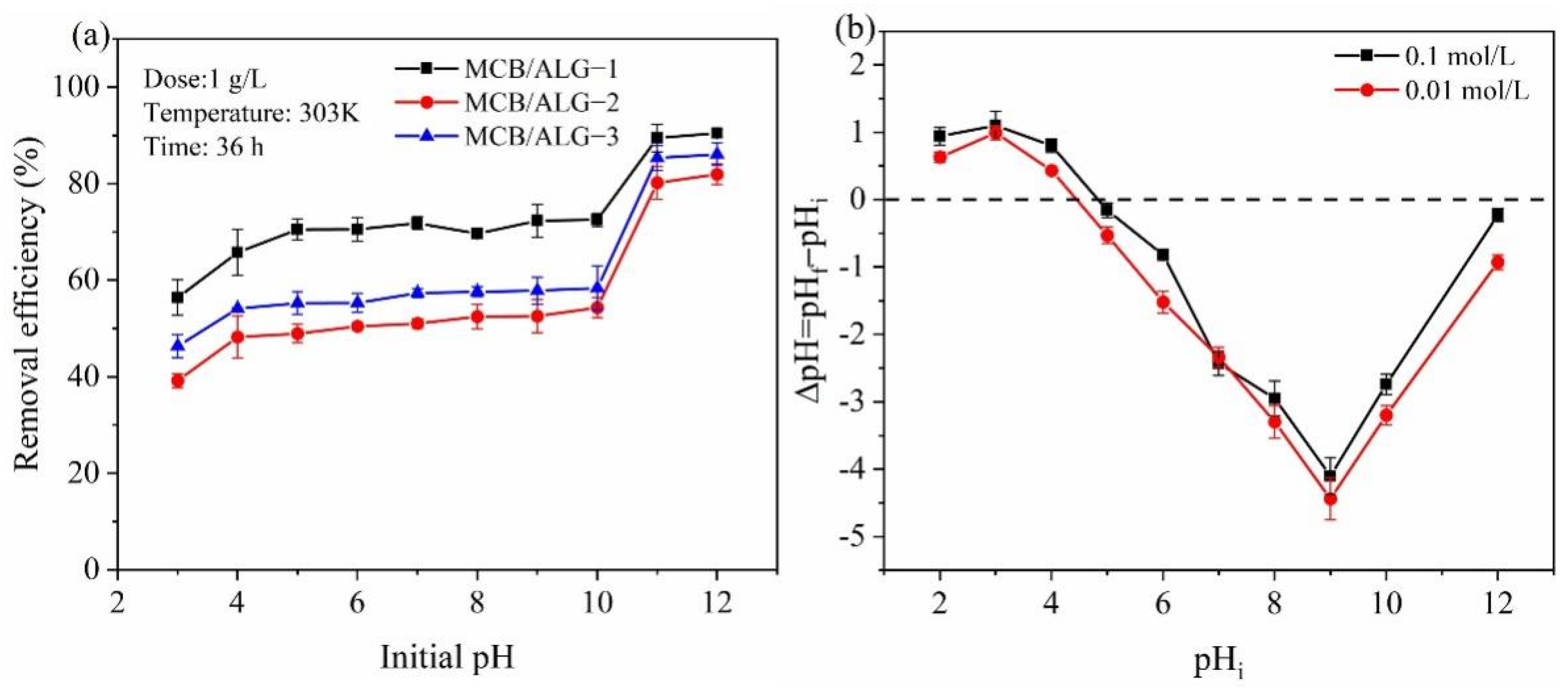
2.2. Effect of pH on Adsorption
2.3. Adsorption Kinetics
2.4. Adsorption Isotherm
2.5. Adsorption Thermodynamics
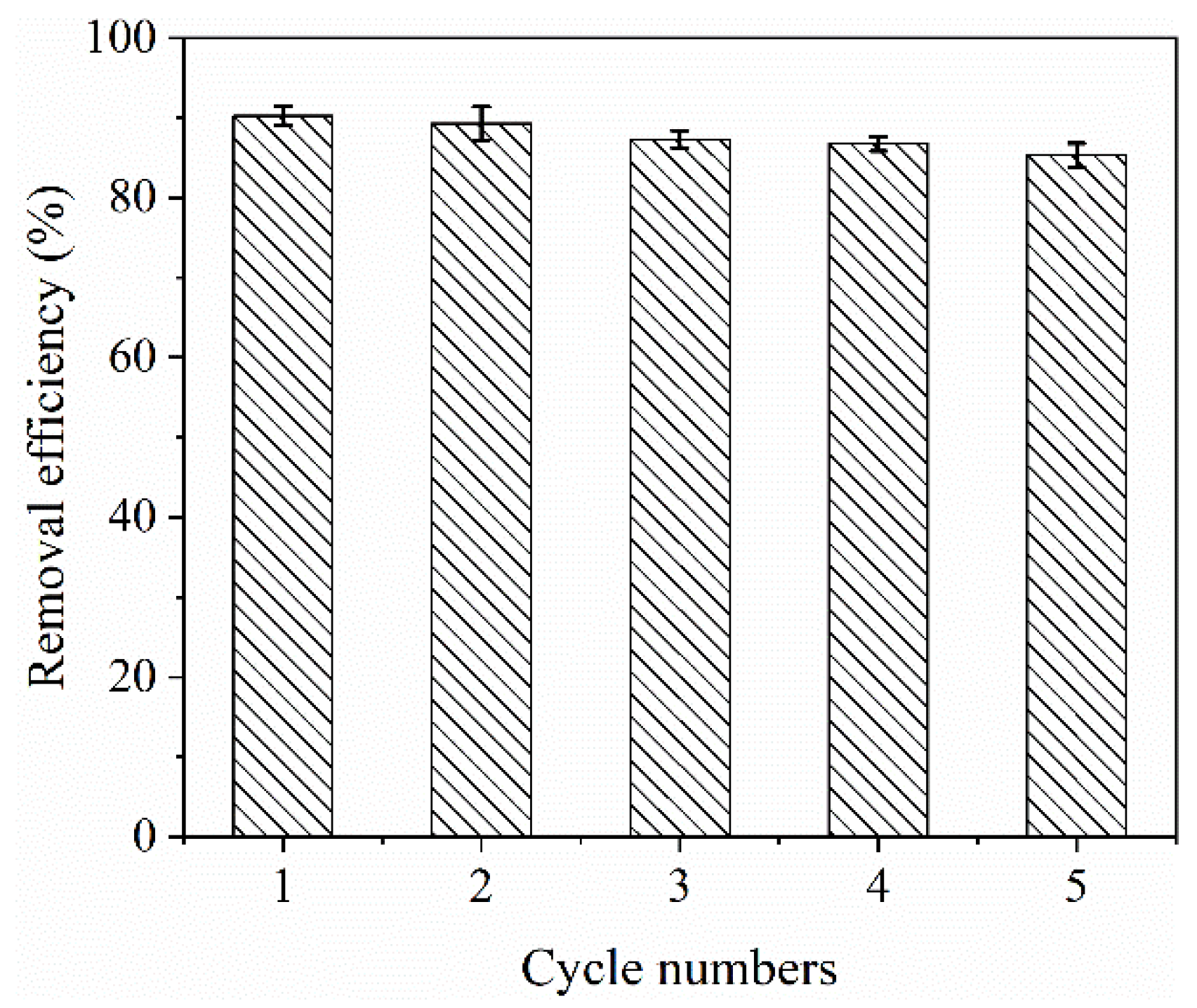
2.6. Regeneration and Reusability
3. Materials and Methods
3.1. Materials
3.2. Preparation of KOH/FeCl3 Modified Corncob Biochar (MCB)
3.3. Preparation of MCB/ALG Composite Beads
3.4. Characteristics of Samples
3.5. Batch Adsorption Experiments
3.6. Regeneration Experiment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, H.; Yang, B.; Liu, Y.; Li, F.; Shen, C.; Ma, C.; Tian, Q.; Song, X.; Sand, W. Recent advances in anaerobic biological processes for textile printing and dyeing wastewater treatment: A mini-review. World J. Microbiol. Biotechnol. 2018, 34, 165. [Google Scholar] [CrossRef]
- Ulu, A.; Alpaslan, M.; Gultek, A.; Ates, B. Eco-friendly chitosan/κ-carrageenan membranes reinforced with activated bentonite for adsorption of methylene blue. Mater. Chem. Phys. 2022, 278, 125611. [Google Scholar] [CrossRef]
- Fayazi, M.; Taher, M.A.; Afzali, D.; Mostafavi, A. Enhanced Fenton-like degradation of methylene blue by magnetically activated carbon/hydrogen peroxide with hydroxylamine as Fenton enhancer. J. Mol. Liq. 2016, 216, 781–787. [Google Scholar] [CrossRef]
- Shi, J.; Bai, X.; Xu, L.; Jin, X.; Shi, X.; Jin, P. Facile preparation of Fe-C3N4 heterojunction for enhanced pollutant degradation in Fenton-like process. J. Water Process. Eng. 2022, 46, 102628. [Google Scholar] [CrossRef]
- Sun, J.; Yu, M.; Kang, R.; Sun, H.; Zhang, Y.; Wang, N. Self-assembled graphene aerogels for removal of methylene blue and copper from aqueous solutions. J. Hazard. Mater. 2021, 4, 100026. [Google Scholar] [CrossRef]
- Kumar, K.V.; Ramamurthi, V.; Sivanesan, S. Modeling the mechanism involved during the sorption of methylene blue onto fly ash. J. Colloid Interface Sci. 2005, 284, 14–21. [Google Scholar] [CrossRef]
- Makhado, E.; Pandey, S.; Nomngongo, P.N.; Ramontja, J. Fast microwave-assisted green synthesis of xanthan gum grafted acrylic acid for enhanced methylene blue dye removal from aqueous solution. Carbohydr. Polym. 2017, 176, 315–326. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, H.; Wang, C.; Li, B.; Yang, H.; Hou, H.; Xiao, C. TiO2-coated glass hollow fiber membranes: Preparation and application for photocatalytic methylene blue removal. J. Eur. Ceram. Soc. 2022, 42, 2496–2504. [Google Scholar] [CrossRef]
- Wang, K.S.; Wei, M.C.; Peng, T.H.; Li, H.C.; Chao, S.J.; Hsu, T.F.; Lee, H.S.; Chang, S.H. Treatment and toxicity evaluation of methylene blue using electrochemical oxidation, fly ash adsorption and combined electrochemical oxidation-fly ash adsorption. J. Environ. Manag. 2010, 91, 1778–1784. [Google Scholar] [CrossRef]
- Tang, X.; Ran, G.; Li, J.; Zhang, Z.; Xiang, C. Extremely efficient and rapidly adsorb methylene blue using porous adsorbent prepared from waste paper: Kinetics and equilibrium studies. J. Hazard. Mater. 2021, 402, 123579. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Xie, X.; Wang, Z. Bifunctional MnFe2O4/chitosan modified biochar composite for enhanced methyl orange removal based on adsorption and photo-Fenton process. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126104. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, F.; Yang, X.; Long, G.; Wu, Y.; Zhang, T.; Leng, K.; Huang, Y.; Ma, Y.; Yu, A.; et al. Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors. Sci. Rep. 2013, 3, 1408. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhang, L.; Wang, Y.; Liu, X.; Rohani, S.; Lu, J. Fe3O4@SiO2@CS-TETA functionalized graphene oxide for the adsorption of methylene blue(MB) and Cu(II). Appl. Surf. Sci. 2017, 420, 970–981. [Google Scholar] [CrossRef]
- Momina; Mohammad, S.; Suzylawati, I. Study of the adsorption/desorption of MB dye solution using bentonite adsorbent coating. J. Water Process. Eng. 2020, 34, 101155. [Google Scholar] [CrossRef]
- Ullah, N.; Ali, Z.; Ullah, S.; Khan, A.S.; Adalat, B.; Nasrullah, A.; Alsaadi, M.; Ahmad, Z. Synthesis of activated carbon-surfactant modified montmorillonite clay-alginate composite membrane for methylene blue adsorption. Chemosphere 2022, 309, 136623. [Google Scholar] [CrossRef]
- Rahmi; Ishmaturrahmi; Mustafa, I. Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchem. J. 2019, 144, 397–402. [Google Scholar] [CrossRef]
- Chen, J.; Ouyang, J.; Cai, X.; Xing, X.; Zhou, L.; Liu, Z.; Cai, D. Removal of ciprofloxacin from water by millimeter-sized sodium alginate/H3PO4 activated corncob-based biochar composite beads. Sep. Purif. Technol. 2021, 276, 119371. [Google Scholar] [CrossRef]
- Dilamian, M.; Noroozi, B. Rice straw agri-waste for water pollutant adsorption: Relevant mesoporous super hydrophobic cellulose aerogel. Carbohydr. Polym. 2021, 251, 117016. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified biochar: Synthesis and mechanism for removal of environmental heavy metals. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Lucaci, A.R.; Bulgariu, D.; Ahmad, I.; Lisă, G.; Mocanu, A.M.; Bulgariu, L. Potential use of biochar from various waste biomass as biosorbent in Co(II) removal processes. Water 2019, 11, 1565. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, J.; Chen, J.; Chen, W.; Zhou, L.; Cai, D.; Ren, C. H3PO4 activated biochars derived from different agricultural biomasses for the removal of ciprofloxacin from aqueous solution. Particuology 2023, 75, 217–227. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Li, J.; Fang, Z.; Bolan, N.; Bhatnagar, A.; Gao, B.; Hou, D.; Wang, S.; Song, H.; et al. Engineered biochar for environmental decontamination in aquatic and soil systems: A review. Carbon Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Luo, M.; Lin, H.; Li, B.; Dong, Y.; He, Y.; Wang, L. A novel modification of lignin on corncob-based biochar to enhance removal of cadmium from water. Bioresour. Technol. 2018, 259, 312–328. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, H.X. Modified-biochar adsorbents(MBAs) for heavy-metal ions adsorption: A critical review. J. Environ. Chem. Eng. 2022, 10, 107393. [Google Scholar] [CrossRef]
- Chen, H.; Yang, X.; Liu, Y.; Lin, X.; Wang, J.; Zhang, Z.; Li, N.; Li, Y.; Zhang, Y. KOH modification effectively enhances the Cd and Pb adsorption performance of N-enriched biochar derived from waste chicken feathers. Waste Manag. 2021, 130, 82–92. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, S.; Qi, Y.; Yang, L.; Wu, L.; He, L.; Li, P.; Qi, X.; Gao, F.; Ding, Y.; et al. An efficient, green and sustainable potassium hydroxide activated magnetic corn cob biochar for imidacloprid removal. Chemosphere 2022, 291, 132707. [Google Scholar] [CrossRef]
- Bedia, J.; Belver, C.; Ponce, S.; Rodriguez, J.; Rodriguez, J.J. Adsorption of antipyrine by activated carbons from FeCl3-activation of Tara gum. Chem. Eng. J. 2018, 333, 58–65. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, X.; Li, Y.; Zhao, C.; Du, Q.; Sun, J.; Wang, Y.; Peng, X.; Xia, Y.; Wang, Z.; et al. Adsorption of ciprofloxacin onto biocomposite fibers of graphene oxide/calcium alginate. Chem. Eng. J. 2013, 230, 389–395. [Google Scholar] [CrossRef]
- Mao, W.; Yue, W.; Xu, Z.; Chang, S.; Hu, Q.; Pei, F.; Huang, X.; Zhang, J.; Li, D.; Liu, G.; et al. Development of a synergistic activation strategy for the pilot-scale construction of hierarchical porous graphitic carbon for energy storage applications. ACS Nano 2020, 14, 4741–4754. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution(IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.Y.; Yang, Q.; Li, X.M.; Luo, K.; Liu, Y.; Zeng, G.M. Preparation of peanut hull-based activated carbon by microwave-induced phosphoric acid activation and its application in Remazol Brilliant Blue R adsorption. Ind. Crops Prod. 2012, 37, 178–185. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Wu, R.; Liu, Y.; Lin, X.; Kan, H.; Zheng, Y. Preparation of acid-and alkali-modified biochar for removal of methylene blue pigment. ACS Omega 2020, 5, 30906–30922. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Xie, F.; Jiang, M.; Ke-Ao, L.; Tian, S.G. Physicochemical properties and lead ion adsorption of biochar prepared from Turkish gall residue at different pyrolysis temperatures. Microsc. Res. Tech. 2021, 84, 1003–1011. [Google Scholar] [CrossRef]
- Yin, Z.; Xu, S.; Liu, S.; Xu, S.; Li, J.; Zhang, Y. A novel magnetic biochar prepared by K2FeO4-promoted oxidative pyrolysis of pomelo peel for adsorption of hexavalent chromium. Bioresour. Technol. 2020, 300, 122680. [Google Scholar] [CrossRef]
- Cheng, L.; Ji, Y.; Liu, X.; Mu, L.; Zhu, J. Sorption mechanism of organic dyes on a novel self-nitrogen-doped porous graphite biochar: Coupling DFT calculations with experiments. Chem. Eng. Sci. 2021, 242, 116739. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, M.F.; Ma, J.-F.; Bian, J.; Peng, F. Chitosan crosslinked composite based on corncob lignin biochar to adsorb methylene blue: Kinetics, isotherm, and thermodynamics. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128621. [Google Scholar] [CrossRef]
- Lin, Q.; Gao, M.; Chang, J.; Ma, H. Adsorption properties of crosslinking carboxymethyl cellulose grafting dimethyldiallylammonium chloride for cationic and anionic dyes. Carbohydr. Polym. 2016, 151, 283–294. [Google Scholar] [CrossRef]
- Yan, L.; Liu, Y.; Zhang, Y.; Liu, S.; Wang, C.; Chen, W.; Liu, C.; Chen, Z.; Zhang, Y. ZnCl2 modified biochar derived from aerobic granular sludge for developed microporosity and enhanced adsorption to tetracycline. Bioresour. Technol. 2020, 297, 122381. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Y.; Tian, X.; Jiang, Z.; Deng, F.; Tao, Y.; Jiang, Q.; Wang, L.; Zhang, Y. KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium(VI) and naphthalene from water: Affecting factors, mechanisms and reusability exploration. J. Hazard. Mater. 2021, 401, 123292. [Google Scholar] [CrossRef]
- Huang, X.Y.; Bu, H.-T.; Jiang, G.B.; Zeng, M.H. Cross-linked succinyl chitosan as an adsorbent for the removal of Methylene Blue from aqueous solution. Int. J. Biol. Macromol. 2011, 49, 643–651. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Liu, H. Insight into the adsorption of tetracycline onto amino and amino-Fe3+ gunctionalized mesoporous silica: Effect of functionalized groups. J. Environ. Sci. 2018, 65, 171–178. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, D.; Xu, Y.; Chang, H.; Shen, H.; Xu, C.; Mou, J.; Zhong, N. Enhanced removal of Cr(VI) from aqueous solution by nano- zero-valent iron supported by KOH activated sludge-based biochar. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129697. [Google Scholar] [CrossRef]
- Jamion, N.A.; Hashim, I. Preparation of activated carbon from tamarind seeds and Methylene blue(MB) removal. J. Fundam. Appl. Sci. 2018, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Li, J.; Wang, L. KOH-activated carbon aerogels derived from sodium carboxymethyl cellulose for high-performance supercapacitors and dye adsorption. Chem. Eng. J. 2017, 310, 300–306. [Google Scholar] [CrossRef]
- Liu, Q.S.; Zheng, T.; Li, N.; Wang, P.; Abulikemu, G. Modification of bamboo-based activated carbon using microwave radiation and its effects on the adsorption of methylene blue. Appl. Surf. Sci. 2010, 256, 3309–3315. [Google Scholar] [CrossRef]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated carbon prepared from NaOH activation of rattan(Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol. Environ. Saf. 2017, 138, 279–385. [Google Scholar] [CrossRef]
- Reffas, A.; Bernardet, V.; David, B.; Reinert, L.; Lehocine, M.B.; Dubois, M.; Batisse, N.; Duclaux, L. Carbons prepared from coffee grounds by H3PO4 activation: Characterization and adsorption of methylene blue and Nylosan Red N-2RBL. J. Hazard. Mater. 2010, 175, 779–788. [Google Scholar] [CrossRef]
- Gokce, Y.; Aktas, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359. [Google Scholar] [CrossRef]
- Yu, Y.; Wan, Y.; Shang, H.; Wang, B.; Zhang, P.; Feng, Y. Corncob-to-xylose residue(CCXR) derived porous biochar as an excellent adsorbent to remove organic dyes from wastewater. Surf. Interface Anal. 2019, 51, 234–245. [Google Scholar] [CrossRef]
- Andersson, K.I.; Eriksson, M.; Norgren, M. Removal of lignin from wastewater generated by mechanical pulping using activated charcoal and fly ash: Adsorption isotherms and thermodynamics. Ind. Eng. Chem. Res. 2011, 50, 7722–7732. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Zhang, X.; Varjani, S.; Liu, Y. Feasibility study on a new pomelo peel derived biochar for tetracycline antibiotics removal in swine wastewater. Sci. Total Environ. 2020, 720, 137662. [Google Scholar] [CrossRef]
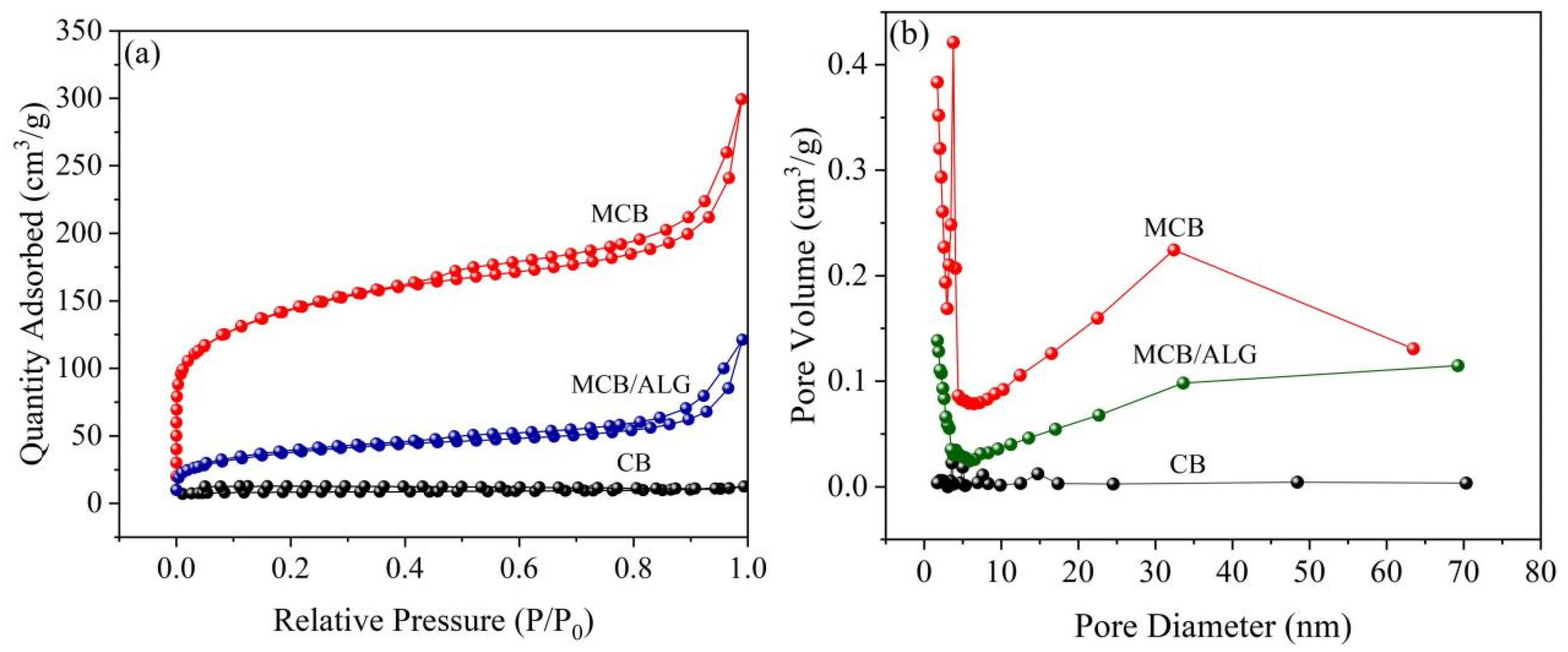



| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| CB | 25.9 | 0.006 | 7.6 |
| MCB | 468.4 | 0.3 | 6.0 |
| MCB/ALG | 128.8 | 0.1 | 8.1 |
| Sample | qe,exp (mg/L) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| qe,cal (mg/g) | k1 (/min) | RMSE | qe,cal (mg/g) | k2 (g/mg·min) | RMSE | ||
| MCB/ALG−1 | 1040.7 | 957.3 | 0.005 | 105.1 | 1055.2 | 7.31 × 10−6 | 81.4 |
| MCB | 585.92 | 525.8 | 0.02 | 46.0 | 562.7 | 6.09 × 10−5 | 25.1 |
| T (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | RMSE | KF (L/mg) | nF | RMSE | |
| 303 | 1373.49 | 0.0030 | 105.05 | 55.93 | 2.37 | 127.88 |
| 313 | 1457.28 | 0.0031 | 94.47 | 59.26 | 2.3 | 116.74 |
| 323 | 1485.03 | 0.0037 | 106.91 | 70.73 | 2.45 | 127.25 |
| Raw | Pyrolysis Temperature (°C) | qm (mg/g) | Reference |
|---|---|---|---|
| Tamarind seed | 500 | 102.77 | [43] |
| Sodium carboxymethyl cellulose | 900 | 249.6 | [44] |
| Bamboo | 600 | 286.1 | [45] |
| Alfalfa | 600 | 326.90 | [35] |
| Rattan stalks | 600 | 359 | [46] |
| Coffee grounds | 600 | 367 | [47] |
| Waste tea | 450 | 683.6 | [48] |
| Corncobs | 800 | 1373.49 | This work |
| Corncob-to-xylose residue | 850 | 1563.9 | [49] |
| T (K) | ΔG (kJ·mol −1) | ΔH (kJ·mol −1) | ΔS (kJ·mol −1 K−1) |
|---|---|---|---|
| 303 | −0.2551 | 9.4163 | 0.0754 |
| 313 | −0.3726 | ||
| 323 | −0.6369 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhu, J.; Li, Q.; Li, L.; Huang, Y.; Wang, Y.; Fan, G.; Zhang, L. Adsorption Performance of Methylene Blue by KOH/FeCl3 Modified Biochar/Alginate Composite Beads Derived from Agricultural Waste. Molecules 2023, 28, 2507. https://doi.org/10.3390/molecules28062507
Liu H, Zhu J, Li Q, Li L, Huang Y, Wang Y, Fan G, Zhang L. Adsorption Performance of Methylene Blue by KOH/FeCl3 Modified Biochar/Alginate Composite Beads Derived from Agricultural Waste. Molecules. 2023; 28(6):2507. https://doi.org/10.3390/molecules28062507
Chicago/Turabian StyleLiu, Heng, Jiaqi Zhu, Qimei Li, Likun Li, Yanjun Huang, Yi Wang, Guozhi Fan, and Lei Zhang. 2023. "Adsorption Performance of Methylene Blue by KOH/FeCl3 Modified Biochar/Alginate Composite Beads Derived from Agricultural Waste" Molecules 28, no. 6: 2507. https://doi.org/10.3390/molecules28062507






