Physicochemical Properties of Plasma-Activated Water and Its Control Effects on the Quality of Strawberries
Abstract
:1. Introduction
2. Results and Discussion
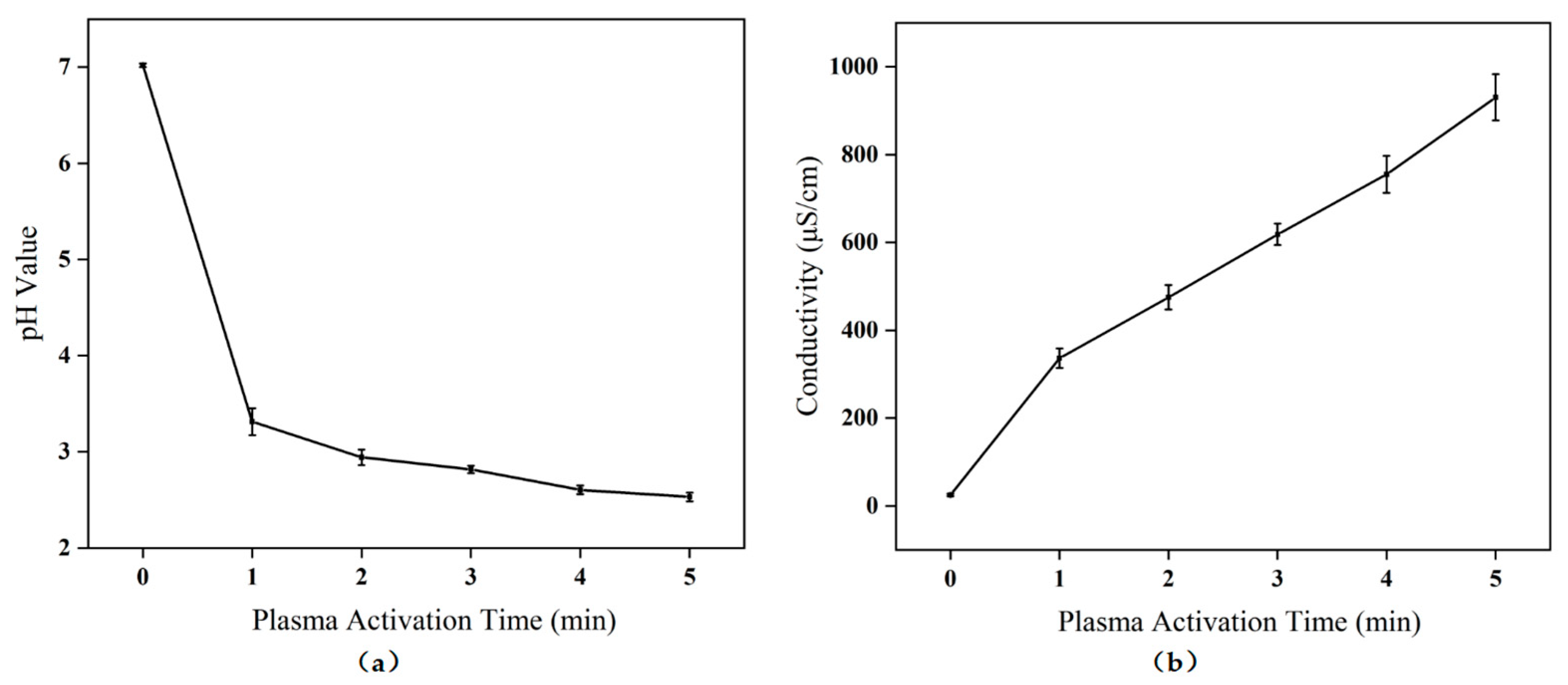
2.1. pH and Electrical Conductivity Changes of PAW
2.2. NO2− and NO3− Concentration Changes of PAW
2.3. H2O2 and O2− Concentration Changes of PAW
2.4. Visual Assessment of Strawberry during Storage
2.5. Strawberry Quality Changes
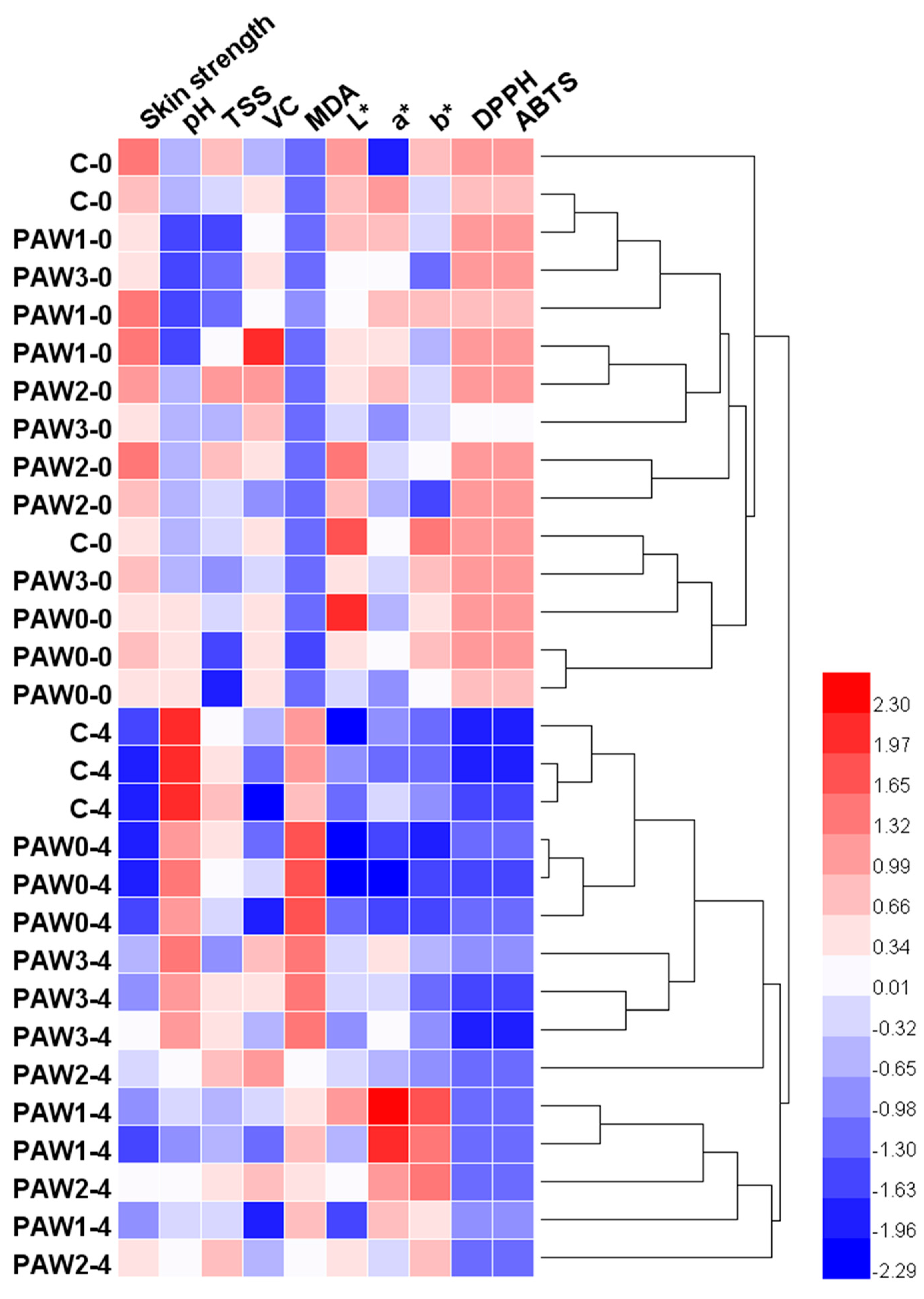
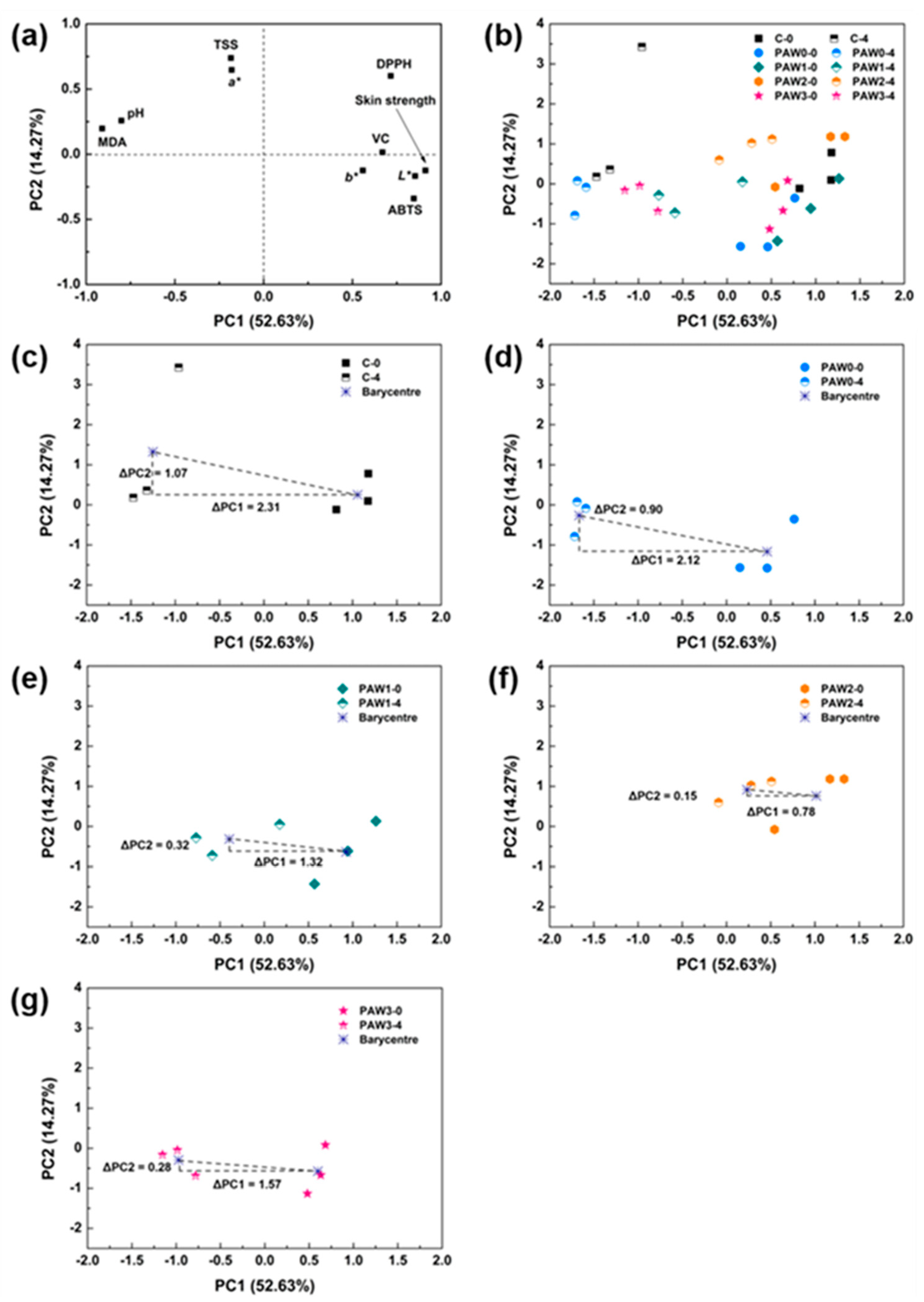
2.6. Optimal Plasma Processing Condition Selection Using Multivariate Analysis Techniques
2.6.1. Hierarchical Cluster Analysis
2.6.2. Principal Component Analysis
3. Material and Methods
3.1. Materials and Chemicals
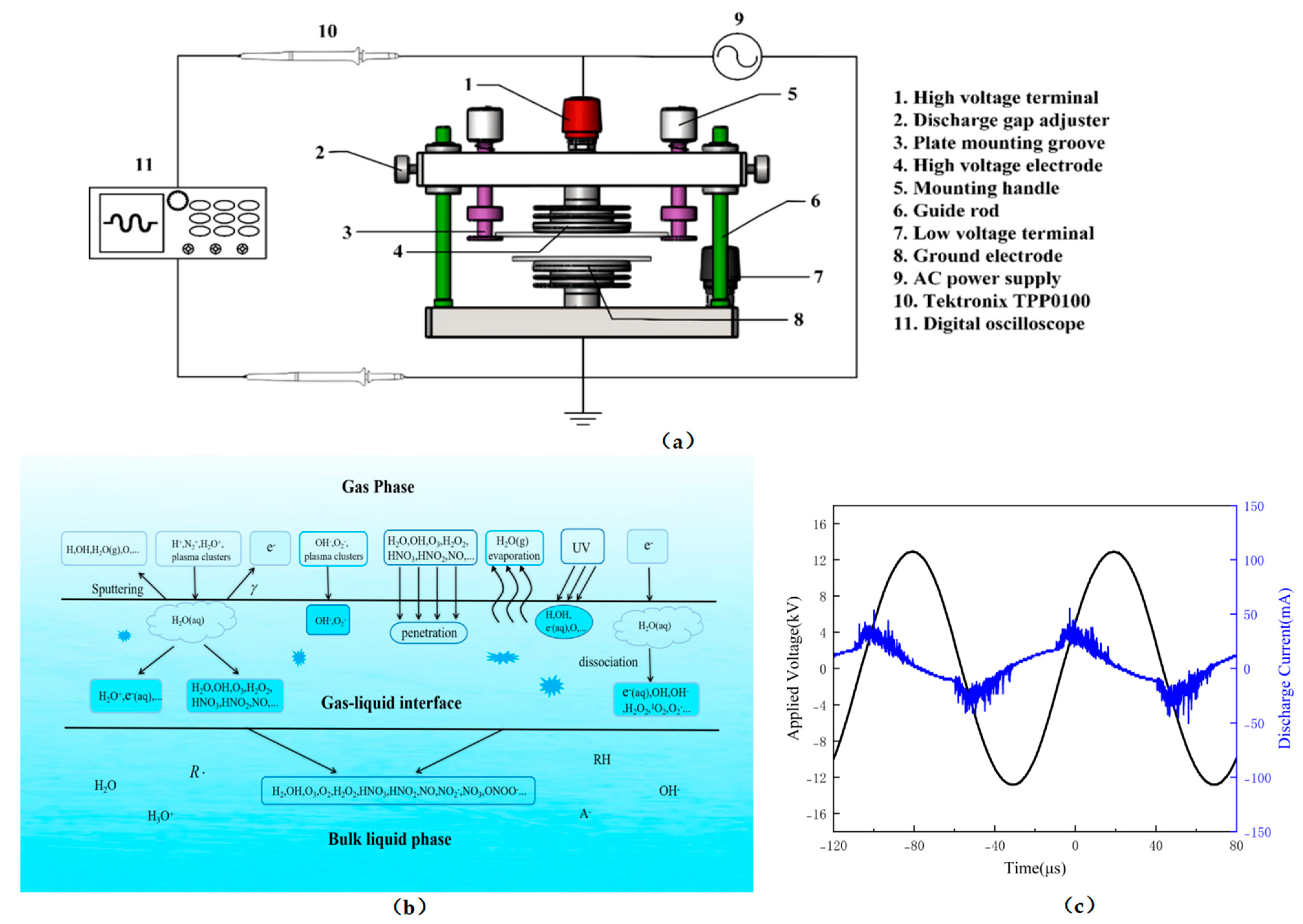
3.2. Plasma Device and Preparation of PAW
3.3. Pretreatment of Strawberries
3.4. Measurement of Physicochemical Properties of PAW
3.5. Quality Analysis of Strawberry
3.5.1. pH Measurement
3.5.2. Firmness
3.5.3. Color Quality
3.5.4. Total Soluble Solids
3.6. Malondialdehyde
3.7. Vitamin C Content
3.8. Determination of Antioxidant Activities
3.8.1. Total Antioxidant Capacity
3.8.2. DPPH Radical Scavenging Activity
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cordenunsi, B.R.; Genovese, M.I.; do Nascimento, J.R.O.; Hassimotto, N.M.A.; dos Santos, R.J.; Lajolo, F.M. Effects of temperature on the chemical composition and antioxidant activity of three strawberry cultivars. Food Chem. 2005, 91, 113–121. [Google Scholar] [CrossRef]
- Sahari, M.A.; Boostani, F.M.; Hamidi, E.Z. Effect of low temperature on the ascorbic acid content and quality characteristics of frozen strawberry. Food Chem. 2004, 86, 357–363. [Google Scholar] [CrossRef]
- Maraei, R.W.; Elsawy, K.M. Chemical quality and nutrient composition of strawberry fruits treated by γ-irradiation. J. Hazard. Mater. 2017, 10, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Teribia, N.; Buvé, C.; Bonerz, D.; Aschoff, J.; Hendrickx, M.; Van Loey, A. Impact of processing and storage conditions on color stability of strawberry puree: The role of PPO reactions revisited. J. Food Eng. 2021, 294, 110402. [Google Scholar] [CrossRef]
- Niemira, B.A. Cold Plasma Decontamination of Foods. Annu. Rev. Food Sci. Technol. 2012, 3, 125–142. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Mir, M.M. Understanding the role of plasma technology in food industry. Food Bioprocess Technol. 2016, 9, 734–750. [Google Scholar] [CrossRef]
- Kolb, J.F.; Mattson, A.M.; Edelblute, C.M.; Hao, X.L.; Malik, M.A.; Heller, L.C. Cold DC-operated air plasma jet for the inactivation of infectious microorganisms. IEEE Trans. Plasma Sci. 2012, 40, 3007–3026. [Google Scholar] [CrossRef]
- Butscher, D.; Zimmermann, D.; Schuppler, M.; von Rohr, P.R. Plasma inactivation of bacterial endospores on wheat grains and polymeric model substrates in a dielectric barrier discharge. Food Control 2016, 60, 636–645. [Google Scholar] [CrossRef]
- Xiang, Q.; Fan, L.; Li, Y.; Dong, S.; Li, K.; Bai, Y. A review on recent advances in plasma-activated water for food safety: Current applications and future trends. Crit. Rev. Food Sci. Nutr. 2022, 62, 2250–2268. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Z.; Cheng, C.; Wei, J.; Lan, Y.; Ni, G.; Sun, Q.; Qian, S.; Zhang, H.; Xia, W.; et al. Bactericidal effects of plasma induced reactive species in dielectric barrier gas-liquid discharge. Plasma Chem. Plasma Process. 2017, 37, 415–431. [Google Scholar] [CrossRef]
- Kelar Tucekova, Z.; Vacek, L.; Krumpolec, R.; Kelar, J.; Zemanek, M.; Cernak, M.; Ruzicka, F. Multi-hollow surface dielectric barrier discharge for bacterial biofilm decontamination. Molecules 2021, 26, 910. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Tian, E.; Song, Y.; Sosnin, E.A.; Skakun, V.S.; Li, T.; Xia, Y.; Zhao, Y.; Lin, X.; Liu, D. Inactivation of shewanella putrefaciens by plasma activated water. Plasma Chem. Plasma Process. 2018, 38, 1035–1050. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, R.; Zhang, Q.; Feng, H.; Liang, Y.; Zhang, J.; Fang, J. Assessment of the physicochemical properties and biological effects of water activated by non-thermal plasma above and beneath the water surface. Plasma Process. Polym. 2015, 12, 439–449. [Google Scholar] [CrossRef]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold atmospheric plasma-activated water irrigation induces defense hormone and gene expression in tomato seedlings. Sci. Rep. 2019, 9, 16080. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Yao, Z.; Yang, L.; Zhang, H.; Qi, Y.; Gou, L.; Xi, W.; Liu, D.; Zhang, L.; Cheng, Y.; et al. Plasma-activated water: An alternative disinfectant for S protein inactivation to prevent SARS-CoV-2 infection. Chem. Eng. J. 2021, 421, 127742. [Google Scholar] [CrossRef]
- Guo, L.; Xu, R.; Gou, L.; Liu, Z.; Zhao, Y.; Liu, D.; Zhang, L.; Chen, H.; Kong, M.G. Mechanism of Virus Inactivation by Cold Atmospheric-Pressure Plasma and Plasma-Activated Water. App. Environ. Microbiol. 2018, 84, e00726-18. [Google Scholar] [CrossRef] [Green Version]
- Guragain, R.P.; Baniya, H.B.; Pradhan, S.P.; Pandey, B.P.; Subedi, D.P. Influence of plasma-activated water (PAW) on the germination of radish, fenugreek, and pea seeds. AIP Adv. 2021, 11, 125304. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, A.; Heroux, P.; Sand, W.; Sun, Z.; Zhan, J.; Wang, C.; Hao, S.; Li, Z.; Li, Z.; et al. Effect of Dielectric Barrier Discharge Cold Plasma on Pea Seed Growth. J. Agric. Food Chem. 2019, 67, 10813–10822. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Oliveira, M.; Burgess, C.M.; Kerry, J.P.; Tiwari, B.K. Plasma-activated water as an alternative nitrite source for the curing of beef jerky: Influence on quality and inactivation of Listeria innocua. Innov. Food Sci. Emerg. Technol. 2020, 59, 102276. [Google Scholar] [CrossRef]
- Ali, M.; Sun, D.-W.; Cheng, J.-H.; Esua, O.J. Effects of combined treatment of plasma activated liquid and ultrasound for degradation of chlorothalonil fungicide residues in tomato. Food Chem. 2022, 371, 131162. [Google Scholar] [CrossRef]
- Sarangapani, C.; Scally, L.; Gulan, M.; Cullen, P.J. Dissipation of pesticide residues on grapes and strawberries using plasma-activated water. Food Bioprocess Technol. 2020, 13, 1728–1741. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Guan, Q.; Sun, X.; Liu, S.; Hao, K.; Hu, W. The effects of cold plasma-activated water treatment on the microbial growth and antioxidant properties of fresh-cut pears. Food Bioprocess Technol. 2019, 12, 1842–1851. [Google Scholar] [CrossRef]
- Guo, J.; Qin, D.; Li, W.; Wu, F.; Li, L.; Liu, X. Inactivation of Penicillium italicum on kumquat via plasma-activated water and its effects on quality attributes. Int. J. Food Microbiol. 2021, 343, 109090. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, C.; Jiang, A.; Sun, X.; Guan, Q.; Hu, W. Effects of plasma-activated water on microbial growth and storage quality of fresh-cut apple. Innov. Food Sci. Emerg. Technol. 2020, 59, 102276. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Liu, D.; Wang, W.; Niu, J.; Xia, Y.; Qi, Z.; Zhao, Z.; Song, Y. Effect of nonthermal plasma-activated water on quality and antioxidant activity of fresh-cut kiwifruit. IEEE Trans. Plasma Sci. 2019, 47, 4811–4817. [Google Scholar] [CrossRef]
- Bruggeman, P.; Leys, C. Non-thermal plasmas in and in contact with liquids. J. Phys. D-Appl. Phys. 2009, 42, 053001. [Google Scholar] [CrossRef]
- Hoeben, W.F.L.M.; van Ooij, P.P.; Schram, D.C.; Huiskamp, T.; Pemen, A.J.M.; Lukes, P. On the possibilities of straightforward characterization of plasma activated water. Plasma Chem. Plasma Process. 2019, 39, 597–626. [Google Scholar] [CrossRef] [Green Version]
- Schnabel, U.; Andrasch, M.; Weltmann, K.-D.; Ehlbeck, J. Inactivation of vegetative microorganisms and bacillus atrophaeus endospores by reactive nitrogen species (RNS). Plasma Process. Polym. 2014, 11, 110–116. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Shen, J.; Tian, Y.; Li, Y.; Ma, R.; Zhang, Q.; Zhang, J.; Fang, J. Bactericidal effects against s-aureus and physicochemical properties of plasma activated water stored at different temperatures. Sci. Rep. 2016, 6, 28505. [Google Scholar] [CrossRef] [Green Version]
- Horsch, A.M.; Sebranek, J.G.; Dickson, J.S.; Niebuhr, S.E.; Larson, E.M.; Lavieri, N.A.; Ruther, B.L.; Wilson, L.A. The effect of pH and nitrite concentration on the antimicrobial impact of celery juice concentrate compared with conventional sodium nitrite on Listeria monocytogenes. Meat Sci. 2014, 96, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Wang, L.; Wu, Y.; Guan, Z.; Jia, Z. Bacterial decontamination of water by bipolar pulsed discharge in a gas-liquid-solid three-phase discharge reactor. IEEE Trans. Plasma Sci. 2006, 34, 1370–1374. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Tiwari, B.K.; Muthukumarappan, K.; O’Donnell, C.P.; Cullen, P.J. Colour degradation and quality parameters of sonicated orange juice using response surface methodology. LWT-Food Sci. Technol. 2008, 41, 1876–1883. [Google Scholar] [CrossRef]
- Huang, J.Y.; Guo, X.P.; Qiu, Y.B.; Chen, Z.Y. Cluster and discriminant analysis of electrochemical noise data. Electrochim. Acta. 2007, 53, 680–687. [Google Scholar] [CrossRef]
- Tian, Y.; Li, D.; Luo, W.; Zhu, Z.; Li, W.; Qian, Z.; Li, G.; Sun, D.-W. Rapid freezing using atomized liquid nitrogen spray followed by frozen storage below glass transition temperature for Cordyceps sinensis preservation: Quality attributes and storage stability. LWT-Food Sci. Technol. 2020, 123, 109066. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, L.; Tanzeela, N.; Liang, D.; Gou, X.; Guo, Y. Multivariate statistical analysis of the quality of apple juice to integrate and simplify juice industrial production technologies. CyTA-J. Food 2018, 16, 190–198. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Ojha, S.; Burgess, C.M.; Sun, D.W.; Tiwari, B.K. Influence of various fish constituents on inactivation efficacy of plasma-activated water. Int. J. Food Sci. Technol. 2020, 55, 2630–2641. [Google Scholar] [CrossRef]
- Illera, A.E.; Chaple, S.; Sanz, M.T.; Ng, S.; Lu, P.; Jones, J.; Carey, E.; Bourke, P. Effect of cold plasma on polyphenol oxidase inactivation in cloudy apple juice and on the quality parameters of the juice during storage. Food Chem. X 2019, 3, 100049. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhang, B.; Xu, R.; Wang, Y.; Ding, X.; Li, P. Antioxidant activity in vitro of the selenium-contained protein from the Se-enriched Bifidobacterium animalis 01. Anaerobe 2010, 16, 380–386. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Storage Time (Day) | Firmness (N) | pH | Color Parameters | |||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE* | ||||
| C | 0 | 74.8 ± 7.10 a | 3.64 ± 0 b | 50.5 ± 1.97 a | 26.0 ± 2.14 a | 17.3 ± 2.22 a | - |
| 4 | 28.8 ± 1.33 c | 3.82 ± 0.01 a | 43.3 ± 2.04 ab | 26.1 ± 0.91 b | 13.4 ± 0.69 b | 9.56 ± 1.46 ab | |
| PAW 0 | 0 | 68.9 ± 5.55 a | 3.71 ± 0 a | 50.0 ± 2.52 ab | 27.9 ± 2.25 a | 17.4 ± 2.53 a | 5.82 ± 1.92 a |
| 4 | 27.3 ± 2.40 c | 3.76 ± 0.01 b | 41.3 ± 1.58 b | 23.7 ± 0.71 c | 11.5 ± 0.77 b | 11.8 ± 1.65 a | |
| PAW 1 | 0 | 77.9 ± 10.95 a | 3.58 ± 0 c | 46.8 ± 2.54 c | 27.2 ± 1.64 a | 15.7 ± 2.94 a | 6.89 ± 2.33 a |
| 4 | 37.5 ± 6.81 b | 3.65 ± 0.03 d | 46.0 ± 3.49 a | 31.0 ± 1.91 a | 20.3 ± 2.20 a | 9.62 ± 0.3 ab | |
| PAW 2 | 0 | 77.3 ± 3.35 a | 3.65 ± 0 b | 48.2 ± 1.74 abc | 26.9 ± 1.29 a | 15.3 ± 1.69 a | 5.89 ± 1.18 a |
| 4 | 60.4 ± 5.16 b | 3.68 ± 0.01 c | 47.2 ± 0.81 a | 27.5 ± 1.72 b | 17.7 ± 3.88 b | 6.21 ± 1.87 c | |
| PAW 3 | 0 | 68.8 ± 4.16 a | 3.63 ± 0.03 b | 47.3 ± 0.9 bc | 26.9 ± 1.23 a | 16.0 ± 2.78 a | 6.00 ± 1.82 a |
| 4 | 49.8 ± 9.01 b | 3.76 ± 0.01 b | 45.9 ± 1.09 a | 27.5 ± 0.82 b | 13.8 ± 0.78 a | 8.08 ± 0.65 bc | |
| Treatments | Storage Time (Day) | TSS (°Brix) | VC (mg/L) | DPPH Radical Scavenging Activity (%) | Antioxidant Activity (mmol/L) | MDA Content (mmol/g) |
|---|---|---|---|---|---|---|
| C | 0 | 10.2 ± 0.55 ab | 113.3 ± 12.5 a | 97.1 ± 1.04 a | 1.31 ± 0.02 a | 6.45 ± 0.09 a |
| 4 | 10.5 ± 0.20 a | 113.3 ± 35.9 ab | 95.0 ± 2.53 ab | 0.61 ± 0.08 b | 8.58 ± 0.21 c | |
| PAW 0 | 0 | 9.00 ± 0.75 b | 120.3 ± 8.0 a | 93.6 ± 1.22 b | 1.31 ± 0.02 a | 6.41 ± 0.18 a |
| 4 | 10.2 ± 0.45 a | 89.6 ± 16.17 a | 92.2 ± 0.62 b | 0.71 ± 0.03 ab | 9.53 ± 0.10 a | |
| PAW 1 | 0 | 9.48 ± 0.97 ab | 127.3 ± 24.0 a | 95.8 ± 1.49 a | 1.31 ± 0.03 a | 6.53 ± 0.24 a |
| 4 | 9.63 ± 0.15 b | 90.8 ± 16.7 ab | 94.9 ± 1.78 ab | 0.77 ± 0.03 a | 8.32 ± 0.13 c | |
| PAW 2 | 0 | 10.5 ± 0.56 a | 107.3 ± 24.1 a | 98.0 ± 1.48 a | 1.31 ± 0.01 a | 6.47 ± 0.14 a |
| 4 | 10.6 ± 0.21 a | 119.0 ± 18.2 b | 97.1 ± 2.00 a | 0.75 ± 0.03 ab | 7.93 ± 0.11 d | |
| PAW 3 | 0 | 9.10 ± 0.34 b | 117.0 ± 10.1 a | 95.6 ± 2.13 ab | 1.26 ± 0.12 a | 6.41 ± 0.05 a |
| 4 | 10.5 ± 0.12 a | 113.0 ± 13.5 ab | 91.8 ± 0.71 b | 0.66 ± 0.11 ab | 9.07 ± 0.07 b |
| Component | Initial Eigenvalues | ||
|---|---|---|---|
| Eigenvalue | % of Variance | Cumulative (%) | |
| 1 | 5.263 | 52.631 | 52.631 |
| 2 | 1.427 | 14.272 | 66.903 |
| 3 | 0.996 | 9.963 | 76.866 |
| 4 | 0.895 | 8.951 | 85.817 |
| 5 | 0.571 | 5.709 | 91.526 |
| 6 | 0.407 | 4.065 | 95.591 |
| 7 | 0.158 | 1.581 | 97.172 |
| 8 | 0.138 | 1.377 | 98.549 |
| 9 | 0.098 | 0.980 | 99.528 |
| 10 | 0.047 | 0.472 | 100.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, C.; Li, Q.; Cheng, J.-H. Physicochemical Properties of Plasma-Activated Water and Its Control Effects on the Quality of Strawberries. Molecules 2023, 28, 2677. https://doi.org/10.3390/molecules28062677
Yang X, Zhang C, Li Q, Cheng J-H. Physicochemical Properties of Plasma-Activated Water and Its Control Effects on the Quality of Strawberries. Molecules. 2023; 28(6):2677. https://doi.org/10.3390/molecules28062677
Chicago/Turabian StyleYang, Xiao, Can Zhang, Qunfang Li, and Jun-Hu Cheng. 2023. "Physicochemical Properties of Plasma-Activated Water and Its Control Effects on the Quality of Strawberries" Molecules 28, no. 6: 2677. https://doi.org/10.3390/molecules28062677






