Research Progress on Chemical Constituents and Pharmacological Activities of Menispermi Rhizoma
Abstract
:1. Introduction
2. Chemical Composition
2.1. Alkaloids
2.1.1. Bisbenzylisoquinoline Alkaloids
2.1.2. Apomorphines and Oxidized Isoporphine Alkaloids
2.1.3. Morphine Alkaloids, Proberberberine, Berberine, and Other Alkaloids
2.2. Other Components
3. Pharmacological Activities
3.1. Anti-Tumour Effect
3.2. Anti-Inflammatory Effect
3.3. Antioxidant Effect
3.4. Antibacterial Effect
3.5. Cardio-Protective Effect
3.6. Anti-Hypoxic Effect
3.7. Anti-Depressant Effect
3.8. Anti-Alzheimer’s Disease Effect
3.9. Toxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Pharmacopoeia Commission. Chinese Pharmacopoeia, 1st ed.; China Medical Science and Technology Press: Beijing, China, 2020; Volume 103, Available online: https://db2.ouryao.com/yd2020/view.php?id=f43d4b9e3a (accessed on 14 March 2023).
- Yu, Y.; Shao, J.; Wei, J.; Li, Y.; Li, X.; Li, L.; Gu, J. Progress in the study of alkaloid components and their pharmacological effects in Menispermi Rhizoma. J. Chin. Med. Mater. 2019, 42, 2453–2461. [Google Scholar] [CrossRef]
- Peng, Y. Chemical constituents and Pharmacological activities of whole herb of Menispermum dauricum DC. Pop. Sci. Technol. 2018, 20, 94–96. Available online: https://kns.cnki.net/kcms2/article/abstract?v=8pLOALknL0b8D3KENhsqwbqaUwHbrXD3qSKEemiJAqLLkuKupKmfgiLXOgHeo0gVqhQX_ON1xY-lR5p9EYrk_rvyQOeLYCJQs_zHeUQ2ZwSAV4YuxDPNHAEs7gPf1DjS&uniplatform=NZKPT&language=CHS (accessed on 14 March 2023).
- Shao, J.; Shi, C.; Wei, J.; Li, Y.; Guo, X. Chemical constituents from rhizome of Menispermum dauricum and their anti-hypoxic activities. China J. Chin. Mater. Med. 2019, 44, 723–729. [Google Scholar] [CrossRef]
- Chen, J.; Xie, Y.; Zhou, T.; Qin, G. Chemical constituents of Menispermum dauricum. Chin. J. Nat. Med. 2012, 10, 292–294. [Google Scholar] [CrossRef]
- Su, Q. Study on the Chemical Composition and Anti-Ulcerative Colitis Activities of Menispermi Rhizoma; Northwest University: Xi’an, China, 2013; Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1014156292.nh&DbName=CMFD2014 (accessed on 14 March 2023).
- Li, X.; Zhao, H.; Huang, W.; Feng, Y.; Li, Z.; Wang, Q.; Yang, S. Rapid Identification of Alkaloids in Menispermi Rhizoma by UPLC-Q-TOF-MS/MS. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 97–102. [Google Scholar] [CrossRef]
- Liu, J. Study on the Spectrum-Effect Relationship of Cytotoxic Activities of Menispermi Rhizoma; Heilongjiang University: Harbin, China, 2012; Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1012408694.nh&DbName=CMFD2012 (accessed on 14 March 2023).
- Chen, X.; Zhang, Y.; Luo, K.; Chang, X.; Lv, H. Trace Alkaloids from Menispermum dauricum. Acta Chin. Med. Pharmacol. 2015, 43, 9–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Y.; Chen, X.; Zhang, N.; Liu, H.; Song, L. Bisbenzylisoquinoline Alkaloids from the Rhizome of Menispermum dauricum. Mod. Chin. Med. 2016, 18, 951–955. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Li, S.; Liu, H.; Wang, A.; Zhang, Y. Alkaloids from Rhizome of Menispermum dauricum and Their Anti-inflammatory Activity. Mod. Chin. Med. 2018, 20, 163–168. [Google Scholar] [CrossRef]
- Wei, H.; Han, Y.; Wang, J.; Hou, T.; Yao, Y.; Jin, J.; Zhao, T.; Zhang, X.; Liu, Y.; Liang, X. Analgesic bisbenzylisoquinoline alkaloids from the rhizoma of Menispermum dauricum DC. Bioorg. Chem. 2021, 107, 104517. [Google Scholar] [CrossRef]
- Wei, H.; Han, Y.; Zhou, H.; Hou, T.; Yao, Y.; Wen, C.; Wang, C.; Wang, J.; Shen, A.; Zhang, X.; et al. Isoquinoline alkaloid dimers with dopamine D1 receptor activities from Menispermum dauricum DC. Phytochemistry 2022, 194, 113015. [Google Scholar] [CrossRef]
- Li, S.; Song, X.; Chai, X.; Wang, Y. Chemical Constituents from the Rhizome of Menispermum dauricum DC. Nat. Prod. Res. Dev. 2013, 25, 60–63. [Google Scholar] [CrossRef]
- Ren, W.; Tian, Z.; Dong, M.; Ma, Y.; Xu, L.; Jiang, H.; Liu, Y. Chemical constituents from the rhizome of Menispermum dauricum DC. and their chemotaxonomic significance. Biochem. Syst. Ecol. 2020, 90, 104047. [Google Scholar] [CrossRef]
- Ren, W.; Zhu, G.; Ma, Y.; Cao, Y.; Duan, B.; Liu, Y. A novel oxoisoaporphine-type alkaloid from the rhizome of Menispermum dauricum. J. Asian Nat. Prod. Res. 2023, 25, 95–101. [Google Scholar] [CrossRef]
- Yao, X. The Studies on Chemical Constituents from the Rhizome of Menispermum dauricum DC. and Pharmacological Activities; Shanxi Medical University: Jinzhong, China, 2022. [Google Scholar] [CrossRef]
- Li, M. The Study on the Chemical Constituents from the Rhizome of Menispermum dauricum DC. & The Study on the Absolute Configurations of the Abietanes from Euphorbia fischeriana Steud; Shanxi Medical University: Jinzhong, China, 2020. [Google Scholar] [CrossRef]
- Wei, J.; Chen, J.; Liang, X.; Guo, X. Microwave-assisted extraction in combination with HPLC-UV for quantitative analysis of six bioactive oxoisoaporphine alkaloids in Menispermum dauricum DC. Biomed Chromatogr. 2016, 30, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shao, J.; Chen, F.; Zhang, T.; Wei, J.; Li, L.; Pan, S.; Yang, Y. Study on oxoisoaporphine alkaloids from rhizome of Menispermum dauricum and their anti-myocardial ischemia activities. J. Logist. Univ. CAPF Med. Sci. 2019, 28, 1–6. [Google Scholar] [CrossRef]
- Wang, A.; Hao, Y.; Zhang, S. Study Progress of Asiatic Moonseed. J. Liaoning Univ. Tradit. Chin. Med. 2012, 14, 194–196. [Google Scholar] [CrossRef]
- Li, X. The Study on the Chemical Constituents and Pharmacological Activities from the Rhizome of Menispermum dauricum DC; Shanxi Medical University: Jinzhong, China, 2021. [Google Scholar] [CrossRef]
- Chen, L.; Li, L.; Cheng, Y.; Liu, Y.; Ma, S.; Li, Y.; Qu, J. Three new alkaloids from Menispermum dauricum. J. Asian Nat. Prod. Res. 2020, 22, 914–919. [Google Scholar] [CrossRef]
- Shang, X.; Yang, C.; Morris-Natschke, S.L.; Li, J.; Yin, X.; Liu, Y.; Guo, X.; Peng, J.; Goto, M.; Zhang, J.; et al. Biologically active isoquinoline alkaloids covering 2014–2018. Med. Res. Rev. 2020, 40, 2212–2289. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zhang, Y.; Bai, Y.; Zhang, C.; Jia, B.; Sun, B.; Du, H.; Fei, H.; Zhou, Z. Research Progress on Pharmacological Effacts of Dau. J. Liaoning Univ. Tradit. Chin. Med. 2015, 17, 91–93. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, K. New Analogues of Aporphine Alkaloids. Mini. Rev. Med. Chem. 2018, 18, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Ma, Y.; Cai, M.; Wang, F.; Li, L.; Liu, Y. Chemical constituents of rhizome of Menispermum dauricum DC. and their anti-inflammatory activities. Guihaia 2022, 1–9. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=GXZW20220829003&DbName=CAPJ2022 (accessed on 14 March 2023).
- Ren, W.; Dong, M.; Feng, J.; Ding, J.; Ma, Y.; Liu, Y.; Zhang, D. Study on Extraction Process and Antioxidant Activity of Menispermi Rhizoma Fatty Oil. Chin. J. Mod. Appl. Pharm. 2021, 38, 420–425. [Google Scholar] [CrossRef]
- Lin, M.; Xia, B.; Yang, M.; Gao, S.; Huo, Y.; Lou, G. Characterization and antitumor activities of a polysaccharide from the rhizoma of Menispermum dauricum. Int. J. Biol. Macromol. 2013, 53, 72–76. [Google Scholar] [CrossRef]
- Lin, M.; Xia, B.; Yang, M.; Gao, S.; Huo, Y.; Lou, G. Anti-ovarian cancer potential of two acidic polysaccharides from the rhizoma of Menispermum dauricum. Carbohydr Polym. 2013, 92, 2212–2217. [Google Scholar] [CrossRef]
- Sun, X.; Cai, J.; Gao, J.; Pu, J.; Ma, Y. Research Progress of Isoquinoline Alkaloids against Liver Cancer Based on Network Pharmacology and Literature. Sci. Technol. Cereals Oils Foods 2021, 29, 122–130. [Google Scholar] [CrossRef]
- Liu, S.; Yi, Y.; Yu, Y.; Zhong, L. Expression of Hedgehog signaling pathway key protein in transplanted tumor of pancreatic cancer in nude mice. Chin. J. Clin. Pharmacol. Ther. 2020, 36, 2432–2435. [Google Scholar] [CrossRef]
- Liu, J. Investigation of the Inhibitory Activities of Daurisoline Ragainst Hepatocellular Carcinoma Cells; Northeast Forestry University: Harbin, China, 2021. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, J.; Zhang, Y.; Li, H.; Li, C. Dauricine inhibiting the cell proliferation and inducing the cell apoptosis of human pancreatic cancer cells line SW1900. Acta Anat. Sin. 2020, 51, 543–547. [Google Scholar] [CrossRef]
- Liu, P.; Bai, Y.; Wu, C.; He, X.; Su, H.; Guo, L. Effection of Dau on the proliferation and migration of pancreatic cancer BxPC-3 cells and its effect on ERK signaling pathways. J. Guangdong Pharm. Univ. 2019, 35, 773–778. [Google Scholar] [CrossRef]
- Yuan, X.; Dou, H.; Chen, S.; Li, X. The effect of autophagy in the apoptosis of cervical cancer Hela cells induced by Dauricine. Lishizhen Med. Mater. Med. Res. 2019, 30, 3031–3033. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=SZGY201912075&DbName=CJFQ2019 (accessed on 14 March 2023).
- Zhu, P.; Lv, J.; Liu, Y.; Zeng, Q.; Ming, L. Effect and mechanism of dauricine on proliferation and apoptosis of hepatoma Huh7 cells. Chin. Herb. Med. 2019, 50, 1151–1156. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZCYO201905019&DbName=CJFQ2019 (accessed on 14 March 2023).
- Jia, H.; Wang, H.; Xia, F. Experimental study on the inhibitory effect of dauricine on human esophageal cancer cell line Eca-109 and its apoptosis. J. Clin. Exp. Med. 2019, 18, 1927–1930. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=SYLC201918007&DbName=CJFQ2019 (accessed on 14 March 2023).
- Deng, B.; Jiang, X.; Tan, Z.; Cai, M.; Deng, S.; Ding, W.; Xu, Y.; Wu, Y.; Zhang, S.; Chen, R.; et al. Dauricine inhibits proliferation and promotes death of melanoma cells via inhibition of Src/STAT3 signaling. Phytother. Res. 2021, 35, 3836–3847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Dauricine Inhibits Viability and Induces Cell Apoptosis via Inhibiting the PI3K/Akt Signaling Pathway in RCC Cells; Nanjing Medical University: Nanjing, China, 2019; Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1019867623.nh&DbName=CDFD2019 (accessed on 14 March 2023).
- Zhang, R.; Zhang, J. Progress on Pharmacological Action of Dauriciny. Food Drug 2022, 24, 81–86. Available online: https://kns.cnki.net/kcms2/article/abstract?v=8pLOALknL0ZuheodKmKXrECROzUSCI1yySNIx1nKMrkT0fUR6cybkg0TWGr2672orQ0p8PFlGl9cBM7TomRz7-GXE2N0DD4VSvzzUTz1U0KKYSGDLvndApzuBx9QMMcJ&uniplatform=NZKPT&language=CHS (accessed on 14 March 2023).
- Lin, D. Dauricine Mediates ROS Generation and Inhibits PI3K/Akt/mTOR Pathway to Induce Autophagic Apoptosis of Bladder Cancer Cells; North Sichuan Medical College: Nanchong, China, 2021. [Google Scholar] [CrossRef]
- Bai, X.; Guo, X.; Cheng, N.; Yang, M.; Zhou, S.; Qin, L.; Huang, Y.; Lin, W. The mechanism of dauricine on nasopharyngeal carcinoma based on network pharmacology and molecular experiment. J. Guangxi Med. Univ. 2022, 39, 424–430. [Google Scholar] [CrossRef]
- Ai, Y.; He, M.; Wang, Y.; Liang, Q. Review of classical prescriptions in treatment of ulcerative colitis. China J. Chin. Mater. Med. 2022, 47, 5797–5805. [Google Scholar] [CrossRef]
- Liu, J.; Liu, D.; Wang, X.; Lu, M. Curative Effect of Rhizoma Menipermi on Model rats with Ulcerative Colitis. Acta Chin. Med. 2018, 33, 1476–1479. [Google Scholar] [CrossRef]
- Zhang, K.; Song, Y.; Li, B.; Gu, D.; Li, Z.; Yuan, H. Mechanism of Menispermi Rhizoma in the treatment of ulcerative colitis based on network pharmacology. Anhui Med. Pharm. J. 2022, 26, 1672–1675+1699. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=AHYY202208044&DbName=CJFQ2022 (accessed on 14 March 2023).
- Lv, L.; Yin, B.; You, Y.; Sun, Z.; He, J.; Cao, Y. Protective Effects of Total Alkaloids from Menispermum dauricum against Airway Inflammation in Asthmatic Mice. Planta Med. 2020, 86, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Jin, Y.; Jia, J.; Li, X. Study on chemical constituents from rhizome of Menispermum dauricum. J. Yanbian Univ. Nat. Sci. Ed. 2017, 43, 128–130. [Google Scholar] [CrossRef]
- Bian, W.; Zhang, Y.; Zhang, C.; Fei, H.; Zhou, Z.; Liu, X.; Deng, Y. Research status of pharmacological action of dauricine. Heilongjiang Sci. 2014, 5, 10–11. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=HELJ201407010&DbName=CJFQ2014 (accessed on 14 March 2023).
- Li, J.; Bi, H.; Du, B.; Sun, Y.; Zhang, H. Study on the Bacteriostasic Action of Menispermi Alkaloid by Microcalorimetry. J. Qufu Norm. Univ. Nat. Sci. 2010, 36, 96–98+102. Available online: https://kns.cnki.net/kcms2/article/abstract?v=8pLOALknL0b42eCsWBxxdeEp3WQKieh4MOEdHamxKU6gP1CBYgCUceugY_C9LjL5kpo6iFtPD4i5smFtYejrTt6hLX1_t58qc-5nR5M1UZMjzVXYE3NknQ==&uniplatform=NZKPT&language=CHS (accessed on 14 March 2023).
- Zhang, T. Dauricine through BBB Reverse Retention Mechanism Research; Heilongjiang University of Chinese Medicine: Harbin, China, 2015; Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1015412815.nh&DbName=CMFD2015 (accessed on 14 March 2023).
- Zhang, G.; Li, Y.; Wang, Y.; Chen, Z.; Yan, L.; He, Z. Neuroprotective effects of phenolic alkaloids of Menispermum dauricum on rats with ischemia reperfusion by regulating expression of p-NR1 and NR2A. Chin. J. Hosp. Pharm. 2017, 37, 579–582. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, L.; Qiu, X.; Qiao, Z.; Yang, X.; Shi, J.; Ning, N. Advances in research on the relationship between 5-hydroxytryptamine and depression and suicidal behavior. Chin. J. Public Health 2010, 26, 496–497. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZGGW201004066&DbName=CJFQ2010 (accessed on 14 March 2023).
- Wang, Y.; Lyu, Y.; Liu, Y.; Chen, G.; Zhang, X.; Ma, S.; Cheng, J.; Zhao, S. Effect of Jianpi Huashi Granule on Tyrosine Hydroxylase, Monoamine Oxidase and Serotonin Transporter Expression in Brain of Rats with Diarrheapredominant Irritable Bowel Syndrome. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 133–138. [Google Scholar] [CrossRef]
- López, M.C.; Fontenla, J.A.; Uriarte, E.; Santana, L.; Sobarzo-Sánchez, E. Comparison of the antidepressive effects of trans-resveratrol and 5-methoxy-7H-dibenzode,hquinolin-7-one. Curr. Top. Med. Chem. 2014, 14, 234–238. [Google Scholar] [CrossRef]
- Pan, S.; Yu, C.; Zhang, Y.; Fei, H.; Zhang, X.; Zhong, L.; Zhou, Z. Effects of batrachine on the expression of Bcl-2 and Bax in the hippocampus of AD rats. Heilongjiang Sci. Technol. Inf. 2015, 32, 74. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=HLKX201532068&DbName=CJFQ2015 (accessed on 14 March 2023).
- Zhang, Y.; Fei, H.; Guo, J. The effects of dauricine on receptor for advanced glycation end products and nuclear transcription factor-κBp65 of the hippocampus in Alzheimer’s disease mice. Chin. J. Gerontol. 2017, 37, 4697–4700. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=ZLXZ201719004&DbName=CJFQ2017 (accessed on 14 March 2023).
- Wang, L. Studies on Antioxidant Activity of Dauricine in Cell Model of Alzheimer’s Disease and Preparation of Brain-Targeted Naonparticles; Guilin Medical University: Guilin, China, 2019. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, W.; Fu, X. Protective function of dauricine on oxidative stress injury induced by APP over-expressing in Alzheimer’s disease cell model. J. Clin. Med. Pract. 2015, 19, 7–9, 16. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=XYZL201501002&DbName=CJFQ2015 (accessed on 14 March 2023).
- Yang, Q.; Luo, D.; Zhao, Y.; Sun, R. Influence of Different Components on Acute Toxicity of Rhizoma Menispermi in mice. Chin. J. Pharmacovigil. 2010, 7, 70–72. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=YWJJ201002003&DbName=CJFQ2010 (accessed on 14 March 2023).
- Luo, D. Research on Toxic Side Effects Based on the Efficacy Caused by Different Components from Menispermum Dauricum DC; Shandong University of Traditional Chinese Medicine: Jinan, China, 2012; Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=1012470129.nh&DbName=CMFD2013 (accessed on 14 March 2023).
- Luan, Y.; Sun, R. Research of Liver Damage Mechanism of the Toxical and Side Effects Accompanied with Anti- inflammation Caused by Different Extracts from Rhizoma Menispermi to Mice. Chin. J. Pharmacovigil. 2013, 10, 513–517. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=YWJJ201309001&DbName=CJFQ2013 (accessed on 14 March 2023).

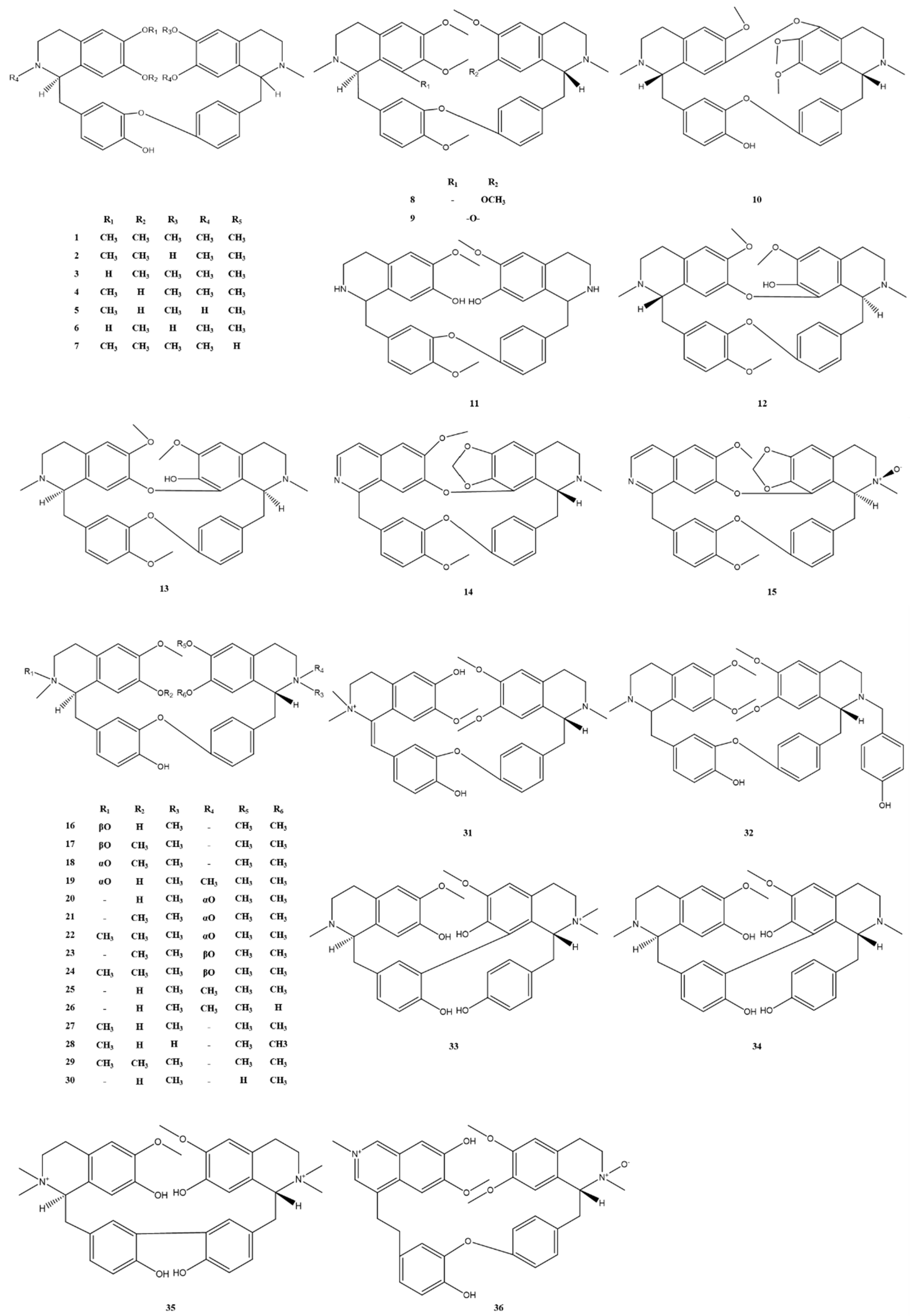


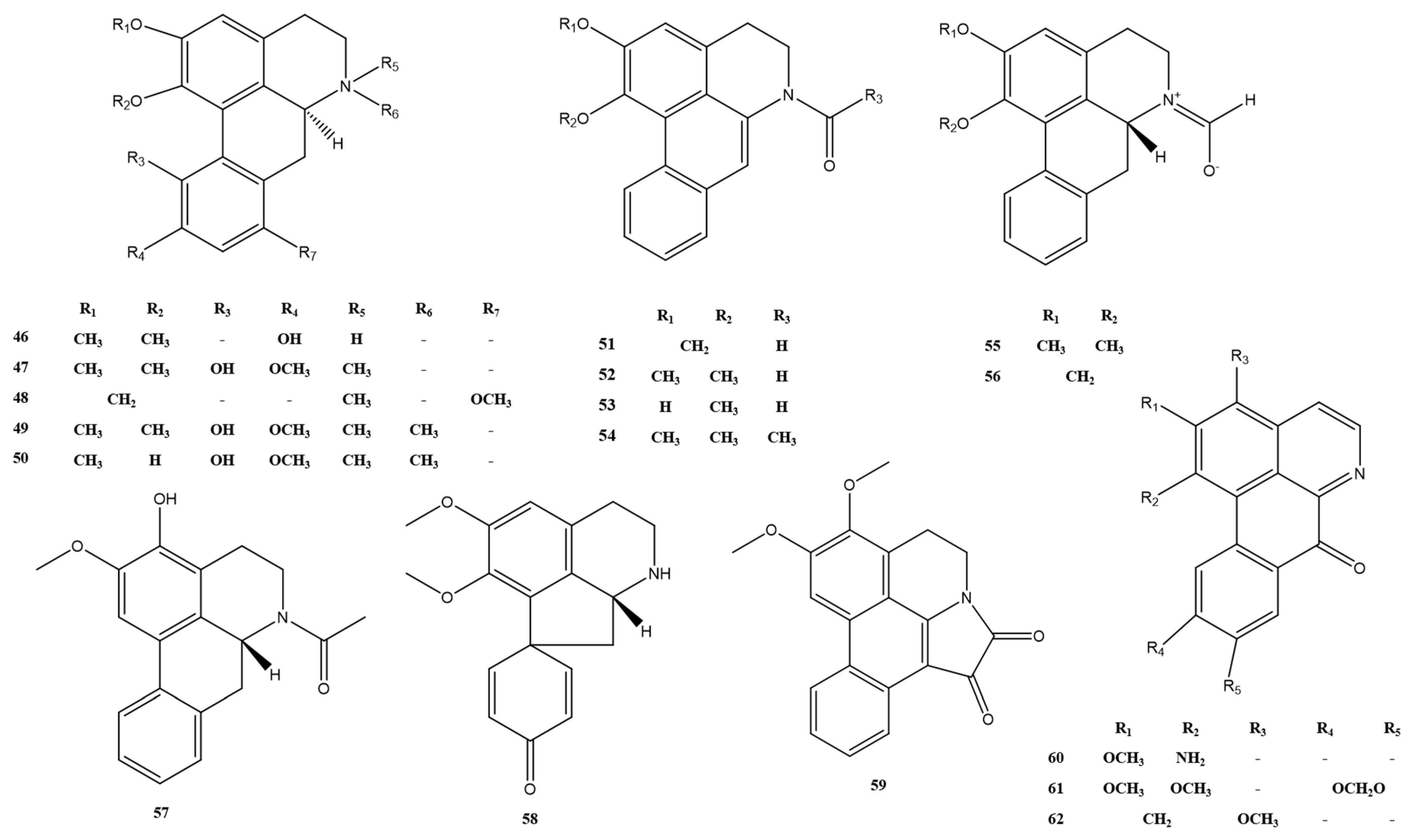
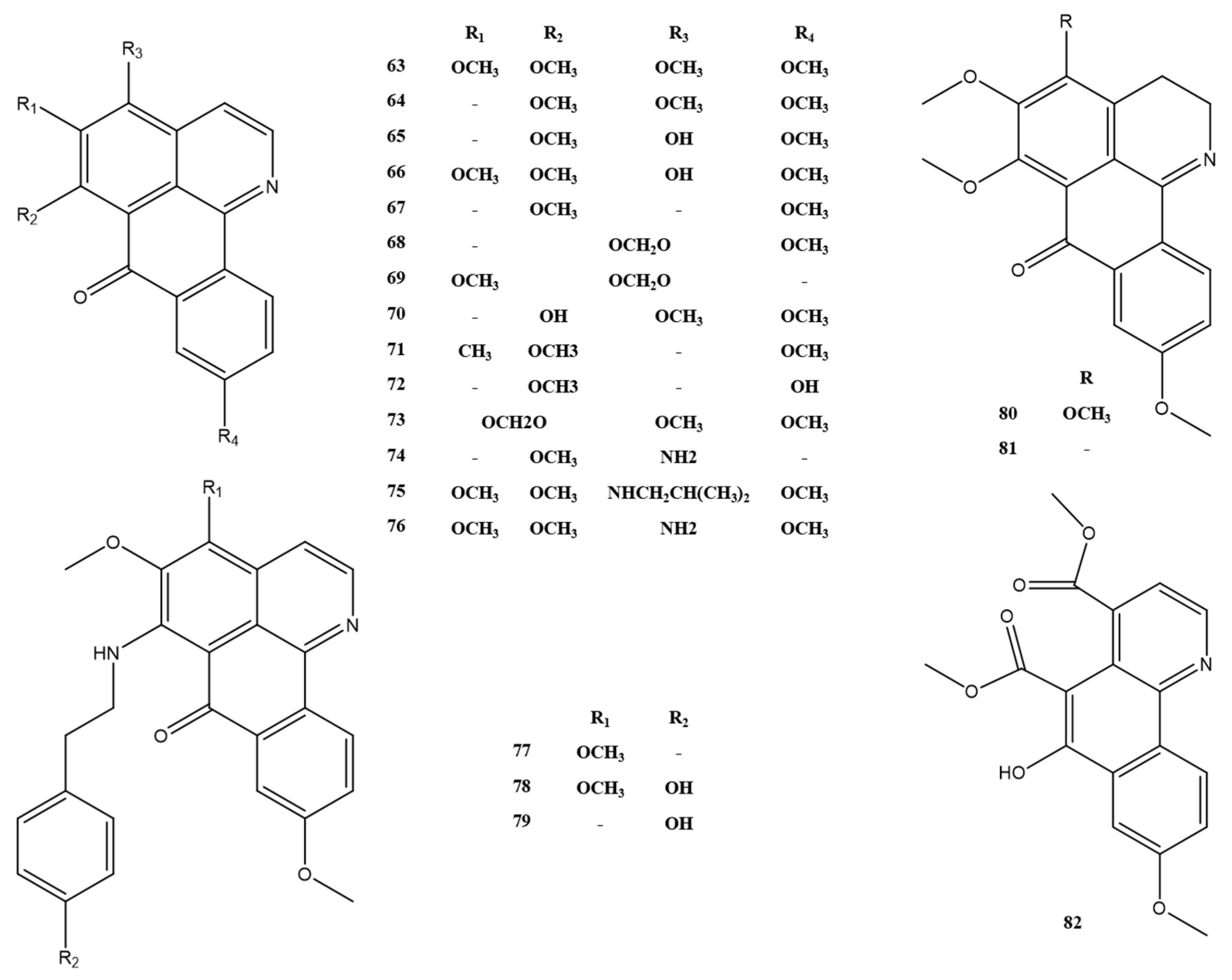
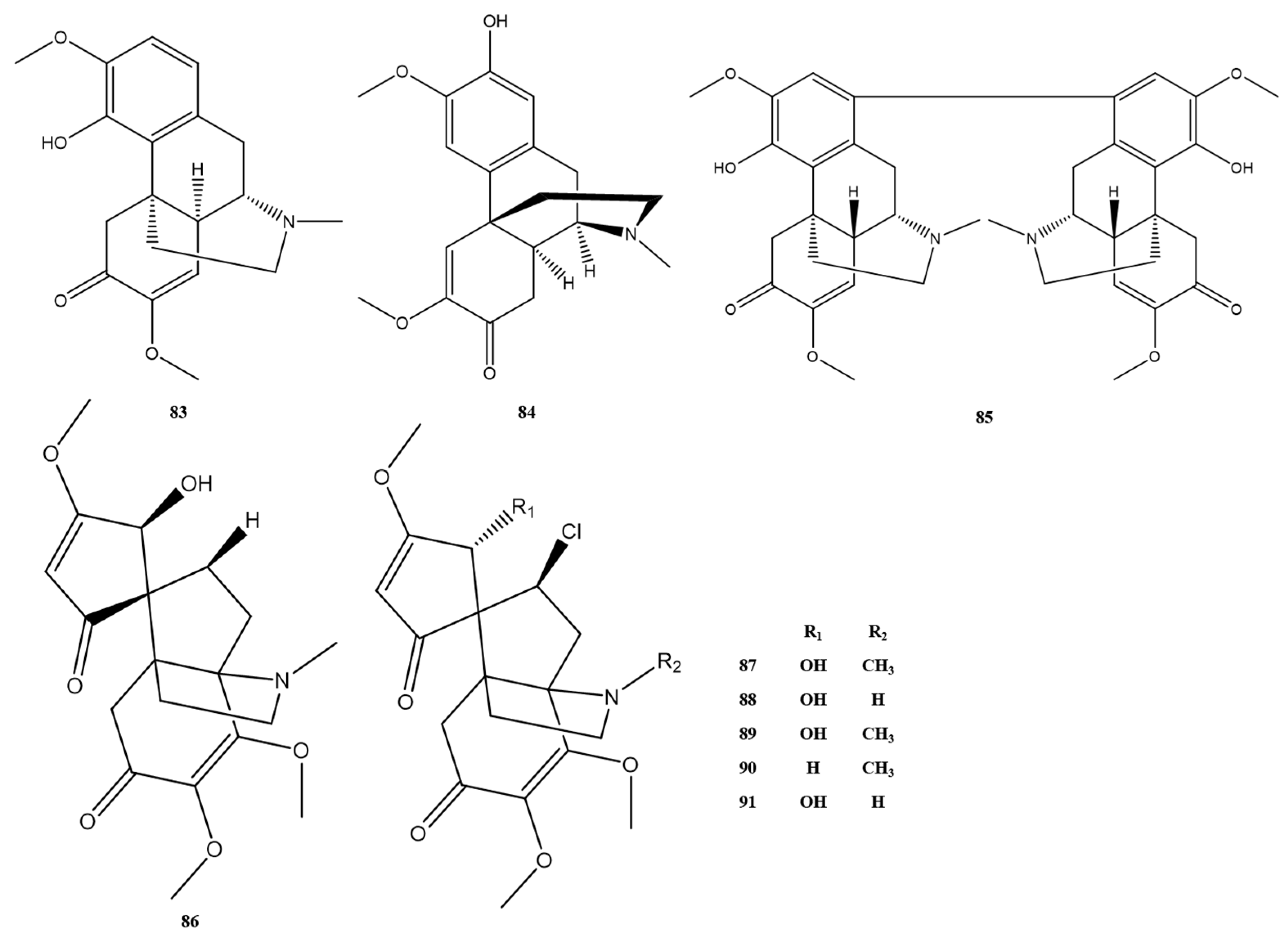


| No. | Alkaloids | Formula | Mass | Reference |
|---|---|---|---|---|
| 1 | dauricine | C38H44N2O6 | 624.3 | [5] |
| 2 | daurinoline | C37H42N2O6 | 610.3 | [6] |
| 3 | dauricinoline | C37H42N2O6 | 610.3 | [6] |
| 4 | daurisoline | C37H42N2O6 | 610.3 | [6] |
| 5 | dauricicline | C36H40N2O6 | 596.3 | [7] |
| 6 | dauricoline | C36H40N2O6 | 596.3 | [7] |
| 7 | (R,R)-N-Desmethlydauricine | C37H42N2O6 | 610.3 | [8] |
| 8 | O-methyldauricine | C39H46N2O6 | 638.3 | [7] |
| 9 | tetrandrine | C38H42N2O6 | 622.3 | [6] |
| 10 | thalifortine | C37H40N2O6 | 608.3 | [7] |
| 11 | costaricine | C35H38N2O6 | 582.3 | [9] |
| 12 | cycleapeltine | C37H40N2O6 | 608.3 | [10] |
| 13 | homoaromoline | C37H40N2O6 | 608.3 | [10] |
| 14 | (+)-1,3,4-dehydrocepharanthine | C36H32N2O6 | 588.2 | [11] |
| 15 | (+)-1,3,4-dehydrocepharanthine-2′β-N-oxide | C36H32N2O7 | 604.2 | [11] |
| 16 | (1R, 1′R)-dauricine-2β-N-oxide | C37H42N2O7 | 626.3 | [12] |
| 17 | (1R, 1′R)-daurisoline-2β-N-oxide | C38H44N2O7 | 640.3 | [12] |
| 18 | (1R, 1′R)-dauricine-2α-N-oxide | C38H44N2O7 | 640.3 | [12] |
| 19 | (1R, 1ʹR)-dauricisoline A-2α-N-oxide | C38H45N2O7+ | 641.3 | [12] |
| 20 | (1R, 1′R)-daurisoline-2′α-N-oxide | C37H42N2O7 | 626.3 | [12] |
| 21 | (1R, 1′R)-dauricine-2′α-N-oxide | C38H44N2O7 | 640.3 | [12] |
| 22 | (1R, 1′R)-dauricisoline C-2′α-N-oxide | C39H47N2O7+ | 655.3 | [12] |
| 23 | (1R, 1′R)-dauricine-2′β-N-oxide | C38H44N2O7 | 640.3 | [12] |
| 24 | (1R, 1′R)-dauricisoline E-2′β-N-oxide | C39H47N2O7+ | 655.3 | [12] |
| 25 | (1R, 1′R)-dauricisoline A | C38H45N2O6+ | 625.3 | [12] |
| 26 | (1R, 1′R)-dauricisoline B | C37H43N2O6+ | 611.3 | [12] |
| 27 | (1R, 1′R)-dauricisoline C | C38H45N2O6+ | 625.3 | [12] |
| 28 | (1R, 1′R)-dauricisoline D | C37H43N2O6+ | 611.3 | [12] |
| 29 | (1R, 1′R)-dauricisoline E | C39H47N2O6+ | 639.3 | [12] |
| 30 | (1R, 1′R)-espinin | C36H40N2O6 | 596.3 | [12] |
| 31 | (1′R)-dauricisoline F | C38H43N2O6+ | 623.3 | [13] |
| 32 | (1R, 1′R)-dauricisoline G | C44H48N2O7 | 716.3 | [13] |
| 33 | (1R, 1′R)-dauricisoline H | C37H43N2O6+ | 611.3 | [13] |
| 34 | (1R, 1′R)-dauricisoline I | C36H40N2O6 | 596.3 | [13] |
| 35 | (1R, 1′R)-dauricisoline J | C38H46N2O62+ | 626.3 | [13] |
| 36 | (1′R)-pavermenidaurine | C38H41N2O7+ | 637.3 | [13] |
| 37 | cissampentin | C37H40N2O6 | 608.3 | [9] |
| 38 | cycleatjehenine | C37H36N2O6 | 604.3 | [9] |
| 39 | neosutchuenenine | C36H40N2O6 | 596.3 | [10] |
| 40 | cissampentine A | C36H38N2O6 | 594.3 | [11] |
| 41 | cissampentine B | C37H40N2O6 | 608.3 | [11] |
| 42 | (−)-pseudocurine | C36H38N2O6 | 594.3 | [11] |
| 43 | sutchueneneonine | C36H40N2O6 | 596.3 | [10] |
| 44 | sutchuenenine | C36H40N2O6 | 596.3 | [10] |
| 45 | secoisotetrandrine | C38H40N2O8 | 652.3 | [10] |
| 46 | tuduranine | C18H19NO3 | 297.1 | [7] |
| 47 | iso-corydine | C20H23NO4 | 341.2 | [7] |
| 48 | cepharanthine | C19H19NO3 | 309.1 | [7] |
| 49 | menisperine | C21H26NO4+ | 356.2 | [14] |
| 50 | magnoflorine | C20H24NO4+ | 342.2 | [14] |
| 51 | N-formyldehydroanonain | C18H13NO3 | 291.1 | [15] |
| 52 | N-demethyl-N-formyldehydronuciferine | C19H17NO3 | 307.1 | [15] |
| 53 | sinotumine G | C18H15NO3 | 293.1 | [16] |
| 54 | 6-acetyl-5,6-dihydro-1,2-dimethoxy-4H-dibenzo[de,g]-quinoline | C20H19NO3 | 321.1 | [17] |
| 55 | N-formylmornuciferin | C19H19NO3 | 309.1 | [15] |
| 56 | N-formylannonaine | C18H15NO3 | 293.1 | [15] |
| 57 | N-acetylasimilobine | C19H19NO3 | 309.1 | [16] |
| 58 | stepharine | C18H19NO3 | 297.1 | [18] |
| 59 | telisatin A | C20H15NO4 | 333.1 | [16] |
| 60 | telazoline | C17H12N2O2 | 276.1 | [11] |
| 61 | oxidized nantenine | C19H13NO5 | 335.1 | [7] |
| 62 | atherospermidine | C18H11NO4 | 305.1 | [16] |
| 63 | dauriporphine | C20H17NO5 | 351.1 | [14] |
| 64 | menisporphine | C19H15NO4 | 321.1 | [14] |
| 65 | 6-O-demethylmenisporphine | C18H13NO4 | 307.1 | [14] |
| 66 | dauriporphinoline | C19H15NO5 | 337.1 | [14] |
| 67 | bianfugecine | C18H13NO3 | 291.1 | [19] |
| 68 | bianfugedine | C18H11NO4 | 305.1 | [19] |
| 69 | oxoisoaporphine A | C18H11NO4 | 305.1 | [20] |
| 70 | oxoisoaporphine B | C18H13NO4 | 307.1 | [20] |
| 71 | menisoxoisoaporphine B | C19H15NO3 | 305.1 | [17] |
| 72 | menispeimin A | C17H11NO3 | 277.1 | [16] |
| 73 | sinotumine D | C19H13NO5 | 335.1 | [16] |
| 74 | lakshminine | C17H12N2O2 | 276.1 | [11] |
| 75 | menisoxoisoaporphine A | C24H26N2O4 | 406.2 | [11] |
| 76 | daurioxoisoporphine B | C19H16N2O4 | 336.1 | [17] |
| 77 | Menisoxoisoaporphine C | C27H24N2O4 | 440.2 | [17] |
| 78 | tyraminoporphine | C27H24N2O5 | 456.2 | [11] |
| 79 | daurioxoisoporphine A | C26H22N2O4 | 426.2 | [7] |
| 80 | 2,3-dihydrodauriporphine | C20H19NO5 | 353.1 | [7] |
| 81 | dihydromenisporphine | C19H17NO4 | 323.1 | [16] |
| 82 | sinotumine F | C18H15NO6 | 341.1 | [7] |
| 83 | sinomenine | C19H23NO4 | 329.2 | [6] |
| 84 | scrodentoside A | C19H23NO4 | 329.2 | [11] |
| 85 | disinomenine | C38H44N2O8 | 656.3 | [21] |
| 86 | dechloroacutumine | C19H25NO6 | 363.2 | [5] |
| 87 | dauricumine | C19H24ClNO6 | 387.1 | [5] |
| 88 | dauricumidine | C18H22ClNO6 | 383.1 | [14] |
| 89 | acutumine | C19H24ClNO6 | 397.1 | [5] |
| 90 | acutuminine | C19H24ClNO5 | 381.1 | [14] |
| 91 | acutumidine | C18H22O6NCl | 383.1 | [14] |
| 92 | stopholidine | C19H21NO4 | 327.1 | [4] |
| 93 | corydalmine | C20H23NO4 | 341.2 | [7] |
| 94 | pessoine | C18H19NO4 | 313.1 | [7] |
| 95 | cheilanthifoline | C19H19NO4 | 325.1 | [7] |
| 96 | stepholidine | C19H21NO4 | 327.1 | [7] |
| 97 | (+)-cheilanthifoline | C19H19NO4 | 325.1 | [17] |
| 98 | epiberberine | C20H18NO4+ | 336.1 | [14] |
| 99 | (6aS, 1′R)-apormenidaurine A | C44H46N2O7 | 714.3 | [13] |
| 100 | (6aS, 1′S)-apormenidaurine B | C46H51N2O10 | 791.4 | [13] |
| 101 | thalifoline | C11H13NO3 | 207.1 | [7] |
| 102 | N-methylcorydaldine | C12H15NO3 | 221.1 | [7] |
| 103 | corypalline | C11H15NO2 | 193.1 | [7] |
| 104 | O-methylcorypalline | C12H17NO2 | 207.1 | [7] |
| 105 | pycnarrhine | C11H14NO2+ | 192.1 | [22] |
| 106 | amurolin | C19H25NO3 | 315.2 | [22] |
| 107 | coclaurine | C17H19NO3 | 285.1 | [7] |
| 108 | lotusine | C19H24NO3+ | 314.2 | [7] |
| 109 | reticuline | C19H23NO4 | 329.2 | [7] |
| 110 | (R)-6-methoxy-1-(4-methoxybenzyl)-2-methyl-1,2,3,4-tetrahydroisoquinolin-7-ol | C19H23NO3 | 313.2 | [7] |
| 111 | pseudolaudanine | C20H25NO4 | 343.2 | [7] |
| 112 | N-methylcoclaurine | C18H21NO3 | 299.2 | [7] |
| 113 | armepavine | C19H23NO3 | 313.2 | [7] |
| 114 | pecrassipine B | C26H27NO5 | 433.2 | [7] |
| 115 | menidaurine A | C26H27NO5 | 433.2 | [23] |
| 116 | menidaurine B | C27H29NO6 | 463.2 | [23] |
| 117 | menidaurine C | C26H27NO5 | 433.2 | [23] |
| No. | Component | Formula | Mass | Reference |
|---|---|---|---|---|
| 1 | p-hydroxyphenethyltrans-ferulate | C18H18O5 | 314.1 | [14] |
| 2 | daucosterol | C35H60O6 | 576.4 | [14] |
| 3 | vanillin | C8H8O3 | 152.0 | [5] |
| 4 | N-trans-feruloyltyramine | C18H19NO4 | 313.1 | [5] |
| 5 | β-sitostenone | C30H52O | 428.4 | [5] |
| 6 | β-sitosterol | C30H52O | 428.4 | [5] |
| 7 | aristoloterpenate I | C32H31NO8 | 557.2 | [5] |
| 8 | aristolochic acid | C17H11NO7 | 341.1 | [4] |
| 9 | aristolactone | C15H20O2 | 232.1 | [4] |
| 10 | eleutheroside d | C34H46O18 | 742.3 | [4] |
| 11 | vanillic acid | C8H8O4 | 168.0 | [27] |
| 12 | 4-hydroxybenzaldehyde | C7H6O2 | 122.0 | [27] |
| 13 | syringaldehyde | C9H10O4 | 182.1 | [27] |
| 14 | 2-hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-1-propanone | C7H6O2 | 226.1 | [27] |
| 15 | methyl 4-hydroxyphenylacetate | C9H10O3 | 166.1 | [27] |
| 16 | 2-(4-hydroxyphenyl)-nitroethane | C8H9NO3 | 167.1 | [27] |
| 17 | 4-hydroxybenzyl cyanide | C8H7NO | 133.1 | [27] |
| 18 | dibutyl phthalate | C16H22O4 | 278.2 | [27] |
| 19 | fragransin b2 | C11H14O5 | 226.1 | [27] |
| 20 | 7-hydroxy-3,6-dimethoxy-1,4-phenanthraquinone | C16H12O5 | 284.1 | [27] |
| 21 | palmitic acid | C16H32O2 | 256.2 | [27] |
| 22 | arachidic acid | C20H40O2 | 312.3 | [27] |
| 23 | β-stigmasterol | C29H48O | 412.4 | [27] |
| 24 | ethyl pentamethylbenzene | C13H26 | 182.2 | [28] |
| 25 | tetradecane | C14H30 | 198.2 | [28] |
| 26 | 2,6,10-trimethylhexadecane | C17H36 | 240.3 | [28] |
| 27 | octadecane | C18H38 | 254.3 | [28] |
| 28 | diheptadecane | C27H56 | 380.4 | [28] |
| 29 | methyldecanoate | C11H22O2 | 186.2 | [28] |
| 30 | 2,4-bis(1,1-dimethylethyl-)phenol | C14H28O | 212.2 | [28] |
| 31 | 12-methyl-methyltridecanoate | C15H30O2 | 242.2 | [28] |
| 32 | 2-dodecen-1-yl(-1)succinic anhydride | C16H25O3 | 265.2 | [28] |
| 33 | 9-hexadecenoic acid | C16H30O2 | 254.2 | [28] |
| 34 | 2-methyl-1-hexadecanol | C17H24O | 244.2 | [28] |
| 35 | 7-methyl-tetradecene(z)-1-ol acetate | C17H29O2 | 265.2 | [28] |
| 36 | methylpalmitate | C17H34O2 | 270.3 | [28] |
| 37 | 14-methyl-methylhexadecanoate | C18H36O2 | 284.3 | [28] |
| 38 | 8,11-methyl-octadecadienoate | C19H32O2 | 292.2 | [28] |
| 39 | methyllinoleate | C19H34O2 | 294.3 | [28] |
| 40 | methyloleate | C19H36O2 | 296.3 | [28] |
| 41 | methylstearate | C19H38O2 | 298.3 | [28] |
| 42 | 16-methyl-methylheptadecanoate | C19H38O2 | 298.3 | [28] |
| 43 | methyleicosanoate | C21H42O2 | 326.3 | [28] |
| 44 | 20-methyl-methyldocosanoate | C22H44O2 | 340.3 | [28] |
| 45 | isopropyl-5,6,19-dioctadecatrienoate | C31H56O2 | 460.4 | [28] |
| 46 | 2,2,2-trifluoroethyl-9-octadecadienoic acid | C20H33F3O2 | 362.2 | [28] |
| 47 | 2-2-amino-n-(3,4,4a,5,6,7-hexahydro-5,6,8-trihydroxy-3-methyl-1-oxo-1h-2-benzopyran-4-yl)-propanamide | C13H20N2O6 | 300.1 | [28] |
| 48 | 3-methylbenzyl alcohol, tert-butyldimethylsilyl ether | C14H30OSi | 242.2 | [28] |
| 49 | hexaethylcyclotrisiloxane | C12H30O3Si3 | 390.1 | [28] |
| 50 | 6,6,8,8,10,10-hexamethyl-2,5,7,9,11,14-hexaoxa-6,8,10-trisilicopentadecane | C12H32O6Si3 | 356.2 | [28] |
| 51 | octamethylcyclotrisiloxane | C8H24O4Si4 | 296.1 | [28] |
| 52 | decamethylcyclotrisiloxane | C10H30O5Si5 | 370.1 | [28] |
| 53 | dodecamethylcyclotrisiloxane | C12H36O6Si6 | 444.1 | [28] |
| 54 | tetradecamethylcyclotrisiloxane | C14H42O7Si7 | 518.1 | [28] |
| 55 | hexadecamethylcyclotrisiloxane | C16H48O8Si8 | 592.2 | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, X.; Wang, K.; Gao, X.; Yan, B. Research Progress on Chemical Constituents and Pharmacological Activities of Menispermi Rhizoma. Molecules 2023, 28, 2701. https://doi.org/10.3390/molecules28062701
Zhai X, Wang K, Gao X, Yan B. Research Progress on Chemical Constituents and Pharmacological Activities of Menispermi Rhizoma. Molecules. 2023; 28(6):2701. https://doi.org/10.3390/molecules28062701
Chicago/Turabian StyleZhai, Xuan, Kangmin Wang, Xingyi Gao, and Bin Yan. 2023. "Research Progress on Chemical Constituents and Pharmacological Activities of Menispermi Rhizoma" Molecules 28, no. 6: 2701. https://doi.org/10.3390/molecules28062701





