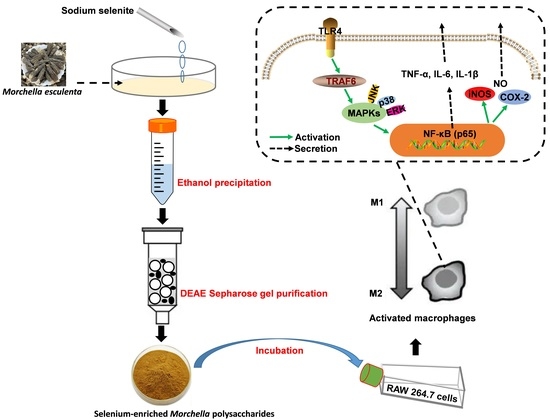
Microanalysis Characterization and Immunomodulatory Effect for Selenium-Enriched Polysaccharide from Morchella esculenta (L.) Pers.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Composition Analysis
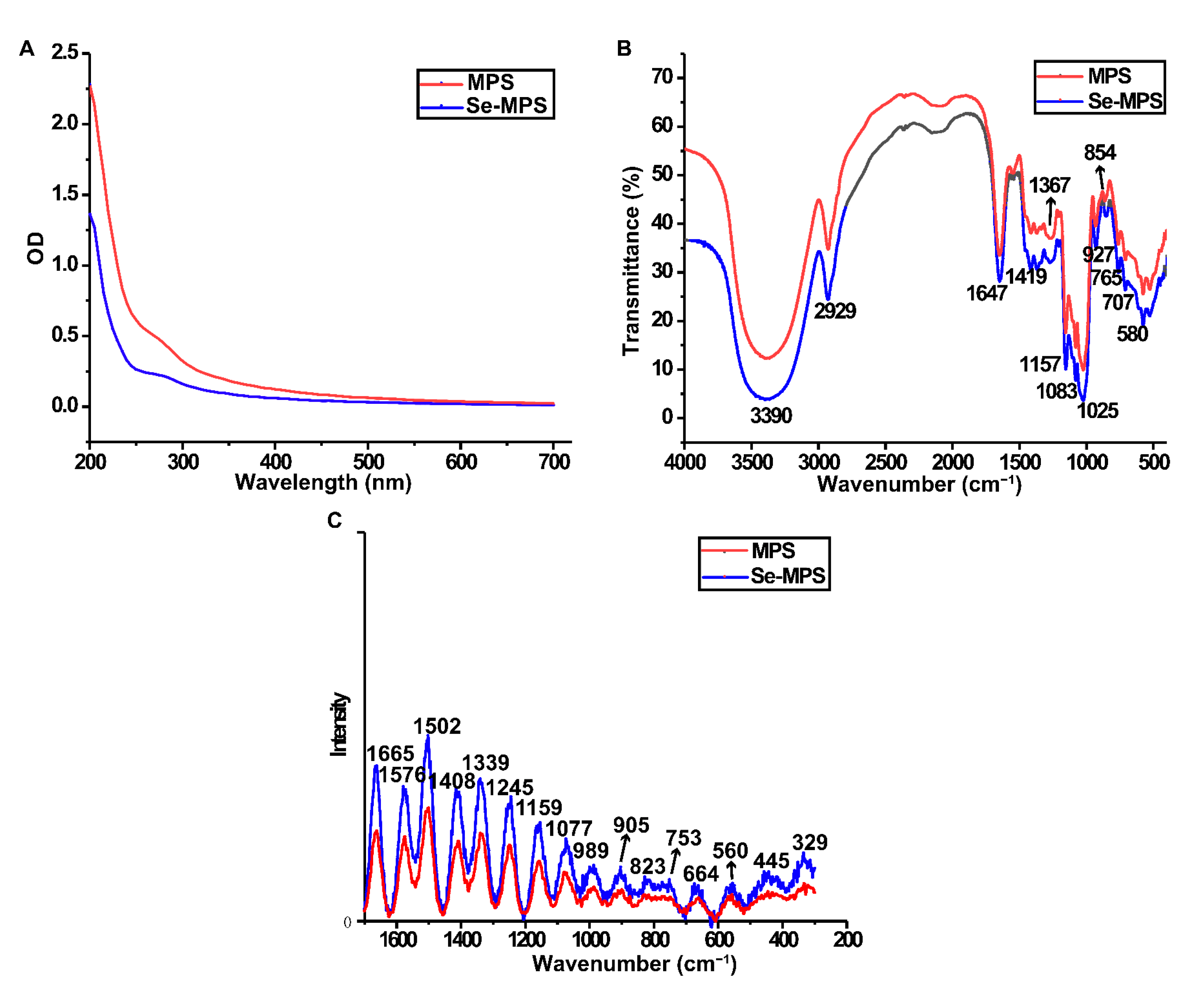
2.2. Chemical Characterization
2.3. Property Analysis
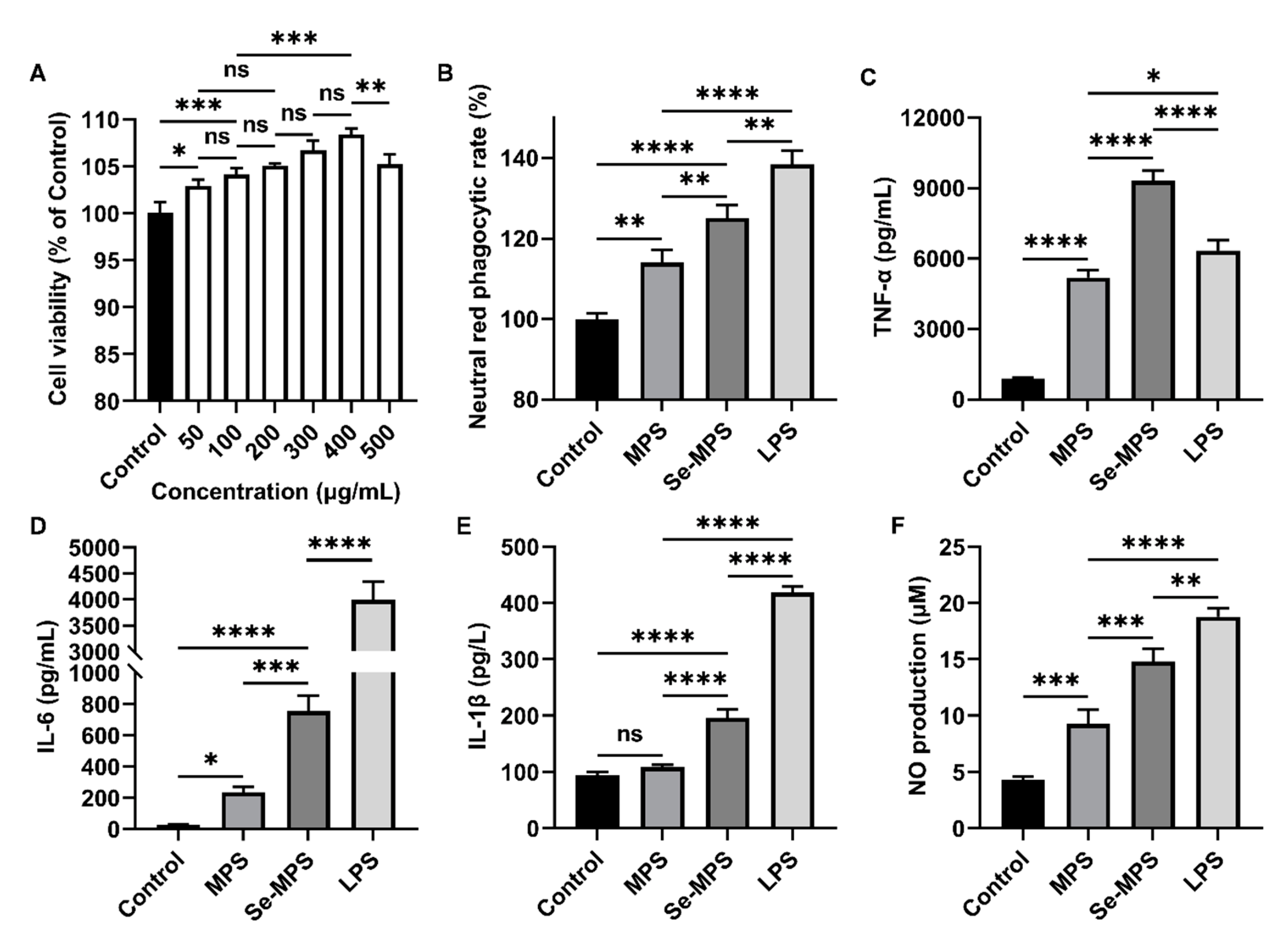
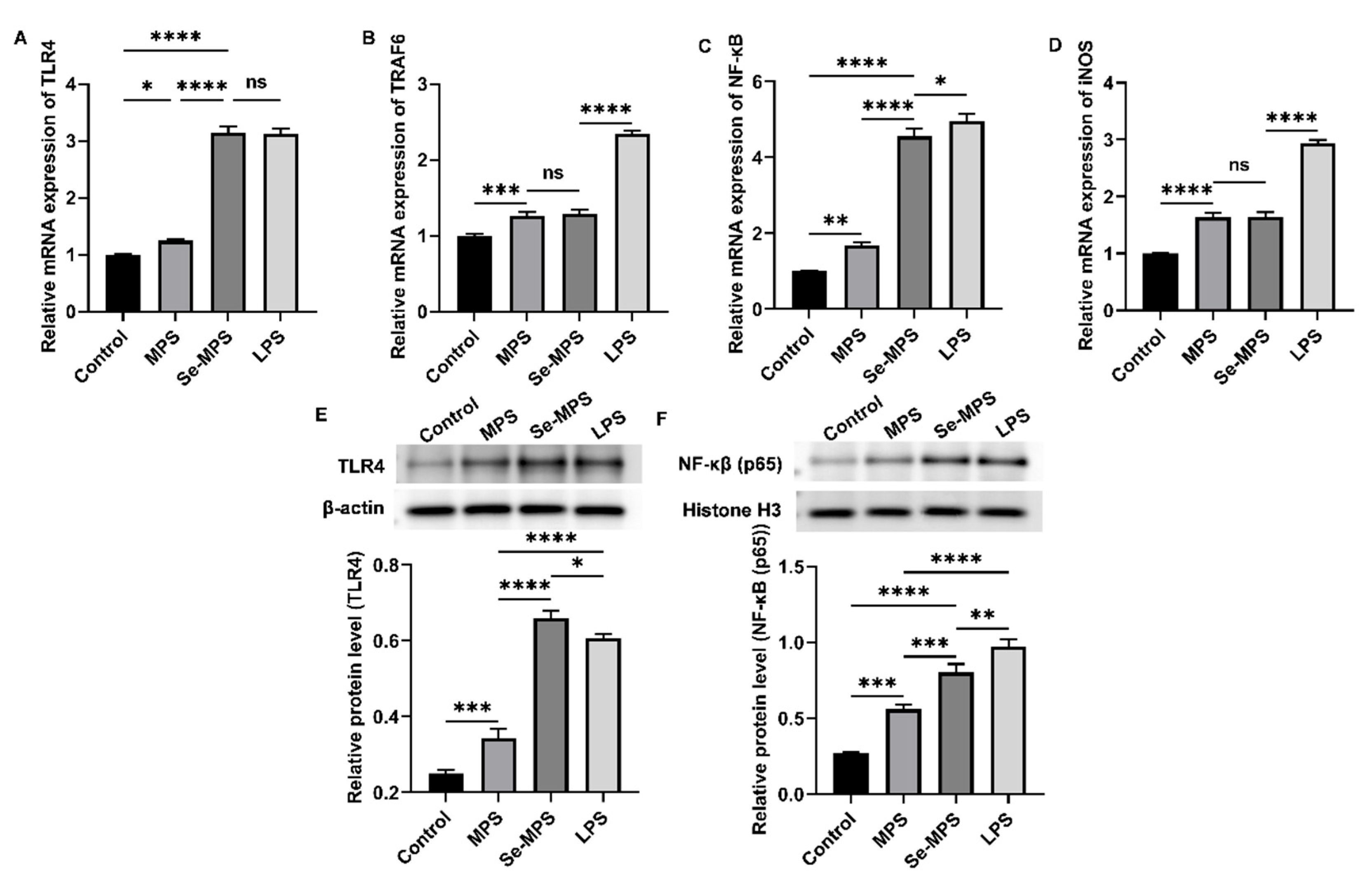
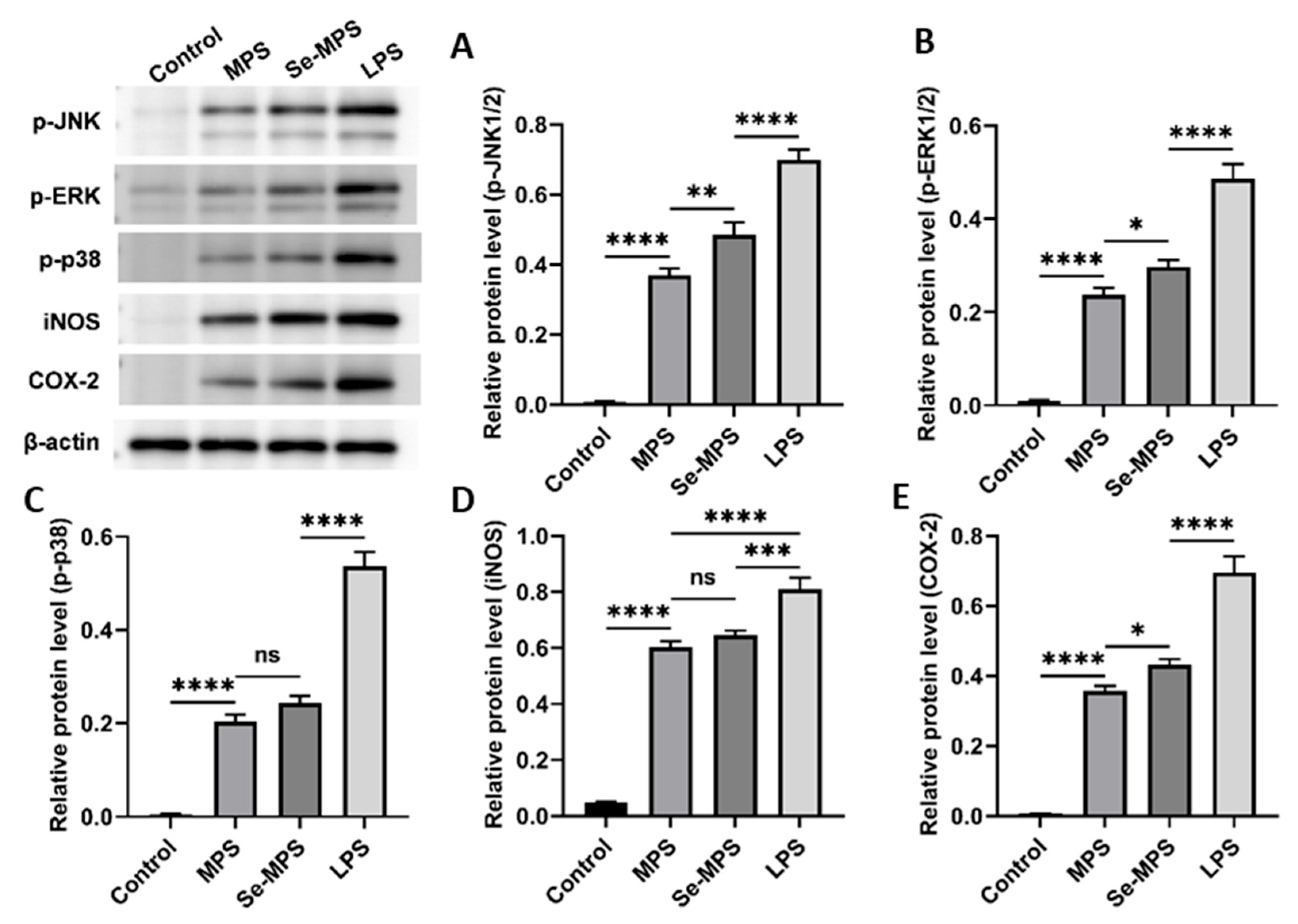
2.4. Immunomodulation Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Polysaccharides Preparation and Purification
3.3. Microchemical Characterization
3.3.1. Preliminary Composition Analysis
3.3.2. Monosaccharide Composition
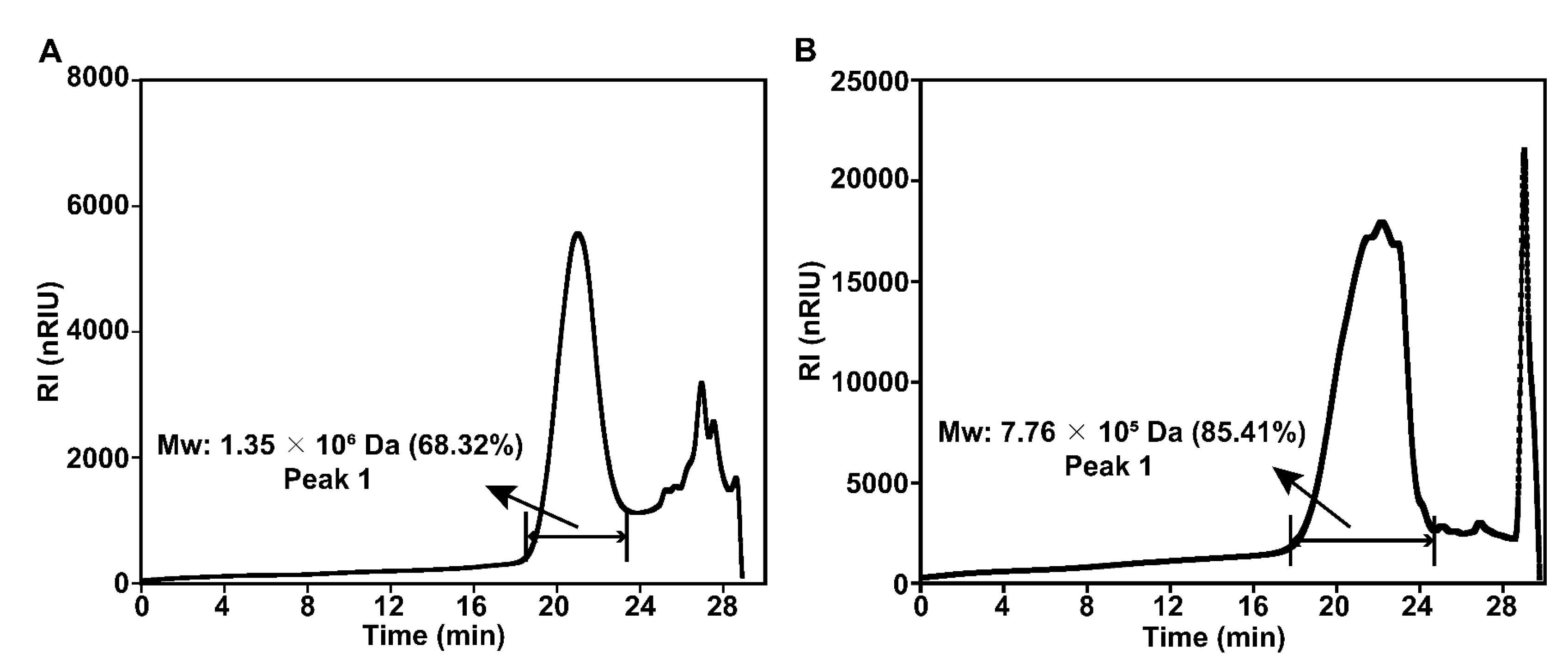
3.3.3. Molecular Weight (Mw) Distribution
3.3.4. Ultraviolet-Visible (UV-vis) and Fourier-Transform Infrared (FT-IR) Analysis
3.3.5. Raman Spectroscopy Analysis
3.3.6. Nuclear Magnetic Resonance (NMR) Spectroscopy Analysis
3.3.7. Scanning Electron Microscopy (SEM) Analysis
3.3.8. X-ray Diffraction (XRD) Analysis
3.3.9. Thermogravimetric (TG) Analysis
3.4. Immunomodulatory Evaluation
3.4.1. Cell Viability
3.4.2. Evaluation of Cellular Phagocytosis
3.4.3. Biochemical Parameters
3.4.4. Real-Time PCR Assay
- Primer TLR4 Forward: CTGGGTGAGAAAGCTGGTAA
- Primer TLR4 Reverse: AGCCTTCCTGGATGATGTTGG
- Primer TRAF6 Forward: CATCTTCAGTTACCGACAGCTCAG
- Primer TRAF6 Reverse: TGGTCGAGAATTGTAAGGCGTAT
- Primer NF-κB Forward: CCAAAGAAGGACACGACAGAATC
- Primer NF-κB Reverse: GGCAGGCTATTGCTCATCACA
- Primer iNOS Forward: CAACCAGTATTATGGCTCCT
- Primer iNOS Reverse: GTGACAGCCCGGTCTTTCCA
- Primer β-actin Forward: TGGAATCCTGTGGCATCCATGAAAC
- Primer β-actin Reverse: TAAAACGCAGCTCAGTAACAGTCCG
3.4.5. Western Blot Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, H.; Chen, J.; Li, J.; Liu, Y.; Park, H.J.; Yang, L. Recent advances on bioactive ingredients of Morchella esculenta. Appl. Biochem. Biotechnol. 2021, 193, 4197–4213. [Google Scholar] [CrossRef]
- Tietel, Z.; Masaphy, S. True morels (Morchella)-nutritional and phytochemical composition, health benefits and flavor: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1888–1901. [Google Scholar] [CrossRef]
- Sullivan, R.; Smith, J.E.; Rowan, N.J. Medicinal mushrooms and cancer therapy: Translating a traditional practice into Western medicine. Perspect. Biol. Med. 2006, 49, 159–170. [Google Scholar] [CrossRef]
- Singla, R.K.; De, R.; Efferth, T.; Mezzetti, B.; Sahab Uddin, M.; Sanusi; Ntie-Kang, F.; Wang, D.; Schultz, F.; Kharat, K.R.; et al. The international natural product sciences taskforce (INPST) and the power of Twitter networking exemplified through #INPST hashtag analysis. Phytomedicine 2023, 108, 154520. [Google Scholar]
- Sun, Y.; He, H.; Wang, Q.; Yang, X.; Jiang, S.; Wang, D. A review of development and utilization for edible fungal polysaccharides: Extraction, chemical characteristics, and bioactivities. Polymers 2022, 14, 4454. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, Z.; Chen, L.; Yu, C.; Wang, H.; Wei, X.; Wang, Y. Comparison and structural characterization of polysaccharides from natural and artificial Se-enriched green tea. Int. J. Biol. Macromol. 2019, 130, 388–398. [Google Scholar] [CrossRef]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium anticancer properties and impact on cellular redox status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Yu, C.; Han, Z.; Chen, Z.; Wei, X.; Wang, Y. Comparative analysis of existence form for selenium and structural characteristics in artificial selenium-enriched and synthetic selenized green tea polysaccharides. Int. J. Biol. Macromol. 2020, 154, 1408–1418. [Google Scholar] [CrossRef]
- Zhu, J.; Du, M.; Wu, M.; Yue, P.; Yang, X.; Wei, X.; Wang, Y. Preparation, physicochemical characterization and identification of two novel mixed ACE-inhibiting peptides from two distinct tea alkali-soluble protein. Eur. Food Res. Technol. 2020, 246, 1483–1494. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Nutritional selenium supplements: Product types, quality, and safety. J. Am. Coll. Nutr. 2001, 20, 1–4. [Google Scholar] [CrossRef]
- Won, D.P.; Lee, J.S.; Kwon, D.S.; Lee, K.E.; Shin, W.C.; Hong, E.K. Immunostimulating activity by polysaccharides isolated from fruiting body of Inonotus obliquus. Mol. Cells. 2011, 31, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Wang, Y.; Wang, Y.; Chen, T.; Tang, H.; Wang, M. Purification, characterization and immuno-modulating properties of polysaccharides isolated from Flammulina velutipes mycelium. Am. J. Chin. Med. 2010, 38, 191–204. [Google Scholar] [CrossRef]
- Su, C.A.; Xu, X.Y.; Liu, D.Y.; Wu, M.; Zeng, F.Q.; Zeng, M.Y.; Wei, W.; Jiang, N.; Luo, X. Isolation and characterization of exopolysaccharide with immunomodulatory activity from fermentation broth of Morchella conica. Daru 2013, 21, 5. [Google Scholar] [CrossRef] [Green Version]
- Malinowska, E.; Klimaszewska, M.; Strączek, T.; Schneider, K.; Kapusta, C.; Podsadni, P.; Łapienis, G.; Dawidowski, M.; Kleps, J.; Górska, S.; et al. Selenized polysaccharides—Biosynthesis and structural analysis. Carbohydr. Polym. 2018, 198, 407–417. [Google Scholar] [CrossRef]
- Duan, W.X.; Yang, X.H.; Zhang, H.F.; Feng, J.; Zhang, M.Y. Chemical structure, hypoglycemic activity, and mechanism of action of selenium polysaccharides. Biol. Trace. Elem. Res. 2022, 200, 4404–4418. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Zhu, C.; Liu, G.; Han, W.; Sun, Y.; Qian, L. Valorization of polysaccharides from Benincasa hispida: Physicochemical, moisturizing, and antioxidant skincare properties. Front. Pharmacol. 2022, 13, 912382. [Google Scholar] [CrossRef]
- Kaleta, B.; Roszczyk, A.; Zych, M.; Kniotek, M.; Zagożdżon, R.; Klimaszewska, M.; Malinowska, E.; Pac, M.; Turło, J. Selective biological effects of selenium-enriched polysaccharide (Se-Le-30) isolated from Lentinula edodes mycelium on human immune cells. Biomolecules 2021, 11, 1777. [Google Scholar] [CrossRef]
- Cai, Z.N.; Li, W.; Mehmood, S.; Pan, W.J.; Wang, Y.; Meng, F.J.; Wang, X.F.; Lu, Y.M.; Chen, Y. Structural characterization, in vitro and in vivo antioxidant activities of a heteropolysaccharide from the fruiting bodies of Morchella esculenta. Carbohydr. Polym. 2018, 195, 29–38. [Google Scholar] [CrossRef]
- Kuang, M.T.; Xu, J.Y.; Li, J.Y.; Yang, L.; Hou, B.; Zhao, Q.; Hu, J.M. Purification, structural characterization and immunomodulatory activities of a polysaccharide from the fruiting body of Morchella sextelata. Int. J. Biol. Macromol. 2022, 213, 394–403. [Google Scholar] [CrossRef]
- Yue, L.; Song, X.; Cui, X.; Zhang, Q.; Tian, X.; Yang, X.; Wu, Q.; Liu, Y.; Ruan, R.; Wang, Y. Synthesis, characterization, and evaluation of microwave-assisted fabricated selenylation Astragalus polysaccharides. Int. J. Biol. Macromol. 2022, 221, 8–15. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, Y.; Zhang, M.; Yang, X.; Yue, P.; Tang, D.; Wei, X. Structural characterization and antioxidant activity of a new polysaccharide from Bletilla striata fibrous roots. Carbohydr. Polym. 2020, 227, 115362. [Google Scholar] [CrossRef]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef]
- Chen, G.; Kan, J. Characterization of a novel polysaccharide isolated from Rosa roxburghii Tratt fruit and assessment of its antioxidant in vitro and in vivo. Int. J. Biol. Macromol. 2018, 107 Pt A, 166–174. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Gao, J.; Huang, J.; Yang, Y.; Xu, Y.; Liu, S.; Yu, W. Characterization, antioxidant and immunomodulatory effects of selenized polysaccharides from dandelion roots. Carbohydr. Polym. 2021, 260, 117796. [Google Scholar] [CrossRef]
- Hou, R.; Chen, J.; Yue, C.; Li, X.; Liu, J.; Gao, Z.; Liu, C.; Lu, Y.; Wang, D.; Li, H.; et al. Modification of lily polysaccharide by selenylation and the immune-enhancing activity. Carbohyd. Polym. 2016, 142, 73–81. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, J.; Chen, X.; Fan, Q.; Zhang, C.; Wang, D.; Li, X.; Chen, X.; Chen, X.; Liu, C.; et al. Optimization of selenylation conditions for lycium barbarum polysaccharide based on antioxidant activity. Carbohydr. Polym. 2014, 103, 148–153. [Google Scholar] [CrossRef]
- Cheung, Y.-C.; Siu, K.-C.; Liu, Y.-S.; Wu, J.-Y. Molecular properties and antioxidant activities of polysaccharide–protein complexes from selected mushrooms by ultrasound-assisted extraction. Process Biochem. 2012, 47, 892–895. [Google Scholar] [CrossRef]
- Salvador, C.; Martins, M.R.; Caldeira, A.T. Microanalysis characterization of bioactive protein-bound polysaccharides produced by Amanita ponderosa cultures. Microsc. Microanal. 2015, 21, 84–90. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Hou, C.; Shi, C.; Lin, Z.; Liao, W.; Yuan, E. A polysaccharide isolated and purified from Platycladus orientalis (L.) Franco leaves, characterization, bioactivity and its regulation on macrophage polarization. Carbohyd. Polym. 2019, 213, 276–285. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Zhu, R.; Yu, J.; Lu, W.; Pan, C.; Yao, W.; Gao, X. Structure characterization, chemical and enzymatic degradation, and chain conformation of an acidic polysaccharide from Lycium barbarum L. Carbohyd. Polym. 2016, 147, 114–124. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Yue, H.; Zhou, L.; Huang, L.; Du, Z.; Ding, K. Structural elucidation of a pectic polysaccharide from Fructus Mori and its bioactivity on intestinal bacteria strains. Carbohyd. Polym. 2018, 186, 168–175. [Google Scholar] [CrossRef]
- Zhang, A.; Deng, J.; Yu, S.; Zhang, F.; Linhardt, R.J.; Sun, P. Purification and structural elucidation of a water-soluble polysaccharide from the fruiting bodies of the Grifola frondosa. Int. J. Biol. Macromol. 2018, 115, 221–226. [Google Scholar] [CrossRef]
- Malfait, T.; Van Dael, H.; Van Cauwelaert, F. Molecular structure of carrageenans and kappa oligomers: A Raman spectroscopic study. Int. J. Biol. Macromol. 1989, 11, 259–264. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Chen, Y.; Xie, J.; Liu, S.; Sun, N.; Hu, X.; Yu, Q. Modification of tea residue dietary fiber by high-temperature cooking assisted enzymatic method: Structural, physicochemical and functional properties. LWT 2021, 145, 111314. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, Y.K.; Chang, Y.H. Effects of selenylation modification on structural and antioxidant properties of pectic polysaccharides extracted from Ulmus pumila L. Int. J. Biol. Macromol. 2017, 104 Pt A, 1124–1132. [Google Scholar] [CrossRef]
- Yang, X.; Huang, M.; Qin, C.; Lv, B.; Mao, Q.; Liu, Z. Structural characterization and evaluation of the antioxidant activities of polysaccharides extracted from Qingzhuan brick tea. Int. J. Biol. Macromol. 2017, 101, 768–775. [Google Scholar] [CrossRef]
- Liu, X.; Hasan, K.M.F.; Wei, S. Immunological regulation, effects, extraction mechanisms, healthy utilization, and bioactivity of edible fungi: A comprehensive review. J. Food Process Eng. 2022, 45, 13970. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in research on immunoregulation of Macrophages by plant polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Li, K.; Xiao, L.; Lei, Z.; Zhang, Z. Characterization of polysaccharide from Helicteres angustifolia L. and its immunomodulatory activities on macrophages RAW264.7. Biomed. Pharmacother. 2019, 109, 262–270. [Google Scholar] [CrossRef]
- Wu, M.J.; Cheng, T.L.; Cheng, S.Y.; Lian, T.W.; Wang, L.; Chiou, S.Y. Immunomodulatory properties of Grifola frondosa in submerged culture. J. Agric. Food Chem. 2006, 54, 2906–2914. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Jakhar, R.; Paul, S.; Kang, S.C. Potentiation of macrophage activity by thymol through augmenting phagocytosis. Int. Immunopharmacol. 2014, 18, 340–346. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Wang, Z.; Wang, Y.; Xie, M.; Xie, J. Sulfated polysaccharide from Cyclocarya paliurus enhances the immunomodulatory activity of macrophages. Carbohydr. Polym. 2017, 174, 669–676. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, C.; Peng, G.; Liu, Y.; Zhang, X.; Meng, Z. Immune-enhancing effects of a polysaccharide PRG1-1 from Russula griseocarnosa on RAW264.7 macrophage cells via the MAPK and NF-κB signalling pathways. Food Agric. Immunol. 2018, 29, 833–844. [Google Scholar] [CrossRef] [Green Version]
- Kitaura, H.; Kimura, K.; Ishida, M.; Kohara, H.; Yoshimatsu, M.; Takano-Yamamoto, T. Immunological reaction in TNF-α-mediated osteoclast formation and bone resorption in vitro and in vivo. Clin. Dev. Immunol. 2013, 2013, 181849. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Liu, Z.; Huang, H. Immune-enhancing activities of chondroitin sulfate in murine macrophage RAW 264.7 cells. Carbohydr. Polym. 2018, 198, 611–619. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xie, J.H.; Kan, L.J.; Wang, J.Q.; Shen, M.Y.; Li, W.J.; Nie, S.P.; Xie, M.Y. Sulfated polysaccharides from Cyclocarya paliurus reduce H2O2-induced oxidative stress in RAW264.7 cells. Int. J. Biol. Macromol. 2015, 80, 410–417. [Google Scholar] [CrossRef]
- Lu, M.; Yang, W.; Peng, Z.; Zhang, J.; Mei, W.; Liu, C.; Tang, J.; Ma, H.; Yuan, X.; Meng, J.; et al. Fluorofenidone inhibits macrophage IL-1β production by suppressing inflammasome activity. Int. Immunopharmacol. 2015, 27, 148–153. [Google Scholar] [CrossRef]
- Ren, Z.; Qin, T.; Qiu, F.; Song, Y.; Lin, D.; Ma, Y.; Li, J.; Huang, Y. Immunomodulatory effects of hydroxyethylated Hericium erinaceus polysaccharide on macrophages RAW264.7. Int. J. Biol. Macromol. 2017, 105 Pt 1, 879–885. [Google Scholar] [CrossRef]
- Suzuki, C.; Aoki-Yoshida, A.; Kimoto-Nira, H.; Kobayashi, M.; Sasaki, K.; Mizumachi, K. Effects of strains of Lactococcus lactis on the production of nitric oxide and cytokines in murine macrophages. Inflammation 2014, 37, 1728–1737. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Xu, R.-H.; Li, P.-L. Expolysaccharides from Bifidobacterium animalis RH activates RAW 264.7 macrophages through toll-like receptor 4. Food Agric. Immunol. 2016, 28, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Liang, Y.; Zhang, C.; Wang, K.; Tu, Y.; Chen, M.; Li, P.; Wan, J.B.; He, C. Polysaccharide PRM3 from Rhynchosia minima root enhances immune function through TLR4-NF-κB pathway. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1751–1759. [Google Scholar] [CrossRef]
- Gupta, P.K.; Rajan, M.G.R.; Kulkarni, S. Activation of murine macrophages by G1-4A, a polysaccharide from Tinospora cordifolia, in TLR4/MyD88 dependent manner. Int. Immunopharmacol. 2017, 50, 168–177. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Long, X.; Li, P.; Chen, S.; Kuang, W.; Guo, J. Sargassum fusiforme polysaccharides inhibit VEGF-A-related angiogenesis and proliferation of lung cancer in vitro and in vivo. Biomed. Pharmacother. 2017, 85, 22–27. [Google Scholar] [CrossRef]
- Shen, T.; Wang, G.; You, L.; Zhang, L.; Ren, H.; Hu, W.; Qiang, Q.; Wang, X.; Ji, L.; Gu, Z.; et al. Polysaccharide from wheat bran induces cytokine expression via the toll-like receptor 4-mediated p38 MAPK signaling pathway and prevents cyclophosphamide-induced immunosuppression in mice. Food Nutr. Res. 2017, 61, 1344523. [Google Scholar] [CrossRef] [Green Version]
- Dong, N.; Li, X.; Xue, C.; Zhang, L.; Wang, C.; Xu, X.; Shan, A. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-κB/MAPK signaling pathway. J. Cell. Physiol. 2020, 235, 5525–5540. [Google Scholar] [CrossRef]
- Qin, G.; Xu, W.; Liu, J.; Zhao, L.; Chen, G. Purification, characterization and hypoglycemic activity of glycoproteins obtained from pea (Pisum sativum L.). Food Sci. Hum. Well. 2021, 10, 297–307. [Google Scholar] [CrossRef]
- Rascón-Cruz, Q.; Espinoza-Sánchez, E.A.; Siqueiros-Cendón, T.S.; Nakamura-Bencomo, S.I.; Arévalo-Gallegos, S.; Iglesias-Figueroa, B.F. Lactoferrin: A glycoprotein involved in immunomodulation, anticancer, and antimicrobial processes. Molecules 2021, 26, 205. [Google Scholar] [CrossRef]
- Ryu, J.H.; Park, H.J.; Jeong, Y.Y.; Han, S.; Shin, J.H.; Lee, S.J.; Kang, M.J.; Sung, N.J.; Kang, D. Aged red garlic extract suppresses nitric oxide production in lipopolysaccharide-treated RAW 264.7 macrophages through inhibition of NF-κB. J. Med. Food 2015, 18, 439–445. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Xanthotoxin suppresses LPS-induced expression of iNOS, COX-2, TNF-α, and IL-6 via AP-1, NF-κB, and JAK-STAT inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 49, 21–29. [Google Scholar] [CrossRef]
- Wen, Y.; Peng, D.; Li, C.; Hu, X.; Bi, S.; Song, L.; Peng, B.; Zhu, J.; Chen, Y.; Yu, R. A new polysaccharide isolated from Morchella importuna fruiting bodies and its immunoregulatory mechanism. Int. J. Biol. Macromol. 2019, 137, 8–19. [Google Scholar] [CrossRef]
- Li, R.; Qin, X.; Liu, S.; Zhang, X.; Zeng, X.; Guo, H.; Wang, T.; Zhang, Y.; Zhang, J.; Zhang, J.; et al. [HNMP]HSO(4) catalyzed synthesis of selenized polysaccharide and its immunomodulatory effect on RAW264.7 cells via MAPKs pathway. Int. J. Biol. Macromol. 2020, 160, 1066–1077. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, X.; Li, F.; Wei, K.; Chen, J.; Wei, X.; Wang, Y. Preparation, physicochemical and hypoglycemic properties of natural selenium-enriched coarse tea glycoproteins. Plant Foods Hum. Nutr. 2022, 77, 258–264. [Google Scholar] [CrossRef]
- Grandy, A.S.; Erich, M.S.; Porter, G.A. Suitability of the anthrone–sulfuric acid reagent for determining water soluble carbohydrates in soil water extracts. Soil. Biol. Biochem. 2000, 32, 725–727. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ventura, M.G.; Stibilj, V.; do Carmo Freitas, M.; Pacheco, A.M.G. Determination of ultratrace levels of selenium in fruit and vegetable samples grown and consumed in Portugal. Food Chem. 2009, 115, 200–206. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, H.; Zhang, J.; Li, F.; Wei, K.; Wei, X.; Wang, Y. Valorization of polysaccharides obtained from dark tea: Preparation, physicochemical, antioxidant, and hypoglycemic properties. Foods 2021, 10, 2276. [Google Scholar] [CrossRef]



| Sample | Total Sugar (%) | Uronic Acid (%) | Protein (%) | Se (μg/g) | Yield (%) |
|---|---|---|---|---|---|
| MPS | 87.8 ± 0.32 | 0.73 ± 0.03 | 0.92 ± 0.06 | 2.71 ± 0.05 | 11.68 ± 0.25 |
| Se-MPS | 98.6 ± 0.22 | 0.37 ± 0.01 | 0.32 ± 0.02 | 214.07 ± 0.78 | 14.65 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, L.; Du, M.; Yang, X.; Wang, Q.; Huang, S.; Ma, Y.; Sun, Y. Microanalysis Characterization and Immunomodulatory Effect for Selenium-Enriched Polysaccharide from Morchella esculenta (L.) Pers. Molecules 2023, 28, 2885. https://doi.org/10.3390/molecules28072885
Qian L, Du M, Yang X, Wang Q, Huang S, Ma Y, Sun Y. Microanalysis Characterization and Immunomodulatory Effect for Selenium-Enriched Polysaccharide from Morchella esculenta (L.) Pers. Molecules. 2023; 28(7):2885. https://doi.org/10.3390/molecules28072885
Chicago/Turabian StyleQian, Lijuan, Mengxiang Du, Xiaoyan Yang, Qian Wang, Shengwei Huang, Yuhan Ma, and Yujun Sun. 2023. "Microanalysis Characterization and Immunomodulatory Effect for Selenium-Enriched Polysaccharide from Morchella esculenta (L.) Pers." Molecules 28, no. 7: 2885. https://doi.org/10.3390/molecules28072885