Graphene Modification by Curcuminoids as an Effective Method to Improve the Dispersion and Stability of PVC/Graphene Nanocomposites
Abstract
:1. Introduction
2. Results and Discussion
2.1. UV-Vis Spectroscopy
2.2. XPS Analysis
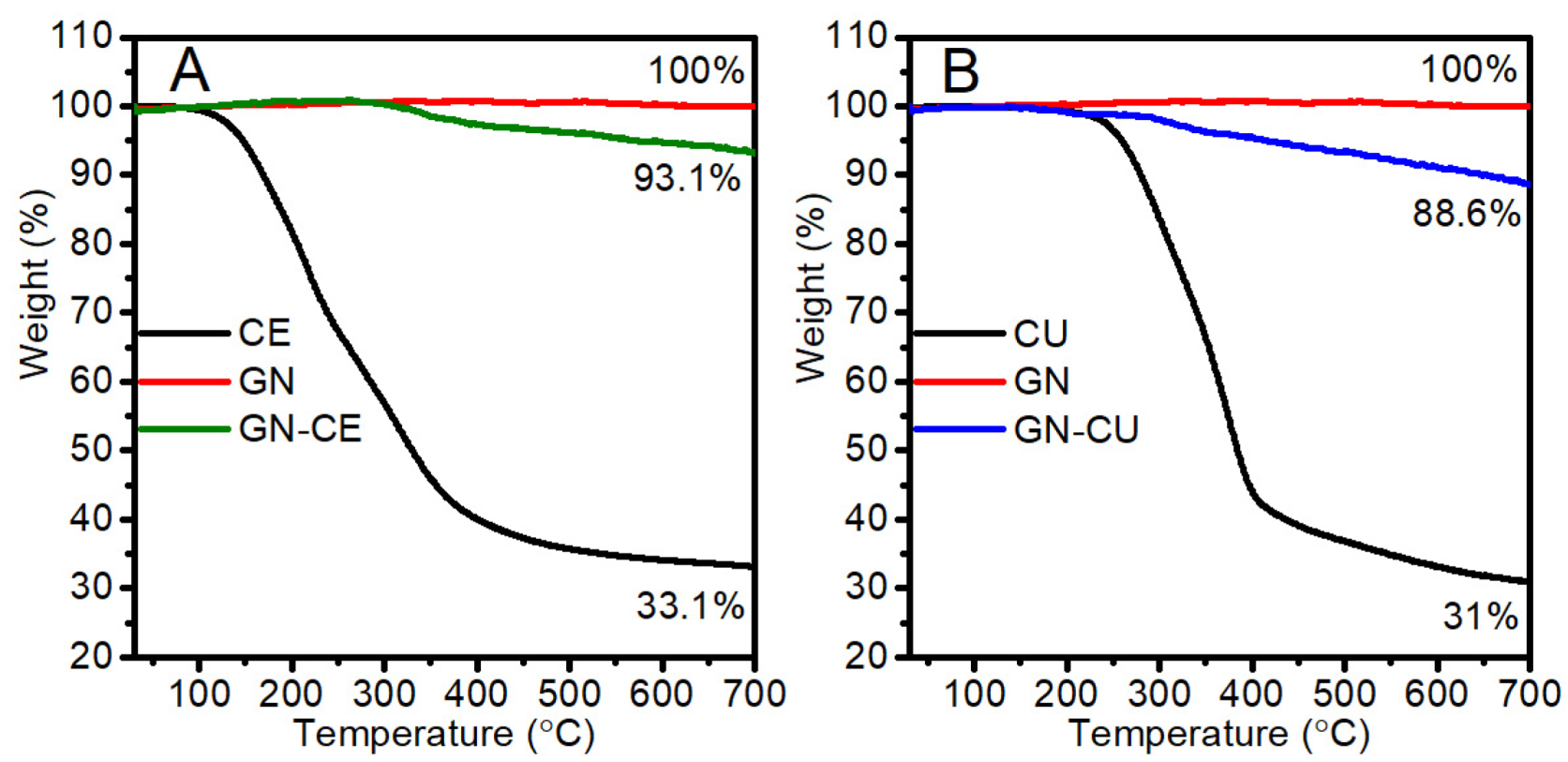
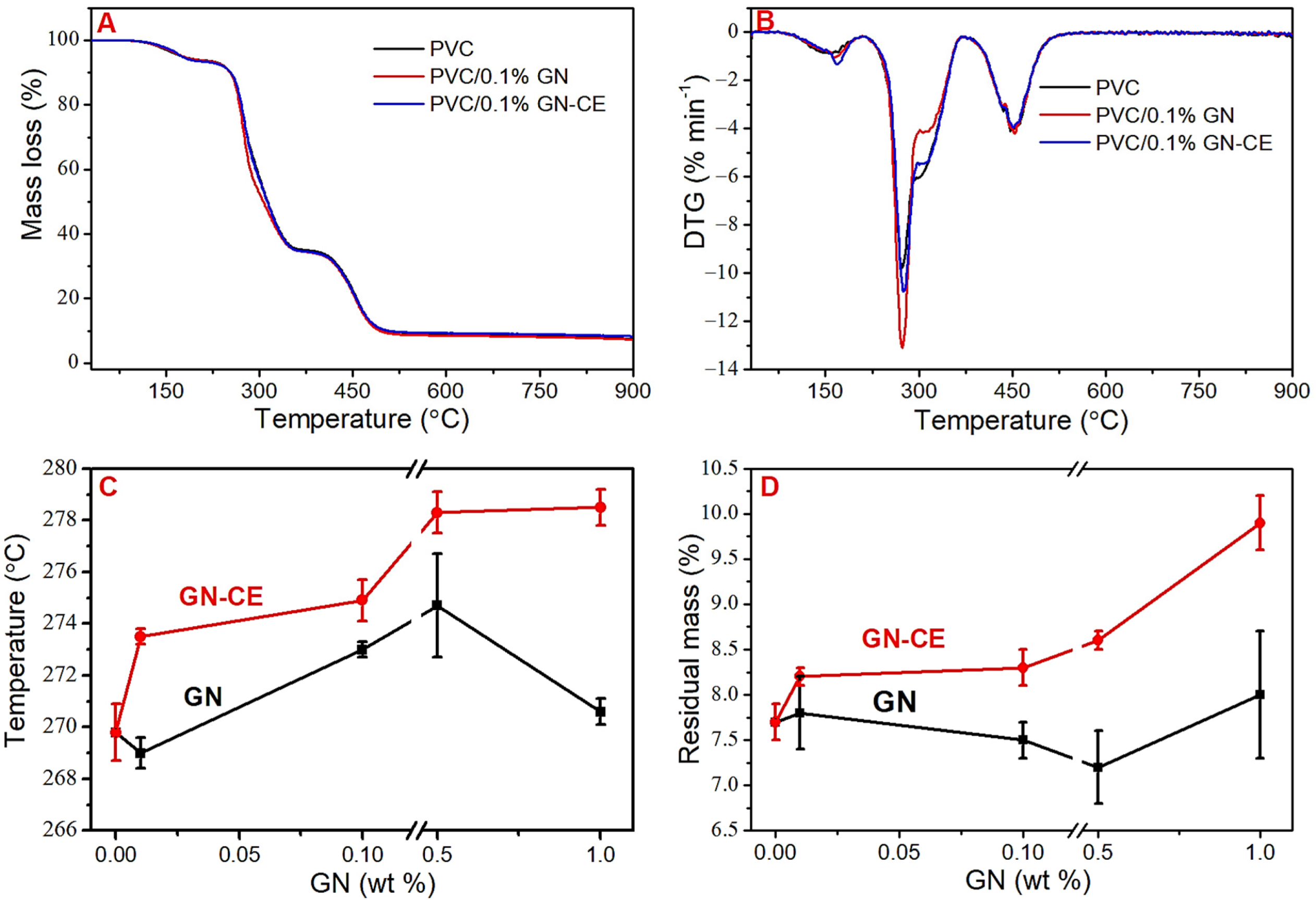
2.3. Thermogravimetric Analysis (TGA)
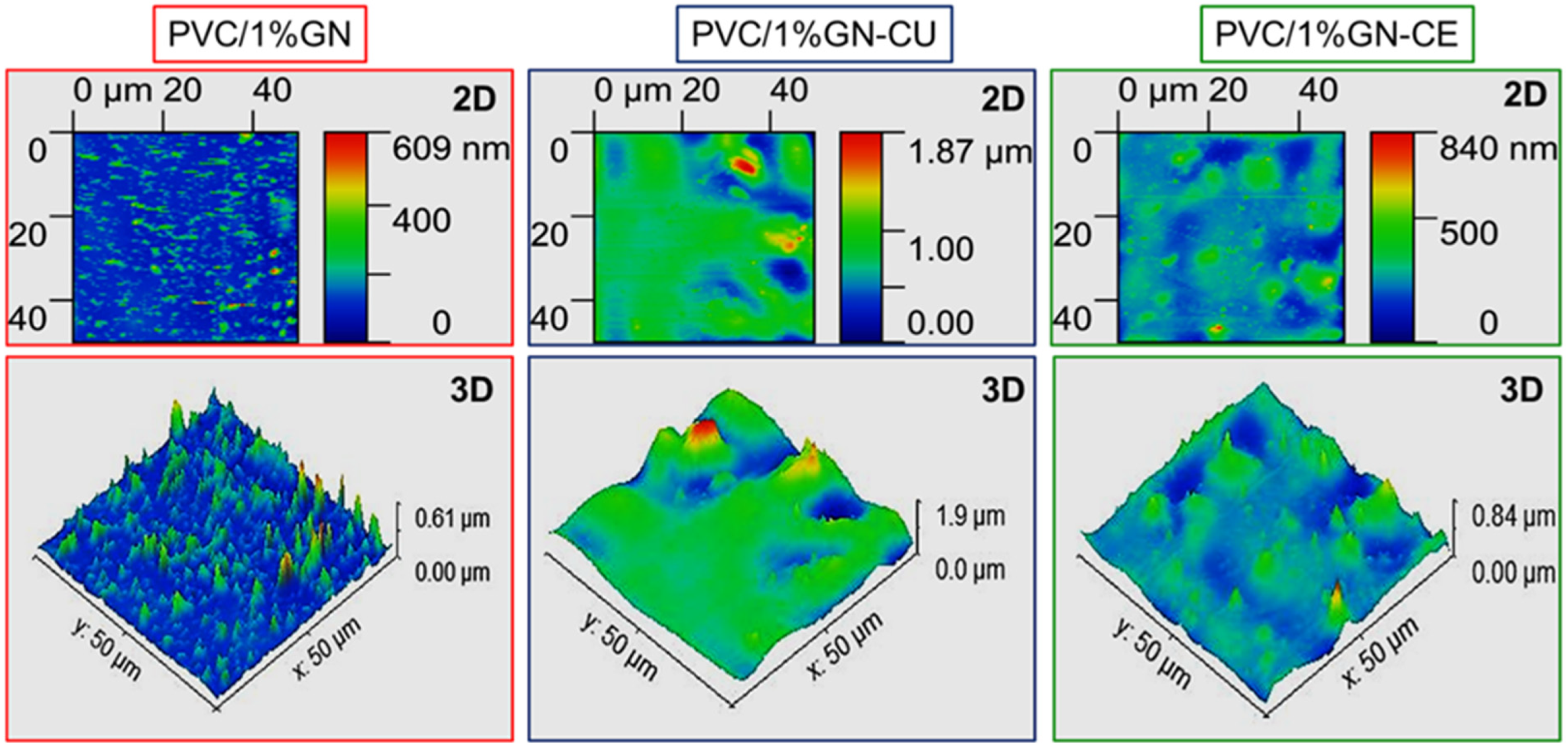
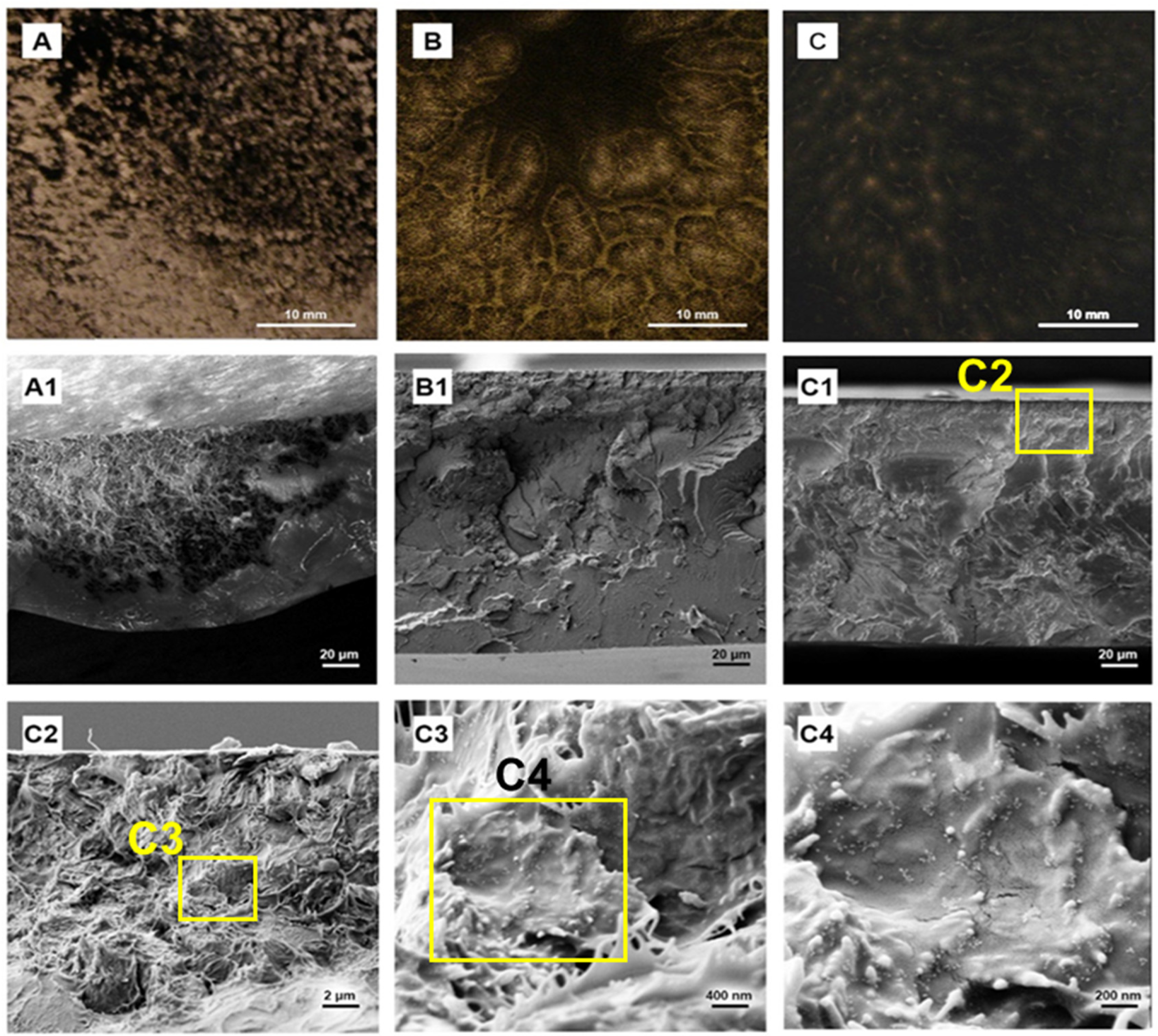
2.4. Morphology Characterization
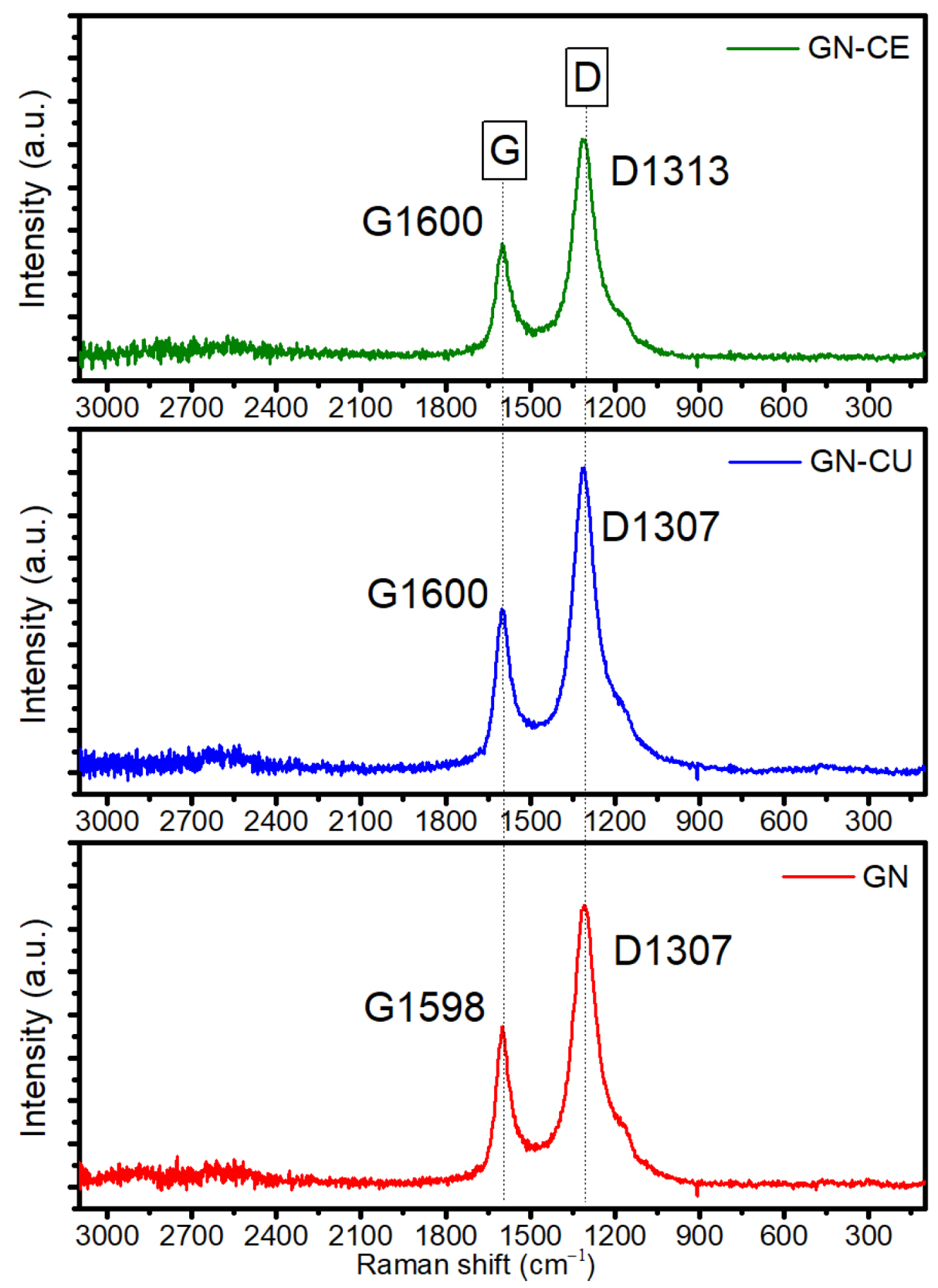
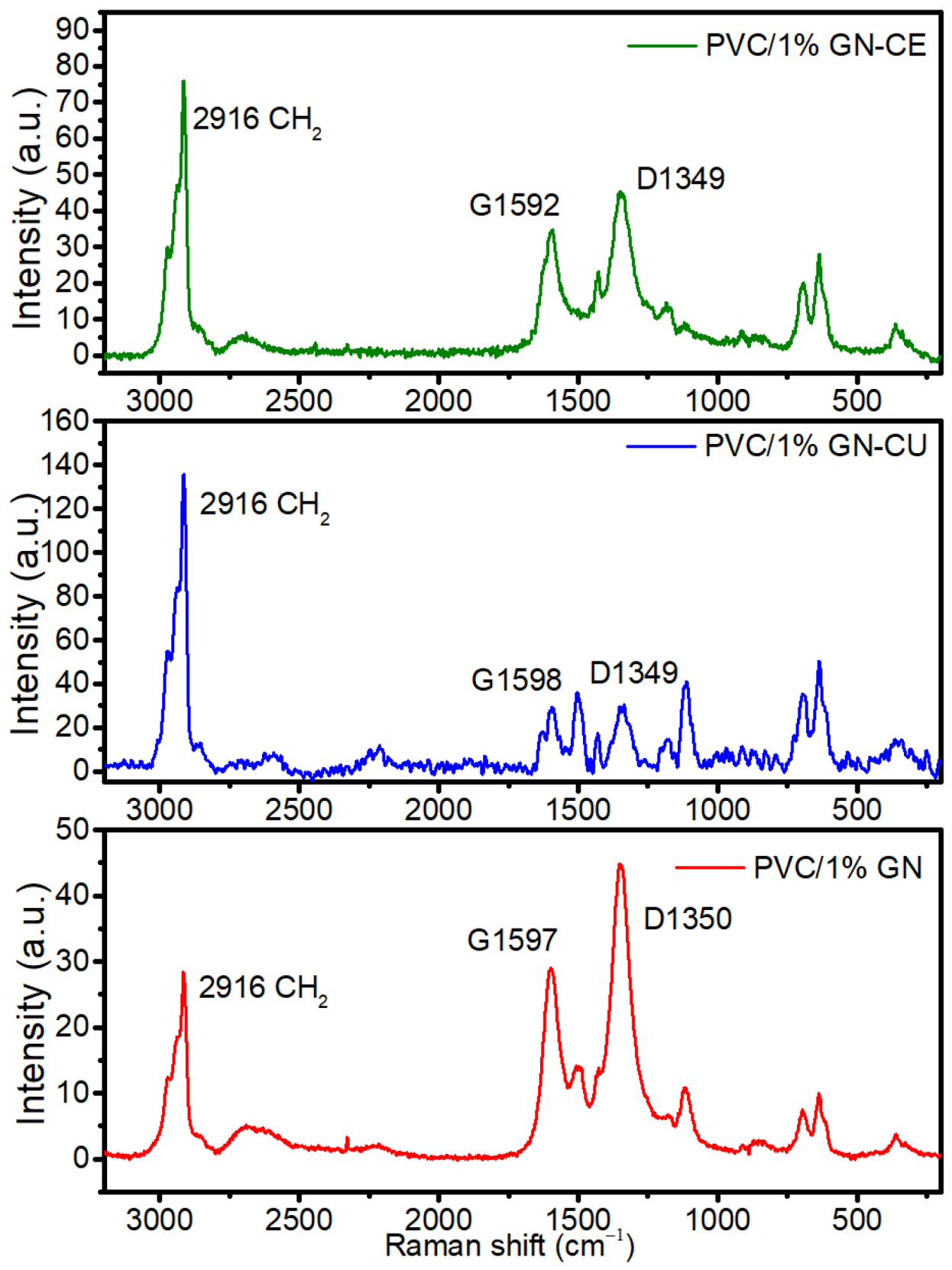
2.5. Raman Spectroscopy
2.6. Dispersion Stability Analysis
2.7. Graphene Dispersion in Nanocomposites
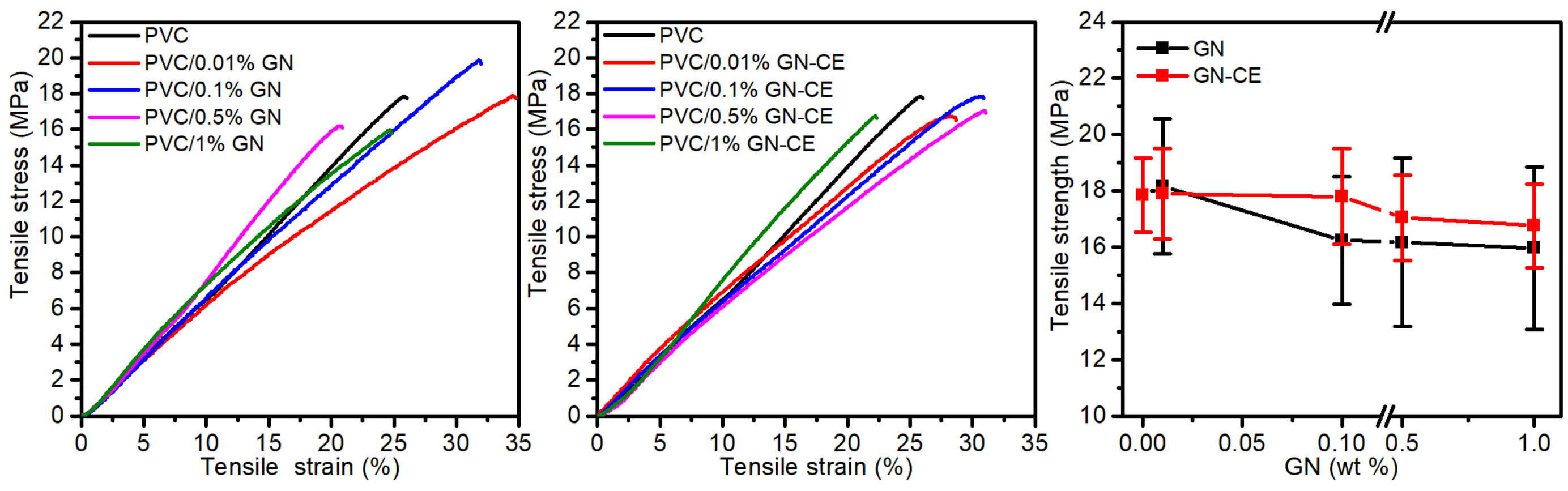
2.8. Thermal and Mechanical Properties of Nanocomposites
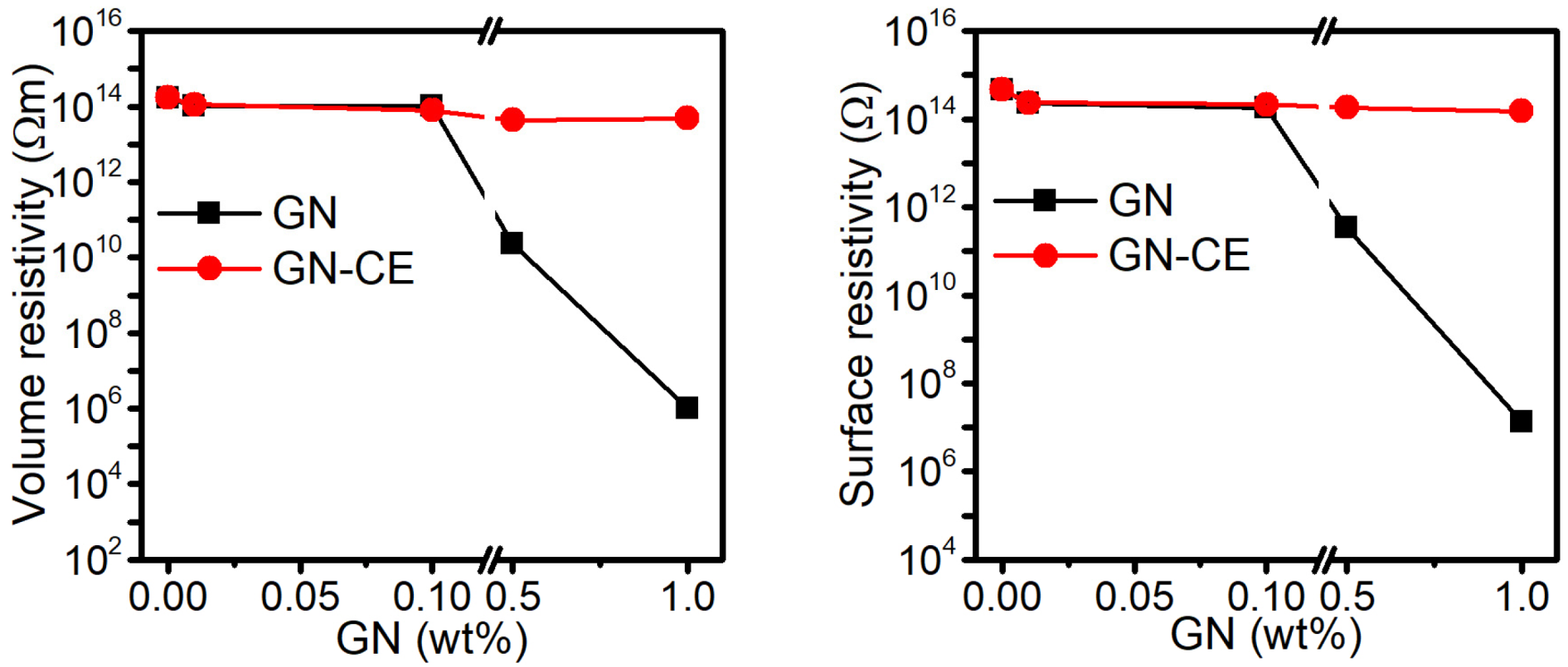
2.9. Electrical Properties of Nanocomposites
2.10. Swelling Behaviour of Nanocomposites
- Sd is swelling degree, %,
- SE is equilibrium swelling, upper asymptote, %,
- tM is time in which the swelling occurs with a maximum rate, s,
- t is time of exposure to the swelling agent, s,
- p is comparison parameter, 1 s−1.
3. Materials and Methods
3.1. Materials
3.2. Graphene Modification
3.3. Preparation of PVC/GN Dispersions and Nanocomposites
3.4. Characterization
- h is sample diameter after time t (mm),
- h0 is initial sample diameter (mm).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Johnson, D.W.; Dobson, B.P.; Coleman, K.S. A manufacturing perspective on graphene dispersions. Curr. Opin. Colloid Interface Sci. 2015, 20, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Abdel Ghany, N.A.; Elsherif, S.A.; Handal, H.T. Revolution of Graphene for different applications: State-of-the-art. Surf. Interfaces 2017, 9, 93–106. [Google Scholar] [CrossRef]
- Drewniak, S.; Muzyka, R.; Stolarczyk, A.; Pustelny, T.; Kotyczka-Morańska, M.; Setkiewicz, M. Studies of reduced graphene oxide and graphite oxide in the aspect of their possible application in gas sensors. Sensors 2016, 16, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikhailov, S. Physics and Applications of Graphene-Experiments; InTech: Rijeka, Croatia, 2011; ISBN 9789533071527. [Google Scholar]
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey, J.T.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: A comparison study between CNT and graphene. Prog. Polym. Sci. 2017, 67, 1–47. [Google Scholar] [CrossRef]
- Galpaya, D.; Wang, M.; Liu, M.; Motta, N.; Waclawik, E.; Yan, C. Recent Advances in Fabrication and Characterization of Graphene-Polymer Nanocomposites. Graphene 2012, 1, 30–49. [Google Scholar] [CrossRef] [Green Version]
- Sreenivasulu, B.; Ramji, B.R.; Nagaral, M. A Review on Graphene Reinforced Polymer Matrix Composites. Mater. Today Proc. 2018, 5, 2419–2428. [Google Scholar] [CrossRef]
- Phiri, J.; Gane, P.; Maloney, T.C. General overview of graphene: Production, properties and application in polymer composites. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 215, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Deshmukh, K.; Joshi, G.M. Thermo-mechanical properties of poly (vinyl chloride)/graphene oxide as high performance nanocomposites. Polym. Test. 2014, 34, 211–219. [Google Scholar] [CrossRef]
- Nawaz, K.; Ayub, M.; Ul-Haq, N.; Khan, M.B.; Niazi, M.B.K.; Hussain, A. The Effect of Graphene Nanosheets on the Mechanical Properties of Polyvinylchloride. Polym. Compos. 2016, 37, 1572–1576. [Google Scholar] [CrossRef]
- Mindivan, F.; Göktaş, M. Preparation of new PVC composite using green reduced graphene oxide and its effects in thermal and mechanical properties. Polym. Bull. 2020, 77, 1929–1949. [Google Scholar] [CrossRef]
- Li, Q.; Shen, F.; Zhang, Y.; Huang, Z.; Muhammad, Y.; Hu, H.; Zhu, Y.; Yu, C.; Qin, Y. Graphene incorporated poly(vinyl chloride) composites prepared by mechanical activation with enhanced electrical and thermo–mechanical properties. J. Appl. Polym. Sci. 2020, 137, 21–23. [Google Scholar] [CrossRef]
- Akhina, H.; Gopinathan Nair, M.R.; Kalarikkal, N.; Pramoda, K.P.; Hui Ru, T.; Kailas, L.; Thomas, S. Plasticized PVC graphene nanocomposites: Morphology, mechanical, and dynamic mechanical properties. Polym. Eng. Sci. 2018, 58, E104–E113. [Google Scholar] [CrossRef]
- Wang, H.; Xie, G.; Fang, M.; Ying, Z.; Tong, Y.; Zeng, Y. Mechanical reinforcement of graphene/poly(vinyl chloride) composites prepared by combining the in-situ suspension polymerization and melt-mixing methods. Compos. Part B Eng. 2017, 113, 278–284. [Google Scholar] [CrossRef]
- Wang, H.; Xie, G.; Yang, C.; Zheng, Y.; Ying, Z.; Ren, W.; Zeng, Y. Enhanced Toughness of Multilayer Graphene-Filled Poly(vinyl chloride) Composites Prepared Using Melt-Mixing Method. Polym. Compos. 2017, 38, 138–146. [Google Scholar] [CrossRef]
- Abd El-Kader, M.F.H.; Awwad, N.S.; Ibrahium, H.A.; Ahmed, M.K. Graphene oxide fillers through polymeric blends of PVC/PVDF using laser ablation technique: Electrical behavior, cell viability, and thermal stability. J. Mater. Res. Technol. 2021, 13, 1878–1886. [Google Scholar] [CrossRef]
- Mindivan, F.; Göktaş, M.; Dike, A.S. Mechanical, thermal, and micro- and nanostructural properties of polyvinyl chloride/graphene nanoplatelets nanocomposites. Polym. Compos. 2020, 41, 3707–3716. [Google Scholar] [CrossRef]
- Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Lewandowski, K. Structure and properties of poly (vinyl chloride)/ graphene nanocomposites. Polym. Test. 2020, 81, 106282. [Google Scholar] [CrossRef]
- Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Lewandowski, K.; Szulc, J.; Runka, T. Manufacturing homogenous PVC/graphene nanocomposites using a novel dispersion agent. Polym. Test. 2020, 91, 106868. [Google Scholar] [CrossRef]
- Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Lewandowski, K.; Studziński, W.; Osial, M.; Jenczyk, P.; Grzywacz, H.; Domańska, A. Curcuma longa L. Rhizome Extract as a Poly(vinyl chloride)/Graphene Nanocomposite Green Modifier. Molecules 2022, 27, 8081. [Google Scholar] [CrossRef]
- Hasan, M.; Banerjee, A.N.; Lee, M. Enhanced thermo-optical performance and high BET surface area of graphene@PVC nanocomposite fibers prepared by simple facile deposition technique: N2 adsorption study. J. Ind. Eng. Chem. 2015, 21, 828–834. [Google Scholar] [CrossRef]
- Wang, L.; Wei, X.; Wang, G.; Zhao, S.; Cui, J.; Gao, A.; Zhang, G.; Yan, Y. A facile and industrially feasible one-pot approach to prepare graphene-decorated PVC particles and their application in multifunctional PVC/graphene composites with segregated structure. Compos. Part B Eng. 2020, 185, 107775. [Google Scholar] [CrossRef]
- Wei, Z.B.; Zhao, Y.; Wang, C.; Kuga, S.; Huang, Y.; Wu, M. Antistatic PVC-graphene Composite through Plasticizer-mediated Exfoliation of Graphite. Chin. J. Polym. Sci. 2018, 36, 1361–1367. [Google Scholar] [CrossRef]
- Ahmed, R.M.; Ibrahiem, A.A.; El-Bayoumi, A.S.; Atta, M.M. Structural, mechanical, and dielectric properties of polyvinylchloride/graphene nano platelets composites. Int. J. Polym. Anal. Charact. 2021, 26, 68–83. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Zhu, W.; Qin, W. Solubility study on the surfactants functionalized reduced graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 79–85. [Google Scholar] [CrossRef]
- Dai, J.; Wang, G.; Ma, L.; Wu, C. Study on the surface energies and dispersibility of graphene oxide and its derivatives. J. Mater. Sci. 2015, 50, 3895–3907. [Google Scholar] [CrossRef]
- Tkalya, E.E.; Ghislandi, M.; de With, G.; Koning, C.E. The use of surfactants for dispersing carbon nanotubes and graphene to make conductive nanocomposites. Curr. Opin. Colloid Interface Sci. 2012, 17, 225–232. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Hatamie, S.; Akhavan, O.; Sadrnezhaad, S.K.; Ahadian, M.M.; Shirolkar, M.M.; Wang, H.Q. Curcumin-reduced graphene oxide sheets and their effects on human breast cancer cells. Mater. Sci. Eng. C 2015, 55, 482–489. [Google Scholar] [CrossRef]
- Grimme, S. Do special noncovalent π-π stacking interactions really exist? Angew. Chemie Int. Ed. 2008, 47, 3430–3434. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Wang, Y.B. Noncovalent π⋅⋅⋅π interaction between graphene and aromatic molecule: Structure, energy, and nature. J. Chem. Phys. 2014, 140, 094302. [Google Scholar] [CrossRef]
- Navik, R.; Gai, Y.; Wang, W.; Zhao, Y. Curcumin-assisted ultrasound exfoliation of graphite to graphene in ethanol. Ultrason. Sonochem. 2018, 48, 96–102. [Google Scholar] [CrossRef]
- Yang, Y.K.; He, C.E.; Peng, R.G.; Baji, A.; Du, X.S.; Huang, Y.L.; Xie, X.L.; Mai, Y.W. Non-covalently modified graphene sheets by imidazolium ionic liquids for multifunctional polymer nanocomposites. J. Mater. Chem. 2012, 22, 5666–5675. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Kim, S.H.; Han, J.T.; Jeong, H.J.; Jeong, S.Y.; Lee, G.W. Highly concentrated and conductive reduced graphene oxide nanosheets by monovalent cation-π interaction: Toward printed electronics. Adv. Funct. Mater. 2012, 22, 3307–3314. [Google Scholar] [CrossRef]
- Texter, J. Graphene dispersions. Curr. Opin. Colloid Interface Sci. 2014, 19, 163–174. [Google Scholar] [CrossRef]
- Achari, A.; Datta, K.; De, M.; Dravid, V.; Eswaramoorthy, M. Amphiphilic aminoclay–RGO hybrids: A simple strategy to disperse a high concentration of RGO in water. Nanoscale 2013, 5, 5316–5320. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Gao, C. Making silica nanoparticle-covered graphene oxide nanohybrids as general building blocks for large-area superhydrophilic coatings. Nanoscale 2011, 3, 519–528. [Google Scholar] [CrossRef]
- Ma, F.; Yuan, N.; Ding, J. The conductive network made up by the reduced graphene nanosheet/polyaniline/polyvinyl chloride. J. Appl. Polym. Sci. 2013, 128, 3870–3875. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Zeng, J.-B.; Gan, L.; Wang, M. Enhancement of Interfacial Interaction between Poly(vinyl chloride) and Zinc Oxide Modified Reduced Graphene Oxide. RSC Adv. 2016, 6, 5784–5791. [Google Scholar] [CrossRef]
- Khaleghi, M.; Didehban, K.; Shabanian, M. Simple and fast preparation of graphene oxide@ melamine terephthaldehyde and its PVC nanocomposite via ultrasonic irradiation: Chemical and thermal resistance study. Ultrason. Sonochem. 2018, 43, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Jia, X.; Li, C.; Ma, Z.; Zhang, G.; Sheng, W.; Zhang, X.; Wei, Z. Effect of interfacial interaction between graphene oxide derivatives and poly(vinyl chloride) upon the mechanical properties of their nanocomposites. J. Mater. Sci. 2014, 49, 2943–2951. [Google Scholar] [CrossRef]
- Khaleghi, M.; Didehban, K.; Shabanian, M. Effect of new melamine-terephthaldehyde resin modified graphene oxide on thermal and mechanical properties of PVC. Polym. Test. 2017, 63, 382–391. [Google Scholar] [CrossRef]
- Piszczek, K. Żelowanie Suspensyjnego, Nieplastyfikowanego Poli(chlorku winylu); Wydawnictwa Uczelniane Uniwersytetu Technologiczno-Przyrodniczego: Bydgoszcz, Poland, 2009. [Google Scholar]
- Thakuria, R.; Nath, N.K.; Saha, B.K. The Nature and Applications of π-π Interactions: A Perspective. Cryst. Growth Des. 2019, 19, 523–528. [Google Scholar] [CrossRef]
- Hatamie, S.; Ahadian, M.M.; Iraji zad, A.; Akhavan, O.; Jokar, E. Photoluminescence and electrochemical investigation of curcumin-reduced graphene oxide sheets. J. Iran. Chem. Soc. 2018, 15, 351–357. [Google Scholar] [CrossRef]
- Alibolandi, M.; Mohammadi, M.; Taghdisi, S.M.; Ramezani, M.; Abnous, K. Fabrication of aptamer decorated dextran coated nano-graphene oxide for targeted drug delivery. Carbohydr. Polym. 2017, 155, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Bani, F.; Adeli, M.; Movahedi, S.; Sadeghizadeh, M. Graphene-polyglycerol-curcumin hybrid as a near-infrared (NIR) laser stimuli-responsive system for chemo-photothermal cancer therapy. RSC Adv. 2016, 6, 61141–61149. [Google Scholar] [CrossRef]
- Saltos, J.A.; Shi, W.; Mancuso, A.; Sun, C.; Park, T.; Averick, N.; Punia, K.; Fatab, J.; Raja, K. Curcumin-derived green plasticizers for Poly(vinyl) chloride. RSC Adv. 2014, 4, 54725–54728. [Google Scholar] [CrossRef]
- Larrañeta, E.; Imízcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Domínguez-Robles, J.; Rodríguez, A.; Donnelly, R.F. Synthesis and Characterization of Lignin Hydrogels for Potential Applications as Drug Eluting Antimicrobial Coatings for Medical Materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef]
- Velho, S.; Brum, L.; Petter, C.; dos Santos, J.H.; Šimunić, Š.; Kappa, W.H. Development of structured natural dyes for use into plastics. Dye. Pigment. 2017, 136, 248–254. [Google Scholar] [CrossRef]
- Thema, F.T.; Moloto, M.J.; Dikio, E.D.; Nyangiwe, N.N.; Kotsedi, L.; Maaza, M.; Khenfouch, M. Synthesis and characterization of graphene thin films by chemical reduction of exfoliated and intercalated graphite oxide. J. Chem. 2013, 3, 150536. [Google Scholar] [CrossRef] [Green Version]
- Marković, Z.M.; Kepić, D.P.; Matijašević, D.M.; Pavlović, V.B.; Jovanović, S.P.; Stanković, N.K.; Milivojević, D.D.; Spitalsky, Z.; Holclajtner-Antunović, I.D.; Bajuk-Bogdanović, D.V.; et al. Ambient light induced antibacterial action of curcumin/graphene nanomesh hybrids. RSC Adv. 2017, 7, 36081–36092. [Google Scholar] [CrossRef] [Green Version]
- Barua, S.; Karak, N.; Chattopadhyay, P.; Islam, J.; Phukan, M.M.; Konwar, B.K. Biocompatible hyperbranched epoxy/silver-reduced graphene oxide-curcumin nanocomposite as an advanced antimicrobial material. RSC Adv. 2014, 4, 47797–47805. [Google Scholar] [CrossRef]
- Husein, S.G.; Hamdani, S.; Rinaldi, K. Optimization of ar-Turmerone Isolation Method from Turmeric Oleoresin using Silica Gel Adsorbent. Lett. Appl. NanoBioSci. 2023, 12, 53. [Google Scholar] [CrossRef]
- Kadam, P.V.; Yadav, K.N.; Bhingare, C.L.; Patil, M.J. Standardization and quantification of curcumin from Curcuma longa extract using UV visible spectroscopy and HPLC. J. Pharmacogn. Phytochem. 2018, 7, 1913–1918. [Google Scholar]
- Pourhajibagher, M.; Parker, S.; Chiniforush, N.; Bahador, A. Photoexcitation triggering via semiconductor Graphene Quantum Dots by photochemical doping with Curcumin versus perio-pathogens mixed biofilms. Photodiagn. Photodyn. Ther. 2019, 28, 125–131. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Hadadzadeh, H.; Farrokhpour, H.; Amirghofran, Z. Immobilization of Gold Nanoparticles on Folate-Conjugated Dendritic Mesoporous Silica-Coated Reduced Graphene Oxide Nanosheets: A New Nanoplatform for Curcumin pH-Controlled and Targeted Delivery. Soft Matter 2018, 14, 2400–2410. [Google Scholar] [CrossRef]
- Fu, C.; Zhao, G.; Zhang, H.; Li, S. Evaluation and characterization of reduced graphene oxide nanosheets as anode materials for lithium-ion batteries. Int. J. Electrochem. Sci. 2013, 8, 6269–6280. [Google Scholar]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectros. Relat. Phenomena 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Henderson, A.E.; Fitzgerald, A.G.; Rickerby, D.S.; Starey, B.E. Electron spectroscopy and electron microscopy of a a-C:H films. J. Electron Spectros. Relat. Phenomena 1990, 52, 475–483. [Google Scholar] [CrossRef]
- Mezzi, A.; Kaciulis, S. Surface investigation of carbon films: From diamond to graphite. Surf. Interface Anal. 2010, 42, 1082–1084. [Google Scholar] [CrossRef]
- Jackson, S.T.; Nuzzo, R.G. Determining hybridization differences for amorphous carbon from the XPS C 1s envelope. Appl. Surf. Sci. 1995, 90, 195–203. [Google Scholar] [CrossRef]
- Díaz, J.; Paolicelli, G.; Ferrer, S.; Comin, F. Separation of thesp3 and sp2 components in the C1s photoemission spectra of amorphous carbon films. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 8064–8069. [Google Scholar] [CrossRef] [PubMed]
- Haerle, R.; Riedo, E.; Pasquarello, A.; Baldereschi, A. sp2/sp3 hybridization ratio in amorphous carbon from C 1s core-level shifts: X-ray photoelectron spectroscopy and first-principles calculation. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 65, 1–9. [Google Scholar] [CrossRef]
- Fujimoto, A.; Yamada, Y.; Koinuma, M.; Sato, S. Origins of sp3C peaks in C1s X-ray Photoelectron Spectra of Carbon Materials. Anal. Chem. 2016, 88, 6110–6114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butenko, Y.V.; Krishnamurthy, S.; Chakraborty, A.K.; Kuznetsov, V.L.; Dhanak, V.R.; Hunt, M.R.C.; Šiller, L. Photoemission study of onionlike carbons produced by annealing nanodiamonds. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 71, 075420. [Google Scholar] [CrossRef] [Green Version]
- Dosoky, N.S.; Setzer, W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef] [Green Version]
- Li, S. Chemical Composition and Product Quality Control of Turmeric (Curcuma longa L.). Pharm. Crop. 2011, 5, 28–54. [Google Scholar] [CrossRef]
- Jantan, I.; Saputri, F.C.; Qaisar, M.N.; Buang, F. Correlation between chemical composition of Curcuma domestica and Curcuma xanthorrhiza and their antioxidant effect on human low-density lipoprotein oxidation. Evidence-based Complement. Altern. Med. 2012, 2012, 438356. [Google Scholar] [CrossRef] [Green Version]
- Qu, S.; Li, M.; Xie, L.; Huang, X.; Yang, J.; Wang, N.; Yang, S. Noncovalent functionalization of graphene attaching [6,6]-phenyl-C61- butyric acid methyl ester (PCBM) and application as electron extraction layer of polymer solar cells. ACS Nano 2013, 7, 4070–4081. [Google Scholar] [CrossRef]
- George, G.; Sisupal, S.B.; Tomy, T.; Kumaran, A.; Vadivelu, P.; Suvekbala, V.; Sivaram, S.; Ragupathy, L. Facile, environmentally benign and scalable approach to produce pristine few layers graphene suitable for preparing biocompatible polymer nanocomposites. Sci. Rep. 2018, 8, 11228. [Google Scholar] [CrossRef] [Green Version]
- Refat, M.S. Synthesis and characterization of ligational behavior of curcumin drug towards some transition metal ions: Chelation effect on their thermal stability and biological activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 105, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.R.K.; Sreelakshmi, G.; Muraleedharan, C.V.; Joseph, R. Water soluble complexes of curcumin with cyclodextrins: Characterization by FT-Raman spectroscopy. Vib. Spectrosc. 2012, 62, 77–84. [Google Scholar] [CrossRef]
- Lestari, M.; Indrayanto, G. Chapter Three—Curcumin. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Tomaszewska, J.; Skórczewska, K.; Jesionowski, T. Preparation and characterization of eco-friendly Mg(OH)2/lignin hybrid material and its use as a functional filler for poly(vinyl chloride). Polymers 2017, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, J.; Sterzyński, T.; Piszczek, K. Rigid Poly(vinyl chloride) Gelation in a Brabender Measuring Mixer. III. Transformation in the Torque Maximum. J. Appl. Polym. Sci. 2007, 106, 3158–3164. [Google Scholar] [CrossRef]
- Lewandowski, K.; Skórczewska, K.; Piszczek, K.; Urbaniak, W. Recycled glass fibres from wind turbines as a filler for poly(Vinyl chloride). Adv. Polym. Technol. 2019, 2019, 8960503. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C. Structure of graphene and its disorders: A review. Sci. Technol. Adv. Mater. 2018, 19, 613–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Huang, X.; Gong, Y.; Xiao, H.; McClements, D.J.; Hu, K. Enhancement of curcumin water dispersibility and antioxidant activity using core-shell protein-polysaccharide nanoparticles. Food Res. Int. 2016, 87, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meabed, O.M.; Shamaa, A.; Abdelrahman, I.Y.; El-Sayyed, G.S.; Mohammed, S.S. The Effect of Nano-chitosan and Nano-curcumin on Radiated Parotid Glands of Albino Rats: Comparative Study. J. Clust. Sci. 2022, 34, 977–989. [Google Scholar] [CrossRef]
- Gupta, V.; Aseh, A.; Ríos, C.N.; Aggarwal, B.B.; Mathur, A.B. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int. J. Nanomed. 2009, 4, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taha, S.; El-Sherbiny, I.; Enomoto, T.; Salem, A.; Nagai, E.; Askar, A.; Abady, G.; Abdel-Hamid, M. Improving the functional activities of curcumin using milk proteins as nanocarriers. Foods 2020, 9, 986. [Google Scholar] [CrossRef]
- Gedde, U.W. Polymer Physics; Chapman & Hall: London, UK; Glasgow, UK; Weinheim, Germany; New York, NY, USA; Tokyo, Japan; Melbourne, Australia; Madras, India, 1995. [Google Scholar]
- Lee, J.W.; Kim, S.W.; Cho, Y.L.; Jeong, H.Y.; Song, Y.I.; Suh, S.J. Characterization and dispersion stabilities of multi-layer graphene- coated copper prepared by electrical wire-explosion method. J. Nanosci. Nanotechnol. 2016, 16, 11286–11291. [Google Scholar] [CrossRef]
- Ferralis, N. Probing mechanical properties of graphene with Raman spectroscopy. J. Mater. Sci. 2010, 45, 5135–5149. [Google Scholar] [CrossRef] [Green Version]
- Delforce, L.; Hofmann, E.; Nardello-Rataj, V.; Aubry, J.M. TiO2 nanoparticle dispersions in water and nonaqueous solvents studied by gravitational sedimentation analysis: Complementarity of Hansen Parameters and DLVO interpretations. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127333. [Google Scholar] [CrossRef]
- Navarrete-Segado, P.; Tourbin, M.; Frances, C.; Grossin, D. Masked stereolithography of hydroxyapatite bioceramic scaffolds: From powder tailoring to evaluation of 3D printed parts properties. Open Ceram. 2022, 9, 100235. [Google Scholar] [CrossRef]
- Yue, M.; Huang, M.; Zhu, Z.; Huang, T.; Huang, M. Effect of ultrasound assisted emulsification in the production of Pickering emulsion formulated with chitosan self-assembled particles: Stability, macro, and micro rheological properties. LWT 2022, 154, 112595. [Google Scholar] [CrossRef]
- Santos, J.; Trujillo-Cayado, L.A.; Carrillo, F.; López-Castejón, M.L.; Alfaro-Rodríguez, M.C. Relation between Droplet Size Distributions and Physical Stability for Zein Microfluidized Emulsions. Polymers 2022, 14, 2195. [Google Scholar] [CrossRef] [PubMed]
- Pascaud, K.; Mercé, M.; Roucher, A.; Destribats, M.; Backov, R.; Schmitt, V.; Sescousse, R.; Brouillet, F.; Sarda, S.; Ré, M.I. Pickering emulsion as template for porous bioceramics in the perspective of bone regeneration. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128748. [Google Scholar] [CrossRef]
- Baibarac, M.; Stingescu, L.; Stroe, M.; Negrila, C.; Matei, E.; Cotet, L.C.; Anghel, I.; Şofran, I.E.; Baia, L. Poly(vinyl chloride) spheres coated with graphene oxide sheets: From synthesis to optical properties and their applications as flame-retardant agents. Polymers 2021, 13, 565. [Google Scholar] [CrossRef]
- Godínez-García, A.; Vallejo-Arenas, D.D.; Salinas-Rodríguez, E.; Gómez-Torres, S.A.; Ruíz, J.C. Spraying synthesis and ion permeation in polyvinyl chloride/graphene oxide membranes. Appl. Surf. Sci. 2019, 489, 962–975. [Google Scholar] [CrossRef]
- Dhakal, S.; Chao, K.; Schmidt, W.; Qin, J.; Kim, M.; Chan, D. Evaluation of Turmeric Powder Adulterated with Metanil Yellow Using FT-Raman and FT-IR Spectroscopy. Foods 2016, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Kugler, S.; Spychaj, T. Nanostruktury wȩglowe i błony lub powłoki polimerowe z ich udziałem: Cz. II. Błony i powłoki polimerowe z udziałem nanostruktur wȩglowych. Polimery/Polymers 2013, 58, 177–180. [Google Scholar] [CrossRef]
- Deshmukh, K.; Khatake, S.M.; Joshi, G.M. Surface properties of graphene oxide reinforced polyvinyl chloride nanocomposites. J. Polym. Res. 2013, 20, 286. [Google Scholar] [CrossRef]
- Xiao, Y.; Xin, B.; Chen, Z.; Lin, L.; Liu, Y.; Hu, Z. Enhanced thermal properties of graphene-based poly (vinyl chloride) composites. J. Ind. Text. 2019, 48, 1348–1363. [Google Scholar] [CrossRef]
- Wang, H.; Xie, G.; Zhu, Z.; Ying, Z.; Zeng, Y. Enhanced tribological performance of the multi-layer graphene filled poly(vinyl chloride) composites. Compos. Part A Appl. Sci. Manuf. 2014, 67, 268–273. [Google Scholar] [CrossRef]
- Vadukumpully, S.; Paul, J.; Mahanta, N.; Valiyaveettil, S. Flexible conductive graphene/poly(vinyl chloride) composite thin films with high mechanical strength and thermal stability. Carbon 2011, 49, 198–205. [Google Scholar] [CrossRef]
- Wang, H.; Xie, G.; Fang, M.; Ying, Z.; Tong, Y.; Zeng, Y. Electrical and mechanical properties of antistatic PVC films containing multi-layer graphene. Compos. Part B Eng. 2015, 79, 444–450. [Google Scholar] [CrossRef]
- Brostow, W.; Hagg Lobland, H.E. Materials: Introduction and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 978-0-470-52379-7. [Google Scholar]
- Broza, G.; Piszczek, K.; Schulte, K.; Sterzynski, T. Nanocomposites of poly(vinyl chloride) with carbon nanotubes (CNT). Compos. Sci. Technol. 2007, 67, 890–894. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent Progress in Graphene/Polymer Nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef] [PubMed]
- Govindaraj, P.; Sokolova, A.; Salim, N.; Juodkazis, S.; Fuss, F.K.; Fox, B.; Hameed, N. Distribution states of graphene in polymer nanocomposites: A review. Compos. Part B Eng. 2021, 226, 109353. [Google Scholar] [CrossRef]
- Reis, T.M.C.; Castro, V.G.; Amurin, L.G.; Silva, G.G. Graphene oxide dispersion in epoxy resin prepared by direct phase transfer from ethanol: Rheology and aging. Compos. Part C Open Access 2023, 10, 100340. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S. Graphene-based polymer coatings for preventing marine corrosion: A review. J. Coatings Technol. Res. 2023, 20, 413–432. [Google Scholar] [CrossRef]

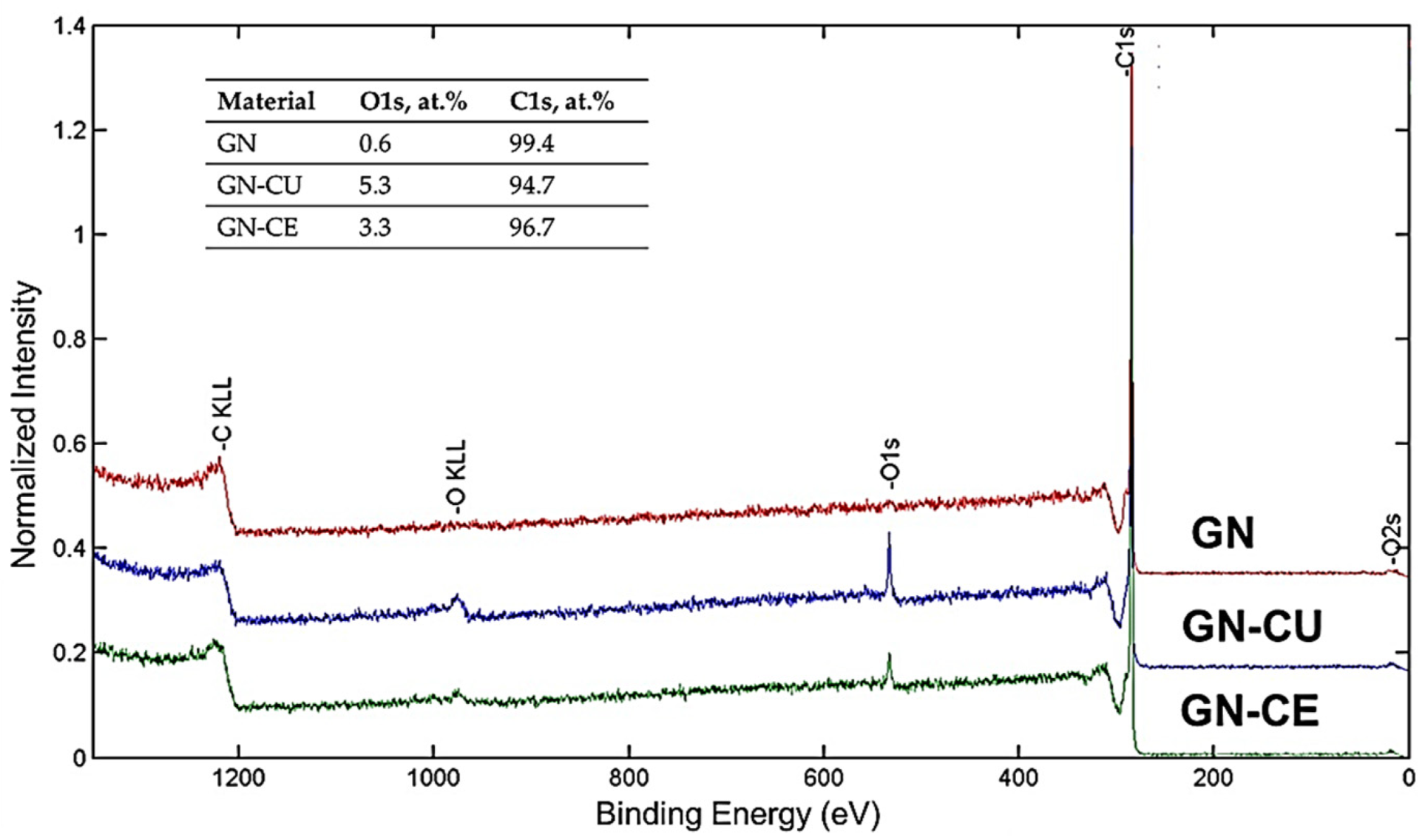




| Binding Energy, eV | Chemical Bonds | FWHM | Atomic, % |
|---|---|---|---|
| GRAPHENE | |||
| O1s | |||
| 530.47 | O1s | 1.47 | 0.2 |
| 532.38 | O1s | 1.47 | 0.2 |
| 533.84 | O1s | 1.47 | 0.2 |
| 0.6 | |||
| C1s | |||
| 284.53 | C-C sp2 | 0.82 | 65.3 |
| 285.33 | C=C sp3 | 0.82 | 7.5 |
| 286.11 | C-O | 1.49 | 10.0 |
| 287.56 | C=O | 1.49 | 4.5 |
| 289.21 | COOR | 1.49 | 3.5 |
| 290.68 | π electrons | 1.49 | 4.6 |
| 292.03 | sp2 loss shakeup | 1.49 | 2.8 |
| 293.87 | sp2 loss shakeup | 1.49 | 1.1 |
| 99.4 | |||
| 100.0 | |||
| GRAPHENE–CURCUMIN | |||
| O1s | |||
| 530.7 | O1s | 1.95 | 0.7 |
| 533.17 | O1s | 1.95 | 4.6 |
| 5.3 | |||
| C1s | |||
| 284.42 | C-C sp2 | 0.73 | 50.9 |
| 284.81 | C=C sp3 | 0.73 | 9.6 |
| 285.72 | C-O | 1.82 | 16.5 |
| 287.18 | C=O | 1.82 | 6.4 |
| 288.99 | COOR | 1.82 | 4.0 |
| 290.73 | π electrons | 1.82 | 4.2 |
| 292.14 | sp2 loss shakeup | 1.82 | 2.1 |
| 294.15 | sp2 loss shakeup | 1.82 | 1.0 |
| 94.7 | |||
| 100 | |||
| GRAPHENE–Curcuma Longa L. EXTRACT | |||
| O1s | |||
| 531.06 | O1s | 1.87 | 0.8 |
| 533.19 | O1s | 1.87 | 2.5 |
| 3.3 | |||
| C1s | |||
| 284.48 | C-C sp2 | 0.76 | 59.4 |
| 284.94 | C=C sp3 | 0.76 | 9.4 |
| 285.92 | C-O | 1.65 | 15.2 |
| 287.35 | C=O | 1.65 | 3.7 |
| 289.66 | COOR | 1.65 | 2.4 |
| 288.45 | π electrons | 1.65 | 2.0 |
| 290.88 | sp2 loss shakeup | 1.65 | 3.2 |
| 292.16 | sp2 loss shakeup | 1.65 | 1.5 |
| 96.7 | |||
| 100.0 | |||
| Material | Cont. of THF, % | Max. DTG I, °C | Max. DTG II, °C | Residual Mass, % | Congo Red Test, min |
|---|---|---|---|---|---|
| PVC | 5.2 (0.4) | 269.8 (2.1) | 452.4 (1.5) | 7.7 (0.2) | 3.5 (0.02) |
| PVC/0.01%GN | 5.8 (0.1) | 269.0 (0.6) | 450.9 (0.6) | 7.8 (0.4) | 3.1 (0.04) |
| PVC/0.01%GN–CE | 5.5 (0.1) | 273.5 (0.3) | 449.9 (1.1) | 8.2 (0.1) | 3.3 (0.08) |
| PVC/0.1%GN | 5.8 (0.4) | 273.0 (0.3) | 454.2 (0.4) | 7.5 (0.2) | 3.1 (0.04) |
| PVC/0.1%GN–CE | 6.4 (0.1) | 274.9 (0.8) | 452.9 (1.4) | 8.3 (0.2) | 2.8 (0.03) |
| PVC/0.5%GN | 5.7 (0.6) | 274.7 (2.0) | 446.8 (0.6) | 7.2 (0.4) | 2.8 (0.04) |
| PVC/0.5%GN–CE | 6.1 (0.2) | 278.3 (0.8) | 451.4 (1.5) | 8.6 (0.1) | 2.4 (0.03) |
| PVC/1%GN | 6.8 (0.5) | 270.6 (0.5) | 438.1 (2.9) | 8.0 (0.7) | 2.6 (0.08) |
| PVC/1%GN–CE | 6.6 (0.2) | 278.5 (0.7) | 453.5 (1.3) | 9.9 (0.3) | 2.3 (0.03) |
| Material | SE, % | tM, s | p, s−1 | R2 |
|---|---|---|---|---|
| PVC | 48.2 (0.3) | 348 (4) | 0.007 (0.0004) | 0.994 |
| PVC/0.01% GN | 43.8 (0.4) | 477 (7) | 0.004 (0.0002) | 0.994 |
| PVC/0.01% GN–CE | 42.9 (0.3) | 570 (7) | 0.003 (0.0001) | 0.996 |
| PVC/0.1% GN | 43.9 (0.4) | 307 (6) | 0.006 (0.0004) | 0.990 |
| PVC/0.1% GN–CE | 42.1 (0.2) | 262 (3) | 0.010 (0.0005) | 0.996 |
| PVC/0.5% GN | 49.5 (0.2) | 262 (2) | 0.009 (0.0003) | 0.998 |
| PVC/0.5% GN–CE | 32.1 (0.3) | 498 (8) | 0.004 (0.0003) | 0.992 |
| PVC/1% GN | 43.5 (0.1) | 267 (1) | 0.010 (0.0002) | 0.999 |
| PVC/1% GN–CE | 27.3 (0.2) | 443 (4) | 0.007 (0.0003) | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilczewski, S.; Skórczewska, K.; Tomaszewska, J.; Osial, M.; Dąbrowska, A.; Nikiforow, K.; Jenczyk, P.; Grzywacz, H. Graphene Modification by Curcuminoids as an Effective Method to Improve the Dispersion and Stability of PVC/Graphene Nanocomposites. Molecules 2023, 28, 3383. https://doi.org/10.3390/molecules28083383
Wilczewski S, Skórczewska K, Tomaszewska J, Osial M, Dąbrowska A, Nikiforow K, Jenczyk P, Grzywacz H. Graphene Modification by Curcuminoids as an Effective Method to Improve the Dispersion and Stability of PVC/Graphene Nanocomposites. Molecules. 2023; 28(8):3383. https://doi.org/10.3390/molecules28083383
Chicago/Turabian StyleWilczewski, Sławomir, Katarzyna Skórczewska, Jolanta Tomaszewska, Magdalena Osial, Agnieszka Dąbrowska, Kostiantyn Nikiforow, Piotr Jenczyk, and Hubert Grzywacz. 2023. "Graphene Modification by Curcuminoids as an Effective Method to Improve the Dispersion and Stability of PVC/Graphene Nanocomposites" Molecules 28, no. 8: 3383. https://doi.org/10.3390/molecules28083383










