Effects of Ammonium Polyphosphate and Organic Modified Montmorillonite on Flame Retardancy of Polyethylene Glycol/Wood-Flour-Based Phase Change Composites
Abstract
:1. Introduction
2. Results and Discussion
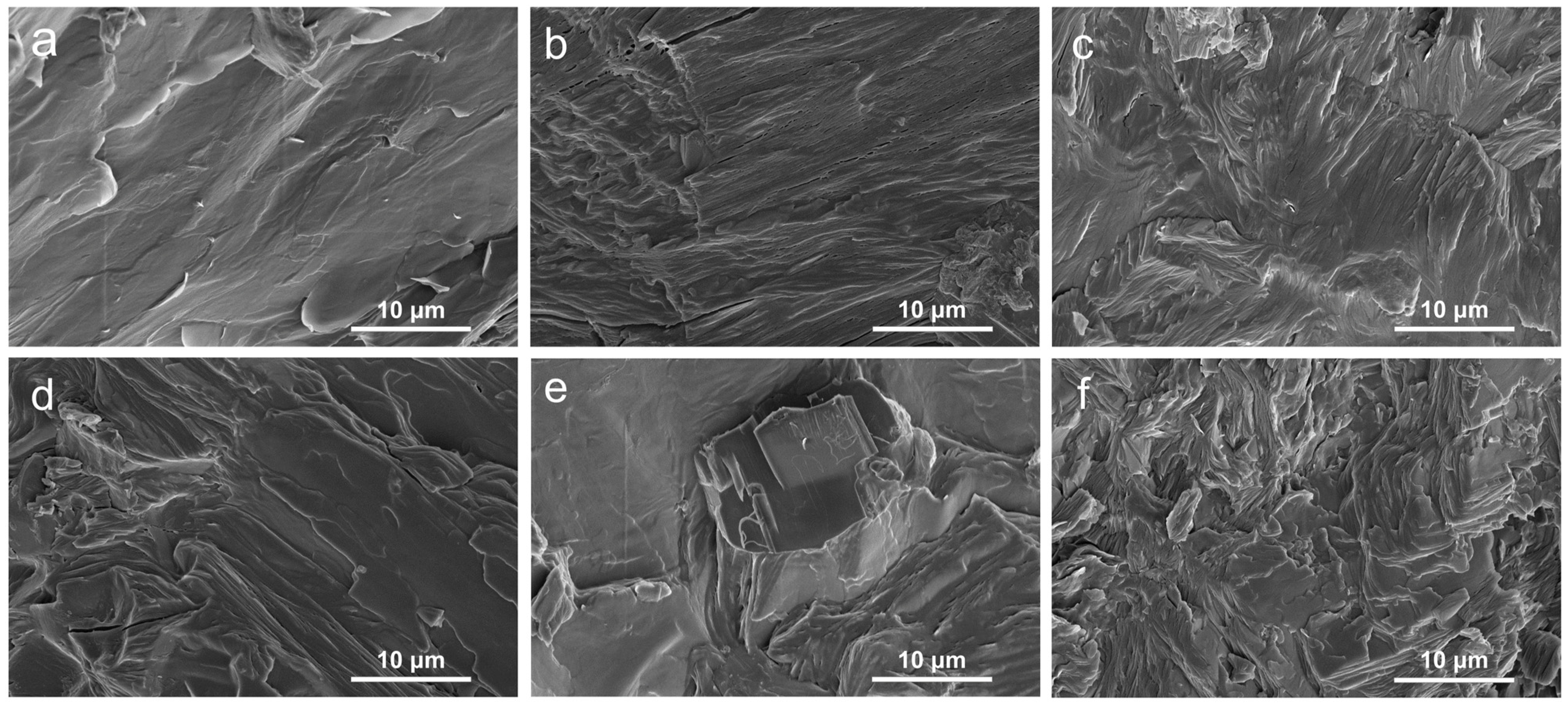
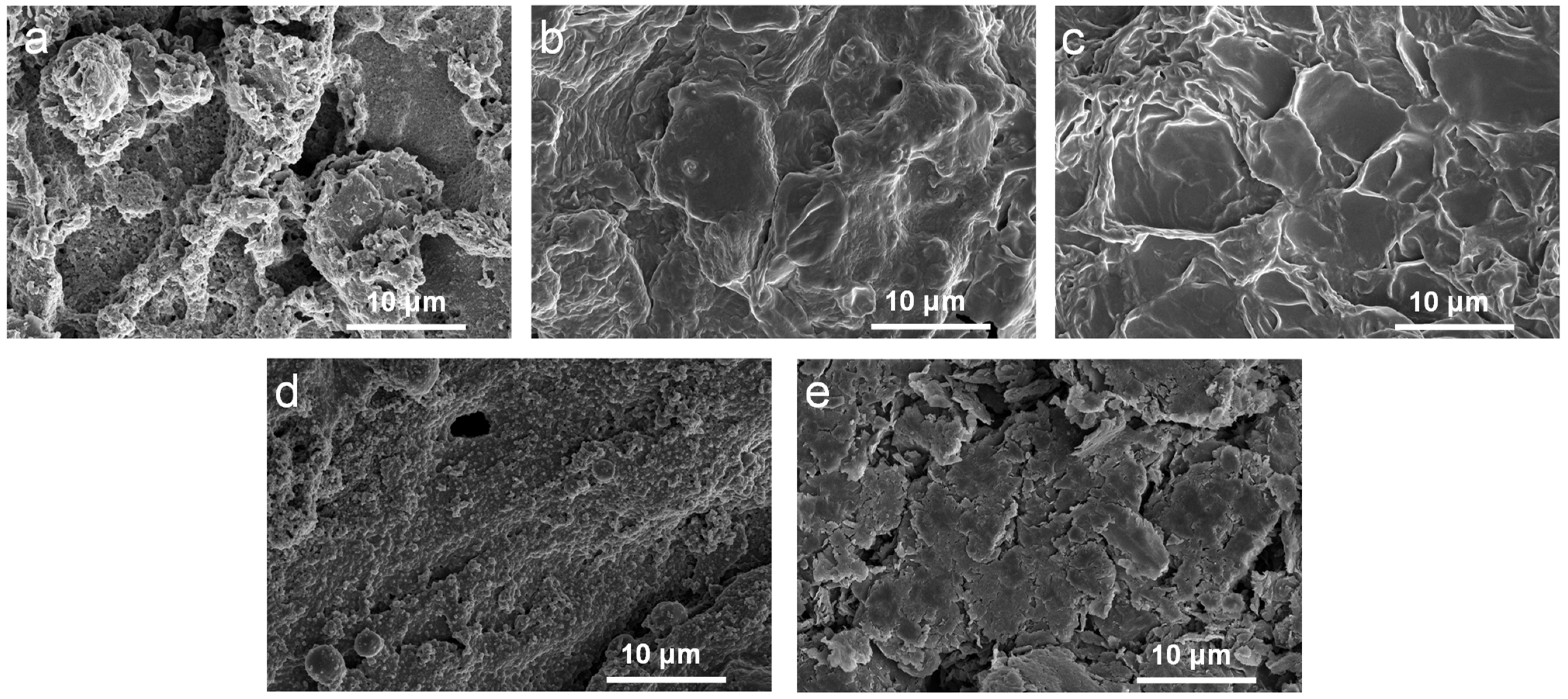
2.1. Morphology and Structure Characterization of PEG/WF and Its Composites
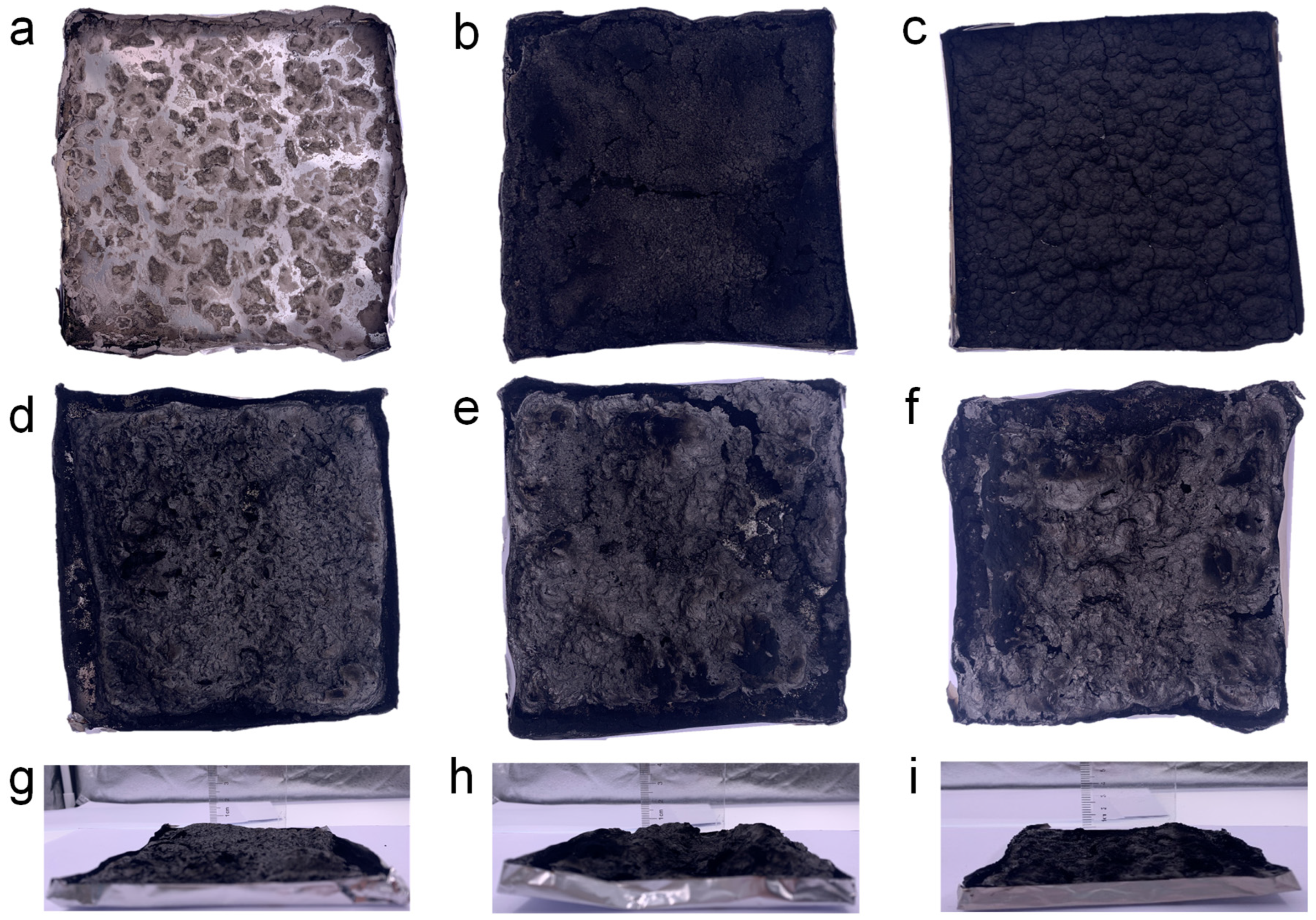
2.2. Flame Retardancy Evaluation of PEG/WF and Its Composites
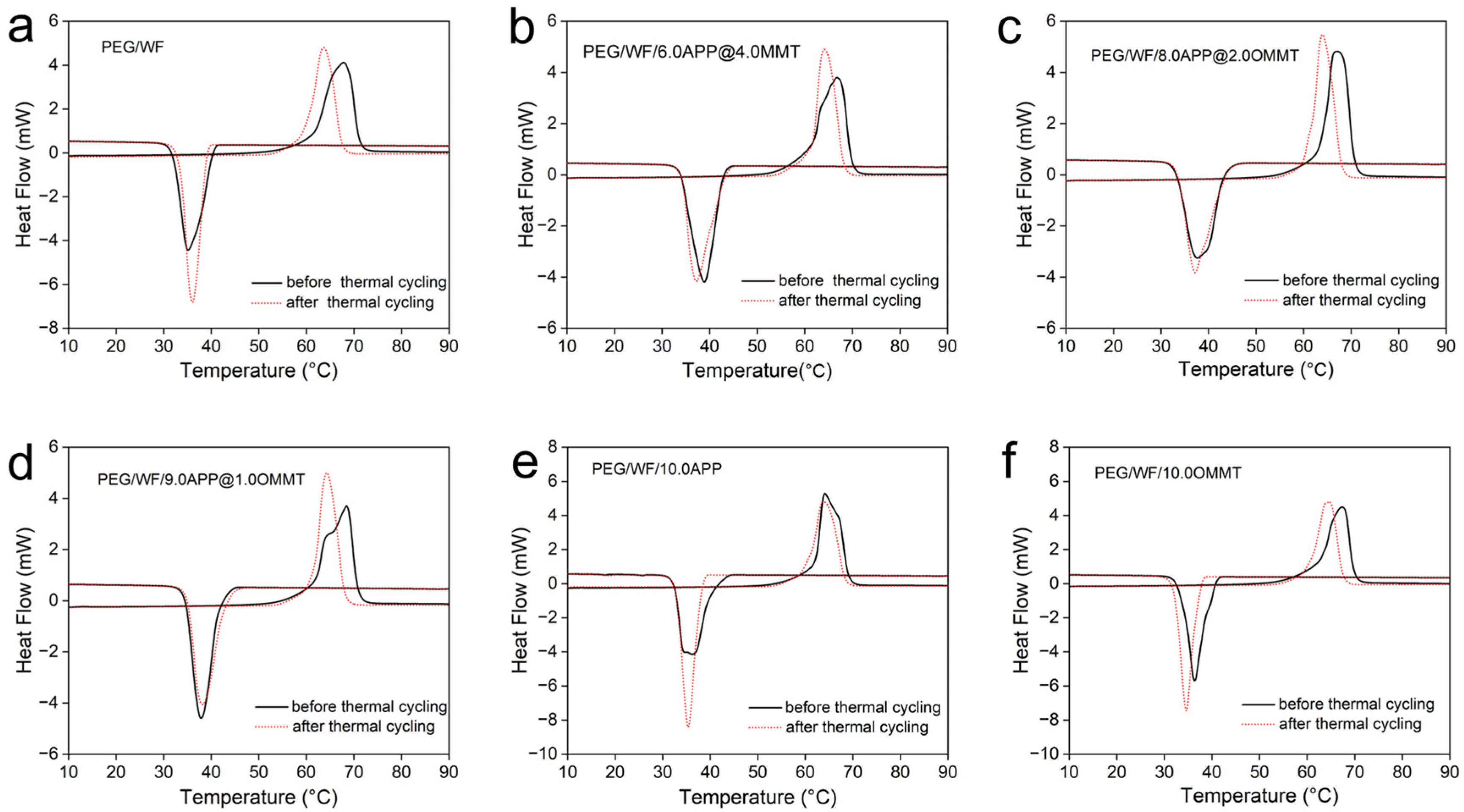
2.3. Phase Change Performance of PEG/WF and Its Composites
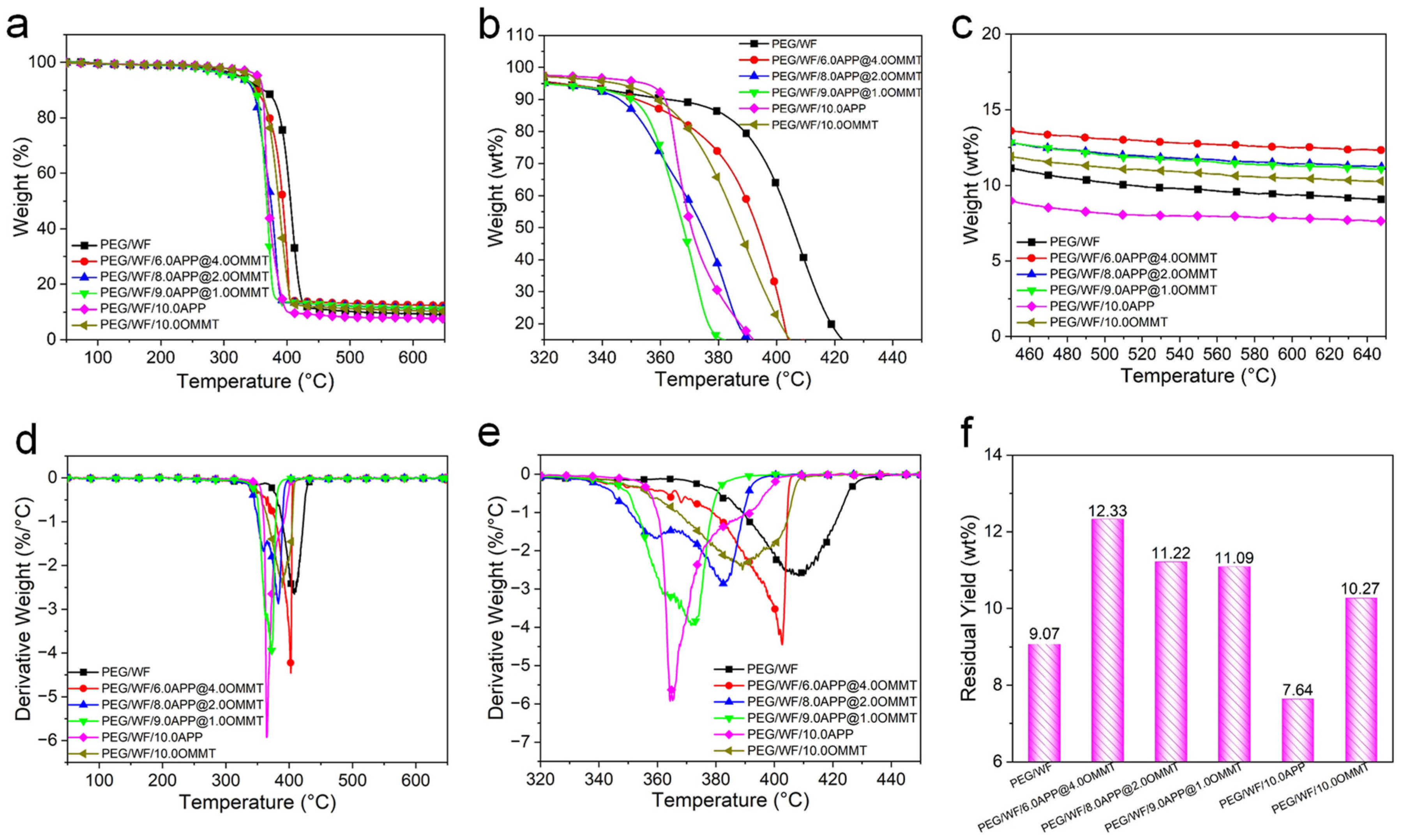
2.4. Thermal Stability Analysis of PEG/WF and Its Composites
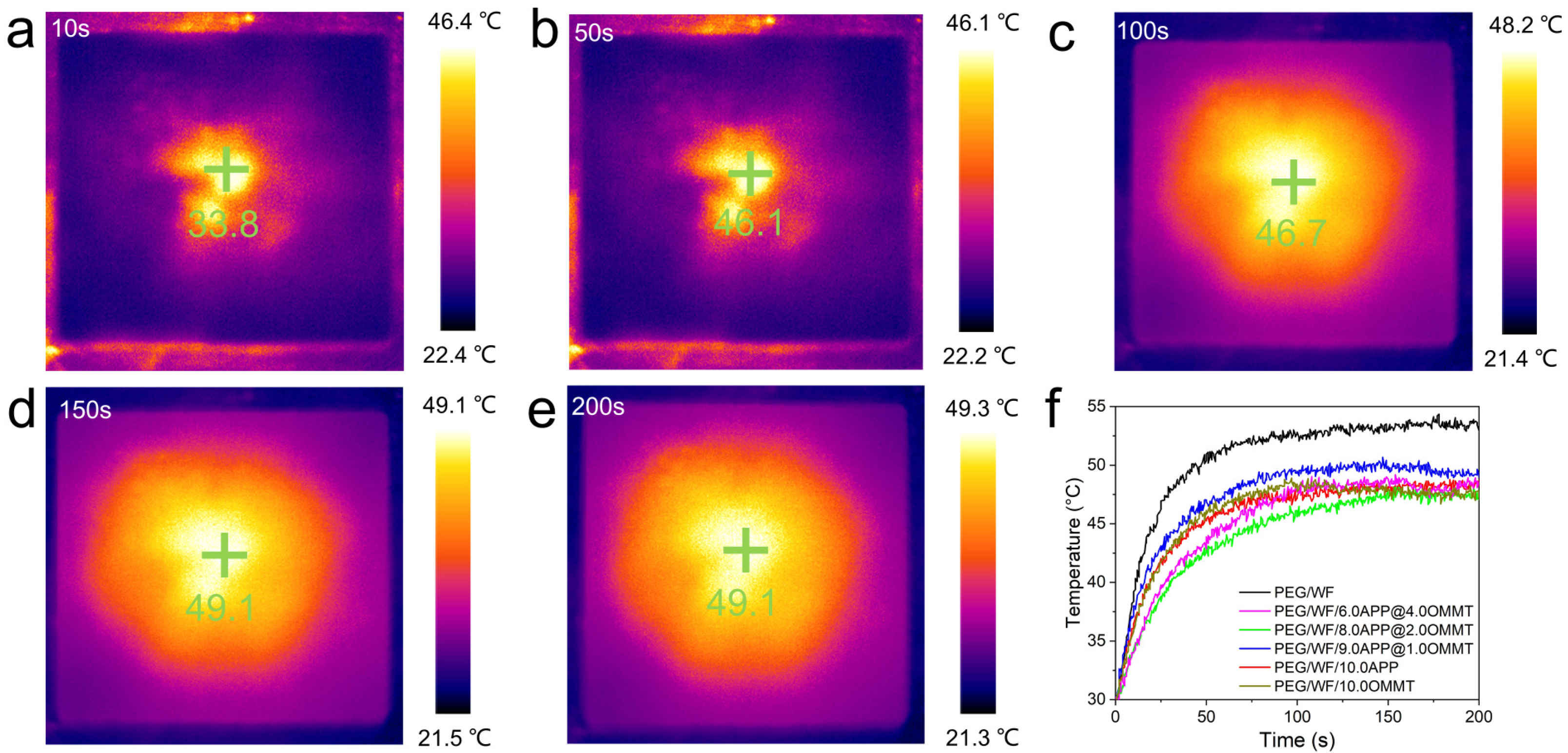
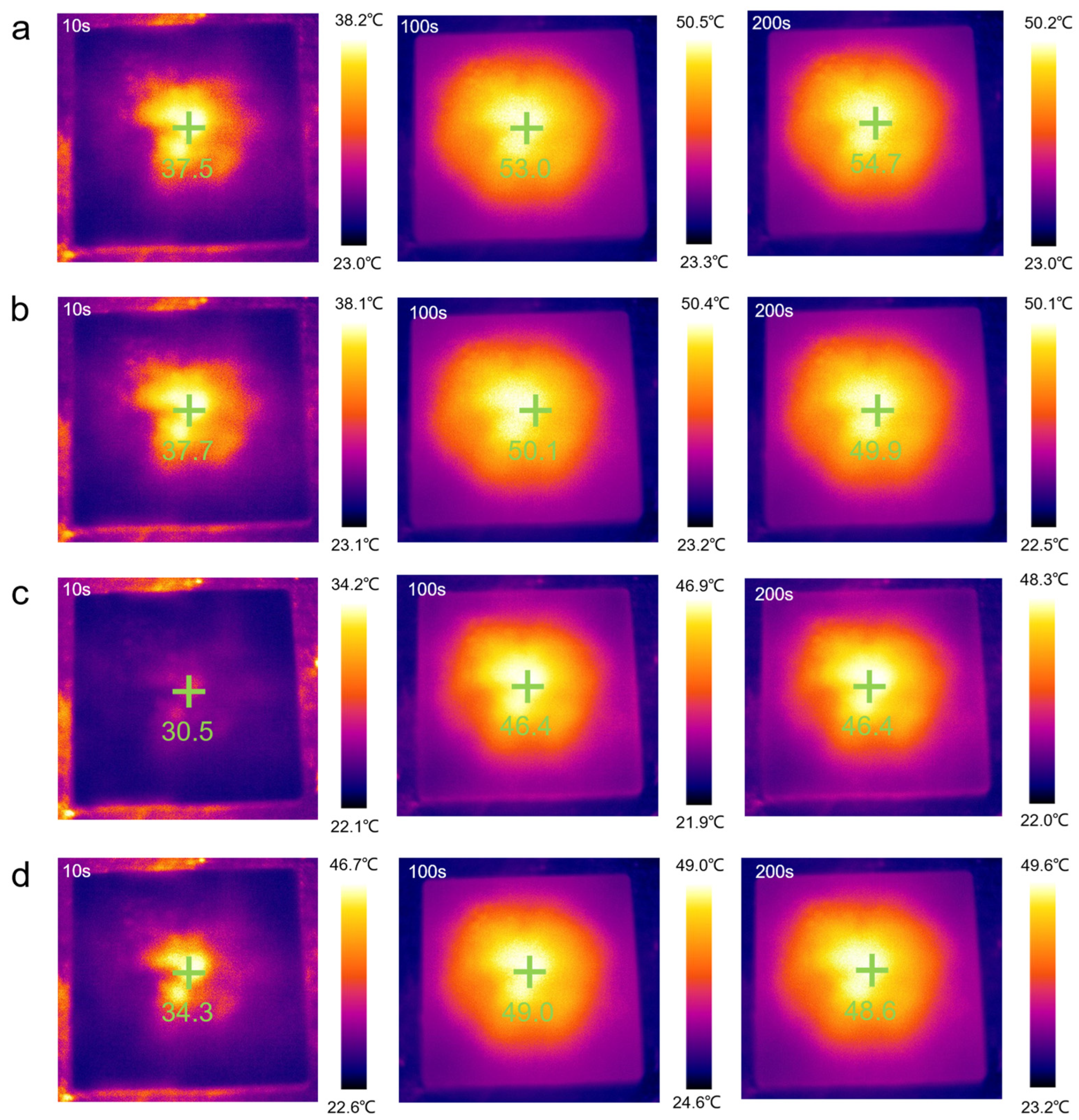
2.5. Temperature Regulation Performance of PEG/WF and Its Composites
3. Materials and Methods
3.1. Materials
3.2. Preparation of PEG/WF and Its Composites
3.3. Instruments and Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, Z.; Zhang, Y.; Hu, K.; Xiao, Y.; Wang, J.; Zhou, C.; Lei, J. Preparation and properties of polyethylene glycol based semi-interpenetrating polymer network as novel form-stable phase change materials for thermal energy storage. Energy Build. 2016, 127, 327–336. [Google Scholar] [CrossRef]
- Lu, X.; Fang, C.; Sheng, X.; Zhang, L.; Qu, J. One-Step and Solvent-Free Synthesis of Polyethylene Glycol-Based Polyurethane As Solid–Solid Phase Change Materials for Solar Thermal Energy Storage. Ind. Eng. Chem. Res. 2019, 58, 3024–3032. [Google Scholar] [CrossRef]
- Lu, X.; Huang, J.; Kang, B.; Yuan, T.; Qu, J.-p. Bio-based poly (lactic acid)/high-density polyethylene blends as shape-stabilized phase change material for thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2019, 192, 170–178. [Google Scholar] [CrossRef]
- Qi, G.-Q.; Liang, C.-L.; Bao, R.-Y.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Polyethylene glycol based shape-stabilized phase change material for thermal energy storage with ultra-low content of graphene oxide. Sol. Energy Mater. Sol. Cells 2014, 123, 171–177. [Google Scholar] [CrossRef]
- Qi, G.-Q.; Yang, J.; Bao, R.-Y.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Enhanced comprehensive performance of polyethylene glycol based phase change material with hybrid graphene nanomaterials for thermal energy storage. Carbon 2015, 88, 196–205. [Google Scholar] [CrossRef]
- Liu, Z.; Wei, H.; Tang, B.; Xu, S.; Shufen, Z. Novel light–driven CF/PEG/SiO2 composite phase change materials with high thermal conductivity. Sol. Energy Mater. Sol. Cells 2018, 174, 538–544. [Google Scholar] [CrossRef]
- Sarı, A.; Biçer, A.; Alkan, C. Thermal energy storage characteristics of poly(styrene-co-maleic anhydride)-graft-PEG as polymeric solid–solid phase change materials. Sol. Energy Mater. Sol. Cells 2017, 161, 219–225. [Google Scholar] [CrossRef]
- Guo, C.; Chen, Y.; Li, L. Investigation on interfacial interaction and thermal properties of flame retarded wood-plastic form-stable phase change material. Compos. Interfaces 2018, 26, 597–610. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Reboredo, M.M.; Aranguren, M.I. Dependence of the mechanical properties of woodflour–polymer composites on the moisture content. J. Appl. Polym. Sci. 1998, 68, 2069–2076. [Google Scholar] [CrossRef]
- Hu, S.; Chen, W.; Liu, W.; Li, H. Microwave irradiation treatment of wood flour and its application in PVC-wood flour composites. J. Wuhan Univ. Technol. -Mater Sci. Ed. 2007, 22, 148–152. [Google Scholar] [CrossRef]
- Liu, R.; Cao, J.; Peng, Y.; Chen, Y. Physical, mechanical, and thermal properties of micronized organo-montmorillonite suspension modified wood flour/poly(lactic acid) composites. Polym. Compos. 2015, 36, 731–738. [Google Scholar] [CrossRef]
- Ma, L.; Guo, C.; Ou, R.; Sun, L.; Wang, Q.; Li, L. Preparation and Characterization of Modified Porous Wood Flour/Lauric-Myristic Acid Eutectic Mixture as a Form-Stable Phase Change Material. Energy Fuels 2018, 32, 5453–5461. [Google Scholar] [CrossRef]
- Jiang, L.; Lei, Y.; Liu, Q.; Wang, Y.; Zhao, Y.; Lei, J. Facile preparation of polyethylene glycol/wood-flour composites as form-stable phase change materials for thermal energy storage. J. Therm. Anal. Calorim. 2019, 139, 137–146. [Google Scholar] [CrossRef]
- Song, J.; Chen, C.; Zhu, S.; Zhu, M.; Dai, J.; Ray, U.; Li, Y.; Kuang, Y.; Li, Y.; Quispe, N.; et al. Processing bulk natural wood into a high-performance structural material. Nature 2018, 554, 224–228. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Nguyen, T.T.; Zhang, A.; Hao, J.; Wang, W. Effect of three tree species on UV weathering of wood flour-HDPE composites. J. For. Res. 2019, 31, 1071–1079. [Google Scholar] [CrossRef]
- Zhao, P.; Guo, C.; Li, L. Exploring the effect of melamine pyrophosphate and aluminum hypophosphite on flame retardant wood flour/polypropylene composites. Constr. Build. Mater. 2018, 170, 193–199. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Liu, C.; Yao, A.; Chen, K.; Shi, Y.; Feng, Y.; Zhang, P.; Yang, F.; Liu, M.; Chen, Z. MXene based core-shell flame retardant towards reducing fire hazards of thermoplastic polyurethane. Compos. Part B Eng. 2021, 226, 109363. [Google Scholar] [CrossRef]
- Liu, C.; Shi, Y.; Feng, Y.; You, X.; Xie, W.; Ke, Y.; Wang, H.; Chen, L.; Wang, Y. Design of core-multi shell flame retardant towards fire safe thermoplastic polyurethane composites with low toxic fumes generation. Compos. Commun. 2022, 35, 101339. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Liu, C.; Wang, H.; Guo, J.; Fu, L.; Feng, Y.; Wang, L.; Yang, F.; Liu, M. Engineering titanium carbide ultra-thin nanosheets for enhanced fire safety of intumescent flame retardant polylactic acid. Compos. Part B Eng. 2022, 236, 109792. [Google Scholar] [CrossRef]
- Wang, W.; Peng, Y.; Chen, H.; Gao, Q.; Li, J.; Zhang, W. Surface microencapsulated ammonium polyphosphate with beta-cyclodextrin and its application in wood-flour/polypropylene composites. Polym. Compos. 2017, 38, 2312–2320. [Google Scholar] [CrossRef]
- Umemura, T.; Arao, Y.; Nakamura, S.; Tomita, Y.; Tanaka, T. Synergy Effects of Wood Flour and Fire Retardants in Flammability of Wood-plastic Composites. Energy Procedia 2014, 56, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Utracki, L.A.; Sepehr, M.; Boccaleri, E. Synthetic, layered nanoparticles for polymeric nanocomposites (PNCs). Polym. Adv. Technol. 2007, 18, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Peng, Y.; Cao, J. Thermal stability of organo-montmorillonite-modified wood flour/poly(lactic acid) composites. Polym. Compos. 2016, 37, 1971–1977. [Google Scholar] [CrossRef]
- Barick, A.K.; Tripathy, D.K. Effect of organically modified layered silicate nanoclay on the dynamic viscoelastic properties of thermoplastic polyurethane nanocomposites. Appl. Clay Sci. 2011, 52, 312–321. [Google Scholar] [CrossRef]
- Giannelis, E.P. Polymer layered silicate nanocomposites. Adv. Mater. 1996, 8, 1–9. [Google Scholar] [CrossRef]
- Madhoushi, M.; Chavooshi, A.; Ashori, A.; Ansell, M.P.; Shakeri, A. Properties of wood plastic composite panels made from waste sanding dusts and nanoclay. J. Compos. Mater. 2013, 48, 1661–1669. [Google Scholar] [CrossRef]
- Liu, R.; Cao, J.; Luo, S.; Wang, X. Effects of two types of clay on physical and mechanical properties of poly(lactic acid)/wood flour composites at various wood flour contents. J. Appl. Polym. Sci. 2013, 127, 2566–2573. [Google Scholar] [CrossRef]
- Albozahid, M.; Naji, H.Z.; Alobad, Z.K.; Saiani, A. Effect of OMMT reinforcement on morphology and rheology properties of polyurethane copolymer nanocomposites. J. Elastomers Plast. 2021, 53, 992–1014. [Google Scholar] [CrossRef]
- Huang, G.; Gao, J.; Wang, X. Preparation and characterization of montmorillonite modified by phosphorus–nitrogen containing quaternary ammonium salts. Appl. Surf. Sci. 2012, 258, 4054–4062. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Qin, Y.; Huang, A.; Liu, R. Organo-montmorillonite modified wood flour/poly (lactic acid) composites via different modification process. Polym. Compos. 2020, 42, 987–994. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. The thermal degradation of polystyrene nanocomposite. Polymer 2005, 46, 2933–2942. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. The effect of clay on the thermal degradation of polyamide 6 in polyamide 6/clay nanocomposites. Polymer 2005, 46, 3264–3274. [Google Scholar] [CrossRef]
- Costache, M.C.; Jiang, D.D.; Wilkie, C.A. Thermal degradation of ethylene–vinyl acetate coplymer nanocomposites. Polymer 2005, 46, 6947–6958. [Google Scholar] [CrossRef]
- Bahreyni, E.; Farid, M.; Fakhari, M.A.; Farid, M. Ammonium Polyphosphate and Organically-Modified Montmorillonite Synergistic Effect on Flame-Retardant and Foaming Properties of High Density Polyethylene/Walnut Shell Powder Biocomposite. Iran. J. Polym. Sci. Technol. 2017, 30, 299–310. [Google Scholar]
- Zheng, J.; Ozisik, R.; Siegel, R.W. Phase separation and mechanical responses of polyurethane nanocomposites. Polymer 2006, 47, 7786–7794. [Google Scholar] [CrossRef]
- Song, Y.; Wang, N.; Li, J.; Shan, X.; Zou, G.; Zhao, C. Synergistic effect of organic montmorillonite and lignin-based charring agent with phosphorus on flame retardant poly(lactic acid). Fuhe Cailiao Xuebao/Acta Mater. Compos. Sin. 2019, 36, 60–68. [Google Scholar]
- Shi, Y.; Liu, C.; Duan, Z.; Yu, B.; Liu, M.; Song, P. Interface engineering of MXene towards super-tough and strong polymer nanocomposites with high ductility and excellent fire safety. Chem. Eng. J. 2020, 399, 125829. [Google Scholar] [CrossRef]
- Liu, C.; Wu, W.; Shi, Y.; Yang, F.; Liu, M.; Chen, Z.; Yu, B.; Feng, Y. Creating MXene/reduced graphene oxide hybrid towards highly fire safe thermoplastic polyurethane nanocomposites. Compos. Part B Eng. 2020, 203, 108486. [Google Scholar] [CrossRef]
- Liu, C.; Xu, K.; Shi, Y.; Wang, J.; Ma, S.; Feng, Y.; Lv, Y.; Yang, F.; Liu, M.; Song, P. Fire-safe, mechanically strong and tough thermoplastic Polyurethane/MXene nanocomposites with exceptional smoke suppression. Mater. Today Phys. 2022, 22, 100607. [Google Scholar] [CrossRef]
- Liu, C.; Yang, D.; Sun, M.; Deng, G.; Jing, B.; Wang, K.; Shi, Y.; Fu, L.; Feng, Y.; Lv, Y.; et al. Phosphorous-Nitrogen flame retardants engineering MXene towards highly fire safe thermoplastic polyurethane. Compos. Commun. 2022, 29, 101055. [Google Scholar] [CrossRef]
- Kamarian, S.; Yu, R.; Song, J.-i. Synergistic effects of halloysite nanotubes with metal and phosphorus additives on the optimal design of eco-friendly sandwich panels with maximum flame resistance and minimum weight. Nanotechnol. Rev. 2021, 11, 252–265. [Google Scholar] [CrossRef]
- Tirri, T.; Aubert, M.; Wilén, C.-E.; Pfaendner, R.; Hoppe, H. Novel tetrapotassium azo diphosphonate (INAZO) as flame retardant for polyurethane adhesives. Polym. Degrad. Stab. 2012, 97, 375–382. [Google Scholar] [CrossRef]
- Mandlekar, N.; Cayla, A.; Rault, F.; Giraud, S.; Salaun, F.; Guan, J. Development of Novel Polyamide 11 Multifilaments and Fabric Structures Based on Industrial Lignin and Zinc Phosphinate as Flame Retardants. Molecules 2020, 25, 4963. [Google Scholar] [CrossRef]
- Schartel, B.; Wilkie, C.A.; Camino, G. Recommendations on the scientific approach to polymer flame retardancy: Part 2—Concepts. J. Fire Sci. 2016, 35, 3–20. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Fu, L.; Feng, Y.; Lv, Y.; Wang, Z.; Liu, M.; Chen, Z. Highly efficient MXene/Nano-Cu smoke suppressant towards reducing fire hazards of thermoplastic polyurethane. Compos. Part A: Appl. Sci. Manuf. 2021, 150, 106600. [Google Scholar] [CrossRef]
- Zhang, S.; Ji, W.; Han, Y.; Gu, X.; Li, H.; Sun, J. Flame-retardant expandable polystyrene foams coated with ethanediol-modified melamine-formaldehyde resin and microencapsulated ammonium polyphosphate. J. Appl. Polym. Sci. 2018, 135, 46471. [Google Scholar] [CrossRef]
- Lu, X.; Liang, B.; Sheng, X.; Yuan, T.; Qu, J. Enhanced thermal conductivity of polyurethane/wood powder composite phase change materials via incorporating low loading of graphene oxide nanosheets for solar thermal energy storage. Sol. Energy Mater. Sol. Cells 2020, 208, 110391. [Google Scholar] [CrossRef]
- Wang, C.; Feng, L.; Li, W.; Zheng, J.; Tian, W.; Li, X. Shape-stabilized phase change materials based on polyethylene glycol/porous carbon composite: The influence of the pore structure of the carbon materials. Sol. Energy Mater. Sol. Cells 2012, 105, 21–26. [Google Scholar] [CrossRef]
- Zhang, D.; Tian, S.; Xiao, D. Experimental study on the phase change behavior of phase change material confined in pores. Sol. Energy 2007, 81, 653–660. [Google Scholar] [CrossRef]
- Liang, B.; Lu, X.; Li, R.; Tu, W.; Yang, Z.; Yuan, T. Solvent-free preparation of bio-based polyethylene glycol/wood flour composites as novel shape-stabilized phase change materials for solar thermal energy storage. Sol. Energy Mater. Sol. Cells 2019, 200, 110037. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Liu, L.; Fu, L.; Yu, B.; Lv, Y.; Yang, F.; Song, P. Strengthening, toughing and thermally stable ultra-thin MXene nanosheets/polypropylene nanocomposites via nanoconfinement. Chem. Eng. J. 2019, 378, 122267. [Google Scholar] [CrossRef]
- Shi, Y.; Gui, Z.; Yuan, B.; Hu, Y.; Zheng, Y. Flammability of polystyrene/aluminim phosphinate composites containing modified ammonium polyphosphate. J. Therm. Anal. Calorim. 2017, 131, 1067–1077. [Google Scholar] [CrossRef]
- Qin, P.; Yi, D.; Xing, J.; Zhou, M.; Hao, J. Study on flame retardancy of ammonium polyphosphate/montmorillonite nanocompound coated cellulose paper and its application as surface flame retarded treatment for polypropylene. J. Therm. Anal. Calorim. 2021, 146, 2015–2025. [Google Scholar] [CrossRef]
- Yi, D.; Yang, R. Ammonium polyphosphate/montmorillonite nanocompounds in polypropylene. J. Appl. Polym. Sci. 2010, 118, 834–840. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Hao, F.; Yang, C. Enhanced flame retardant performance of rigid polyurethane foam by using the modified OMMT layers with large surface area and ammonium polyphosphate. Mater. Today Commun. 2022, 32, 104021. [Google Scholar] [CrossRef]
- Liu, C.; Shi, Y.; Ye, H.; He, J.; Lin, Y.; Li, Z.; Lu, J.; Tang, Y.; Wang, Y.; Chen, L. Functionalizing MXene with Hypophosphite for Highly Fire Safe Thermoplastic Polyurethane composites. Compos. Part A Appl. Sci. Manuf. 2023, 168, 107486. [Google Scholar] [CrossRef]
- Bee, S.-T.; Lim, K.-S.; Sin, L.T.; Ratnam, C.T.; Bee, S.L.; Rahmat, A.R. Interactive effect of ammonium polyphosphate and montmorillonite on enhancing flame retardancy of polycarbonate/acrylonitrile butadiene styrene composites. Iran. Polym. J. 2018, 27, 899–911. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, P.; Shi, Y.; Rao, X.; Cai, S.; Fu, L.; Feng, Y.; Wang, L.; Zheng, X.; Yang, W. Enhanced Fire Safety of Rigid Polyurethane Foam via Synergistic Effect of Phosphorus/Nitrogen Compounds and Expandable Graphite. Molecules 2020, 25, 4741. [Google Scholar] [CrossRef]
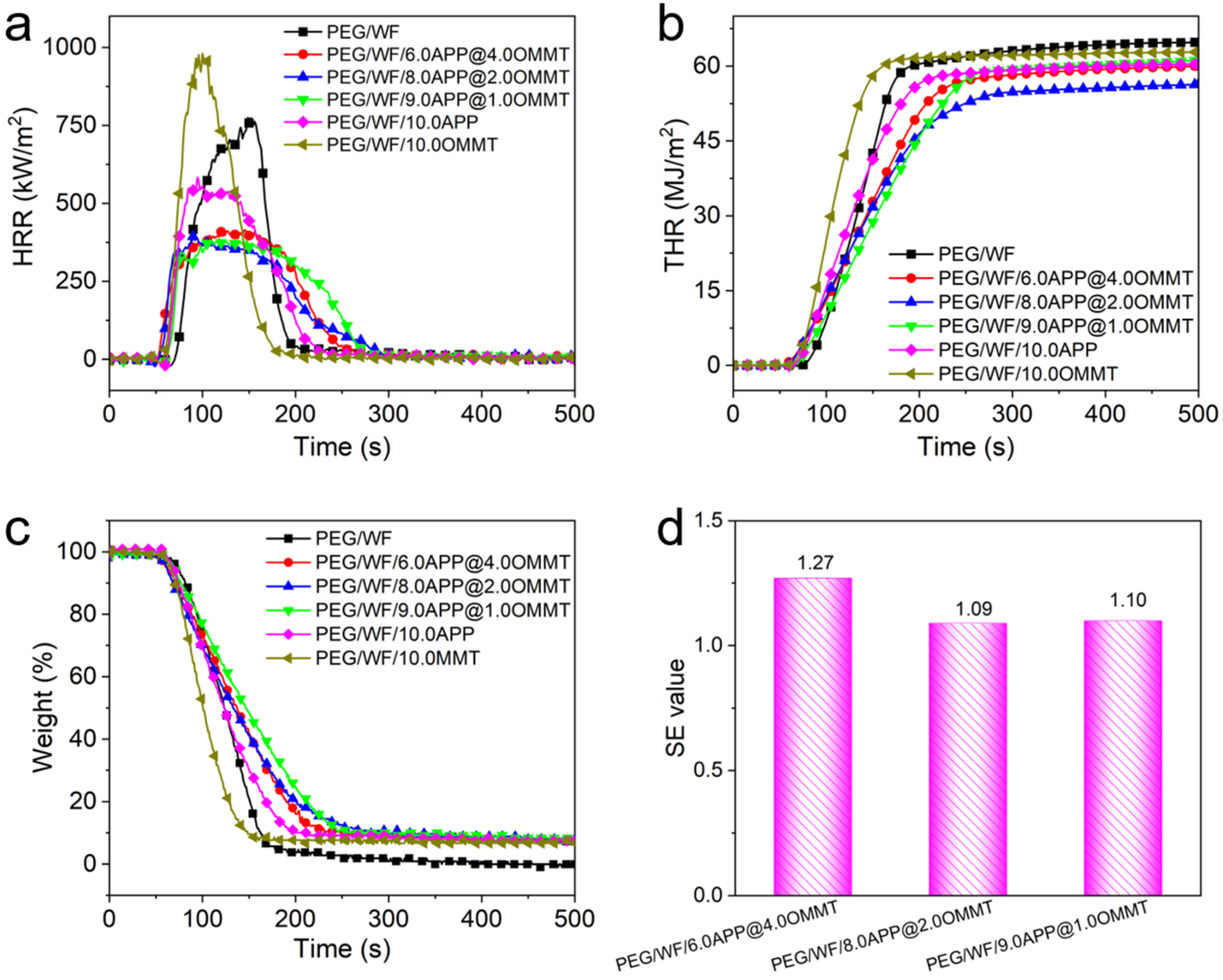

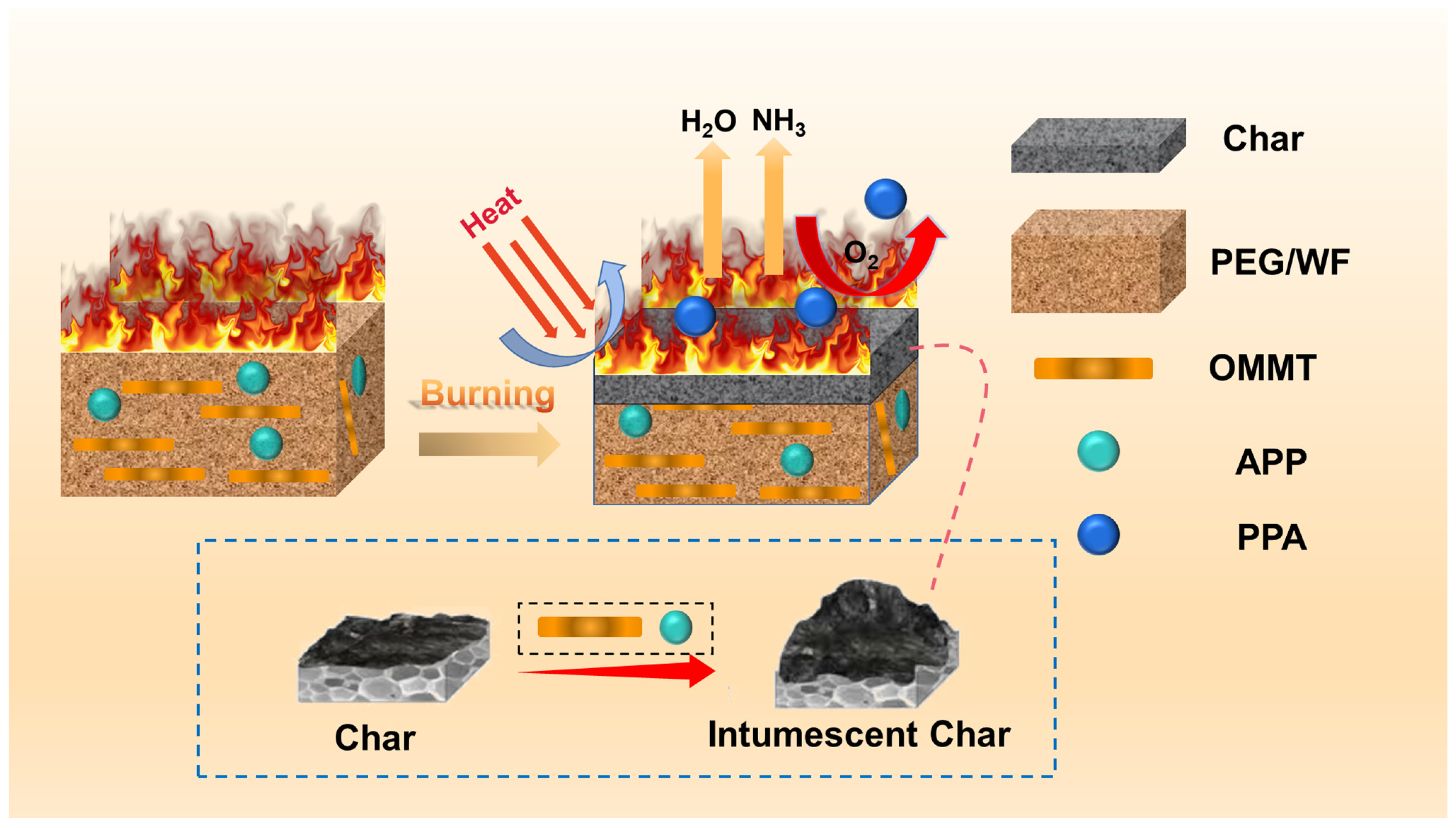
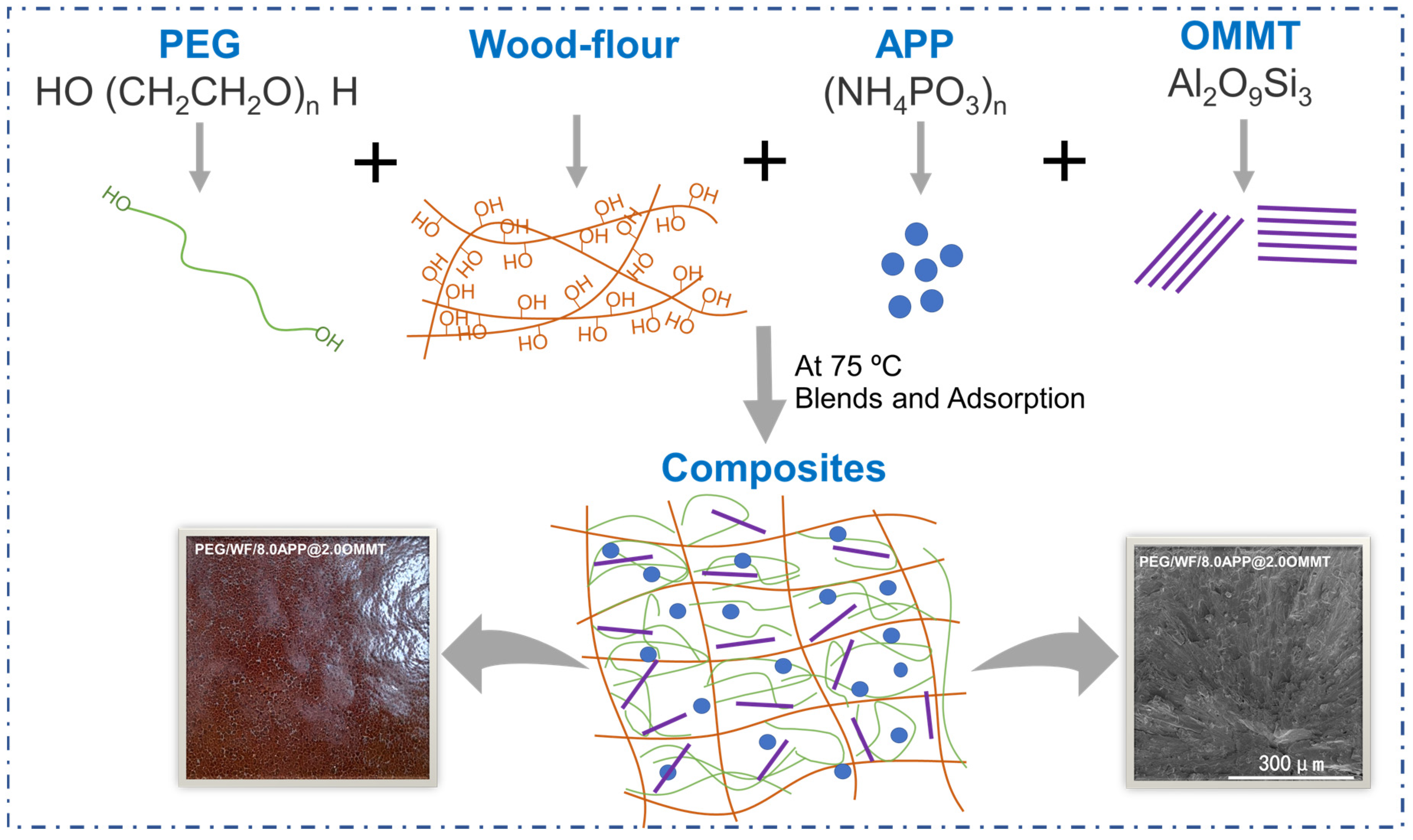
| Sample No. | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | MARHE (kW/m2) | Char Residues (wt.%) |
|---|---|---|---|---|---|
| Error | ±3.6 | ±24 | ±1.1 | ±27.4 | ±1.8 |
| PEG/WF | 66 | 795 | 64.8 | 313.2 | 0.0 |
| PEG/WF/[email protected] | 50 | 425 | 60.0 | 252.6 | 7.6 |
| PEG/WF/[email protected] | 51 | 399 | 56.3 | 232.1 | 7.9 |
| PEG/WF/[email protected] | 59 | 397 | 61.2 | 233.0 | 7.5 |
| PEG/WF/10.0APP | 58 | 590 | 60.4 | 289.9 | 7.4 |
| PEG/WF/10.0OMMT | 53 | 989 | 62.8 | 388.9 | 6.7 |
| Sample No. | Melting Process | Freezing Process | ||
|---|---|---|---|---|
| (°C) | (J/g) | (°C) | (J/g) | |
| PEG/WF | 61.3 | 179.7 | 40.5 | 153.1 |
| PEG/WF/[email protected] | 61.2 | 163.9 | 42.9 | 147.4 |
| PEG/WF/[email protected] | 63.7 | 176.6 | 43.2 | 155.7 |
| PEG/WF/[email protected] | 61.9 | 159.7 | 41.8 | 145.4 |
| PEG/WF/10.0APP | 62.1 | 185.0 | 40.2 | 168.6 |
| PEG/WF/10.0OMMT | 62.6 | 166.7 | 39.7 | 148.3 |
| Sample No. | (J/g) | (J/g) | η (%) |
|---|---|---|---|
| PEG/WF/[email protected] | 163.9 | 179.7 | 91.2 |
| PEG/WF/[email protected] | 176.6 | 179.7 | 98.3 |
| PEG/WF/[email protected] | 159.7 | 179.7 | 88.9 |
| PEG/WF/10.0APP | 185.0 | 179.7 | 102.9 |
| PEG/WF/10.0OMMT | 166.7 | 179.7 | 92.8 |
| Sample No. | Melting Process | Freezing Process | ||
|---|---|---|---|---|
| (°C) | (J/g) | (°C) | (J/g) | |
| PEG/WF | 60.0 | 161.9 | 39.0 | 153.6 |
| PEG/WF/[email protected] | 61.5 | 151.8 | 43.2 | 146.8 |
| PEG/WF/[email protected] | 61.3 | 161.6 | 43.8 | 155.2 |
| PEG/WF/[email protected] | 61.5 | 150.2 | 42.8 | 145.9 |
| PEG/WF/10.0APP | 60.5 | 174.4 | 38.0 | 169.1 |
| PEG/WF/10.0OMMT | 60.3 | 154.3 | 37.1 | 146.7 |
| Sample No. | T5% (°C) | T50% (°C) | Tmax (°C) | Residual Yield at 650 °C (wt.%) |
|---|---|---|---|---|
| PEG/WF | 325 | 405 | 408 | 9.07 |
| PEG/WF/[email protected] | 324 | 393 | 402 | 12.33 |
| PEG/WF/[email protected] | 320 | 374 | 383 | 11.22 |
| PEG/WF/[email protected] | 318 | 367 | 371 | 11.09 |
| PEG/WF/10.0APP | 351 | 370 | 364 | 7.64 |
| PEG/WF/10.0OMMT | 343 | 386 | 390 | 10.27 |
| No. | Sample No. | PEG | WF | APP | OMMT |
|---|---|---|---|---|---|
| 1# | PEG/WF | 83.3 | 6.7 | 0.0 | 0.0 |
| 2# | PEG/WF/[email protected] | 83.3 | 6.7 | 6.0 | 4.0 |
| 3# | PEG/WF/[email protected] | 83.3 | 6.7 | 8.0 | 2.0 |
| 4# | PEG/WF/[email protected] | 83.3 | 6.7 | 9.0 | 1.0 |
| 5# | PEG/WF/10.0APP | 83.3 | 6.7 | 10.0 | 0.0 |
| 6# | PEG/WF/10.0OMMT | 83.3 | 6.7 | 0.0 | 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Liu, C.; Xie, W.; Ke, Y.; You, X.; Jing, B.; Shi, Y. Effects of Ammonium Polyphosphate and Organic Modified Montmorillonite on Flame Retardancy of Polyethylene Glycol/Wood-Flour-Based Phase Change Composites. Molecules 2023, 28, 3464. https://doi.org/10.3390/molecules28083464
Wang K, Liu C, Xie W, Ke Y, You X, Jing B, Shi Y. Effects of Ammonium Polyphosphate and Organic Modified Montmorillonite on Flame Retardancy of Polyethylene Glycol/Wood-Flour-Based Phase Change Composites. Molecules. 2023; 28(8):3464. https://doi.org/10.3390/molecules28083464
Chicago/Turabian StyleWang, Ke, Chuan Liu, Wenxi Xie, Yihan Ke, Xiaoyong You, Binghao Jing, and Yongqian Shi. 2023. "Effects of Ammonium Polyphosphate and Organic Modified Montmorillonite on Flame Retardancy of Polyethylene Glycol/Wood-Flour-Based Phase Change Composites" Molecules 28, no. 8: 3464. https://doi.org/10.3390/molecules28083464





