Synthesis of Carboxyl Modified Polyether Polysiloxane Surfactant for the Biodegradable Foam Fire Extinguishing Agents
Abstract
:1. Introduction
2. Results and Discussions
2.1. Determination of the Optimum Experimental Condition
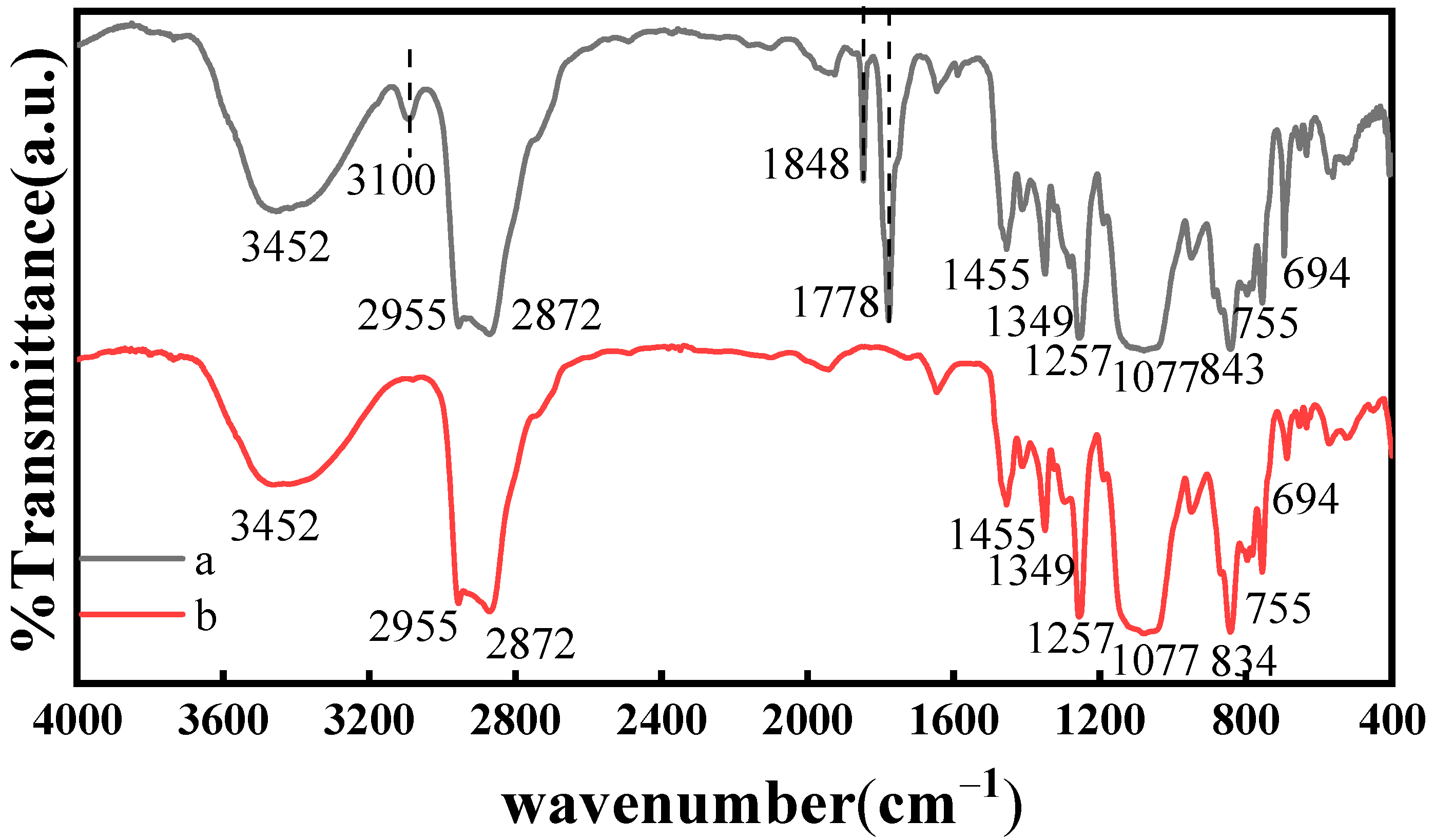
2.2. FT-IR Spectra
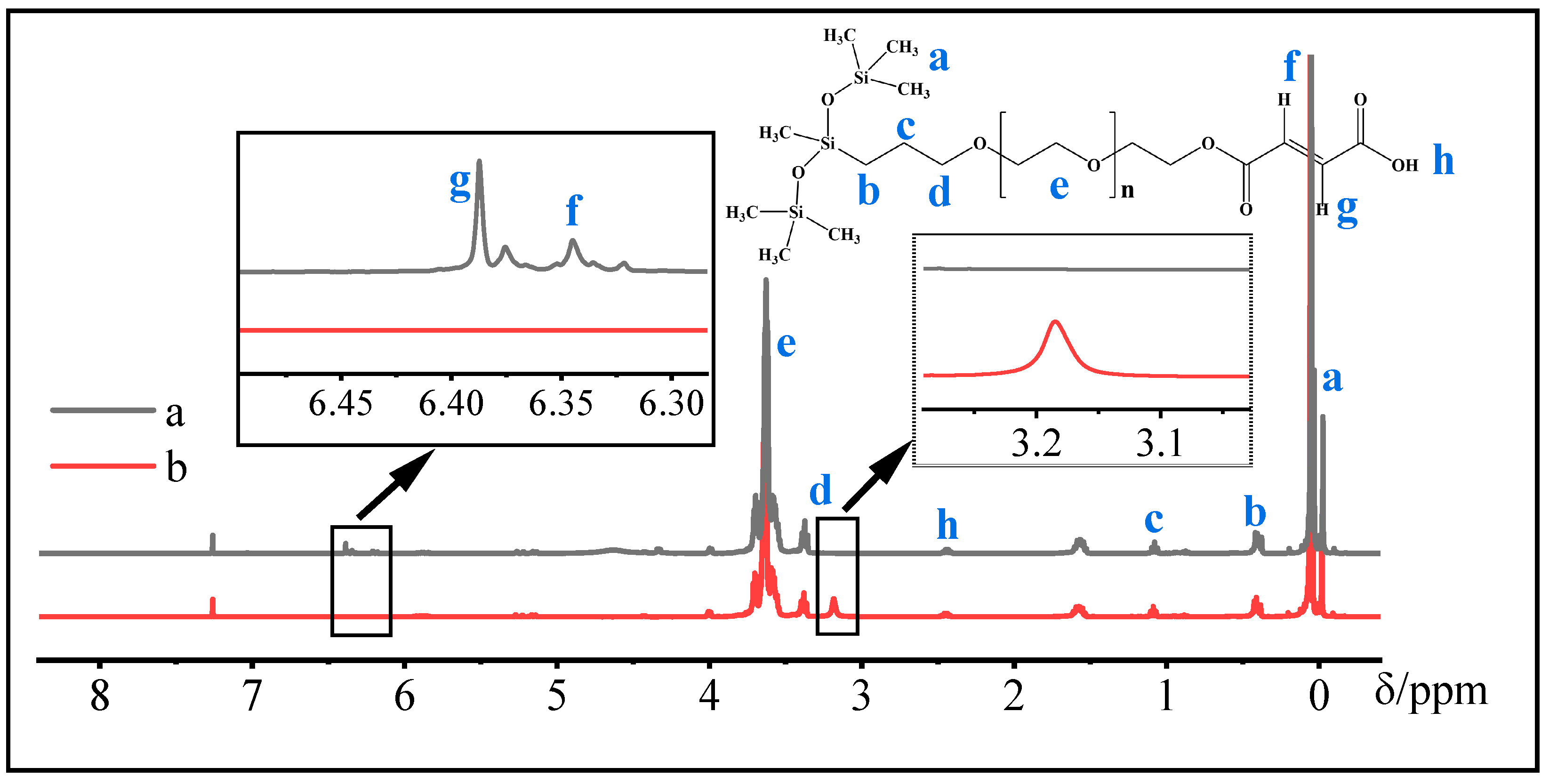
2.3. H-NMR Spectra
2.4. UV-Vis Absorption Spectra
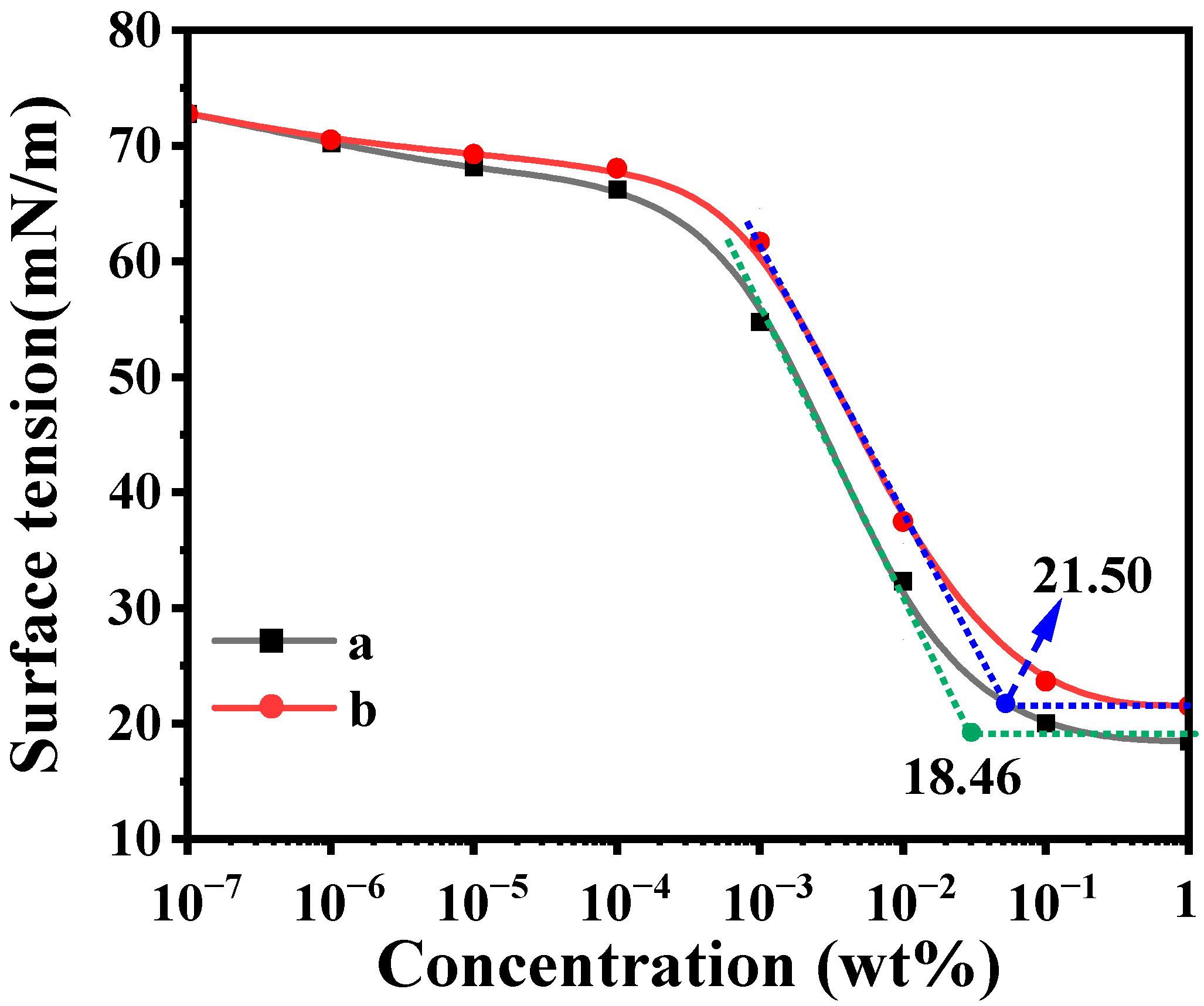
2.5. Surface Activity
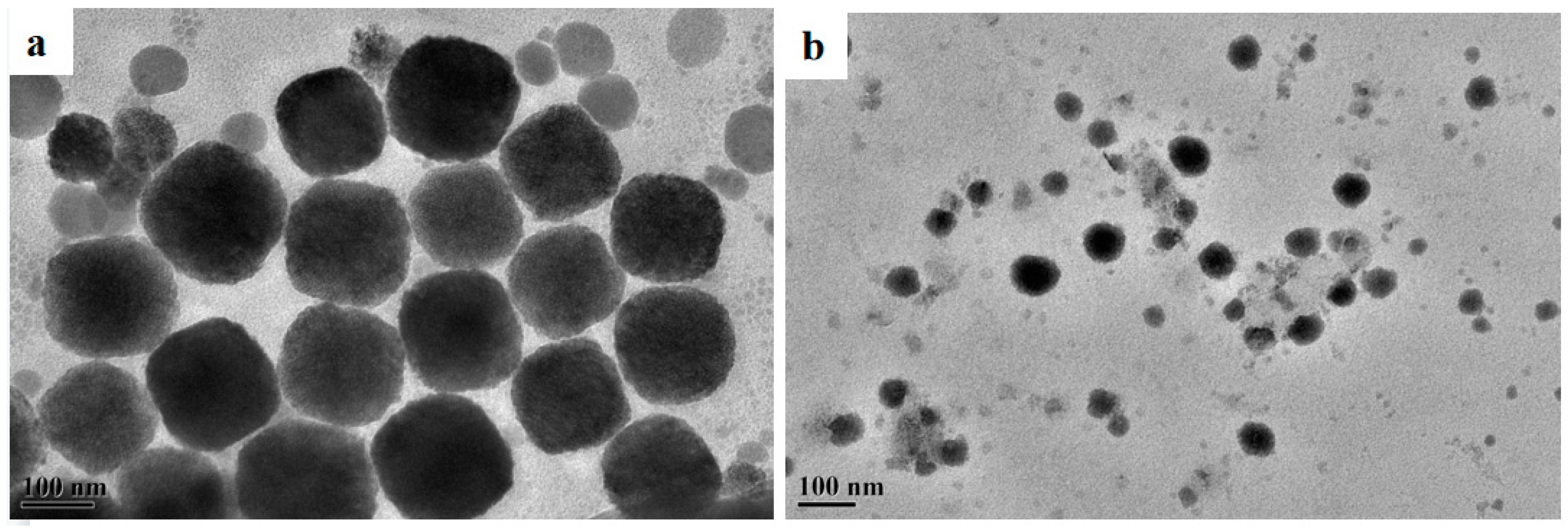
2.6. TEM Results
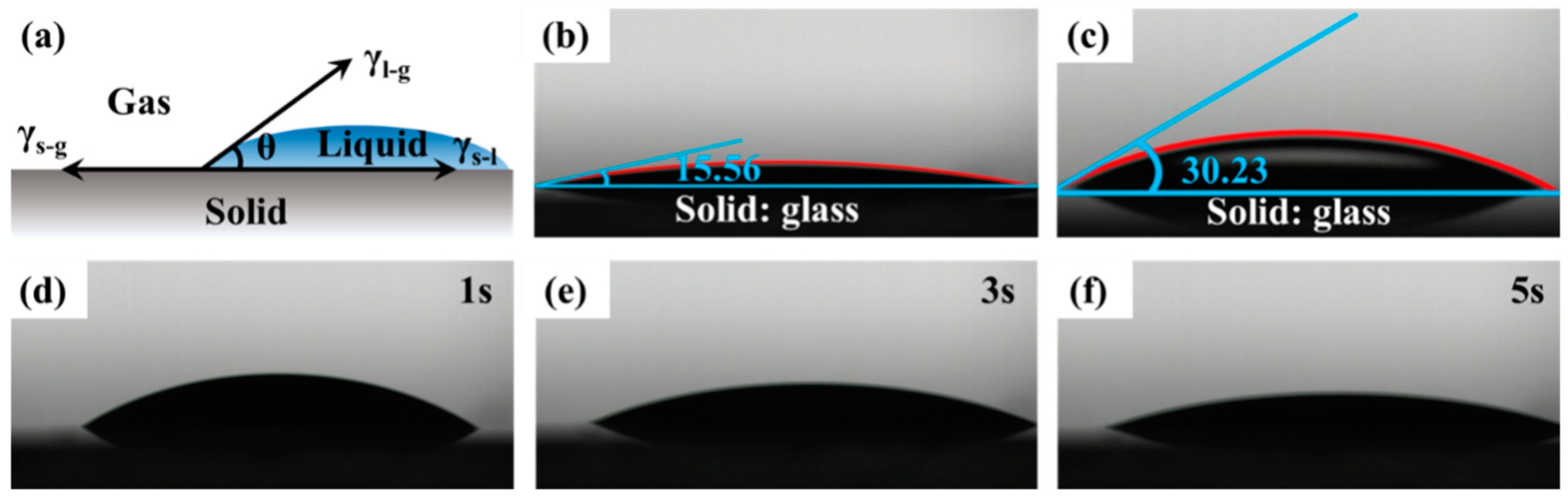
2.7. Wetting Properties
2.8. Foam Property
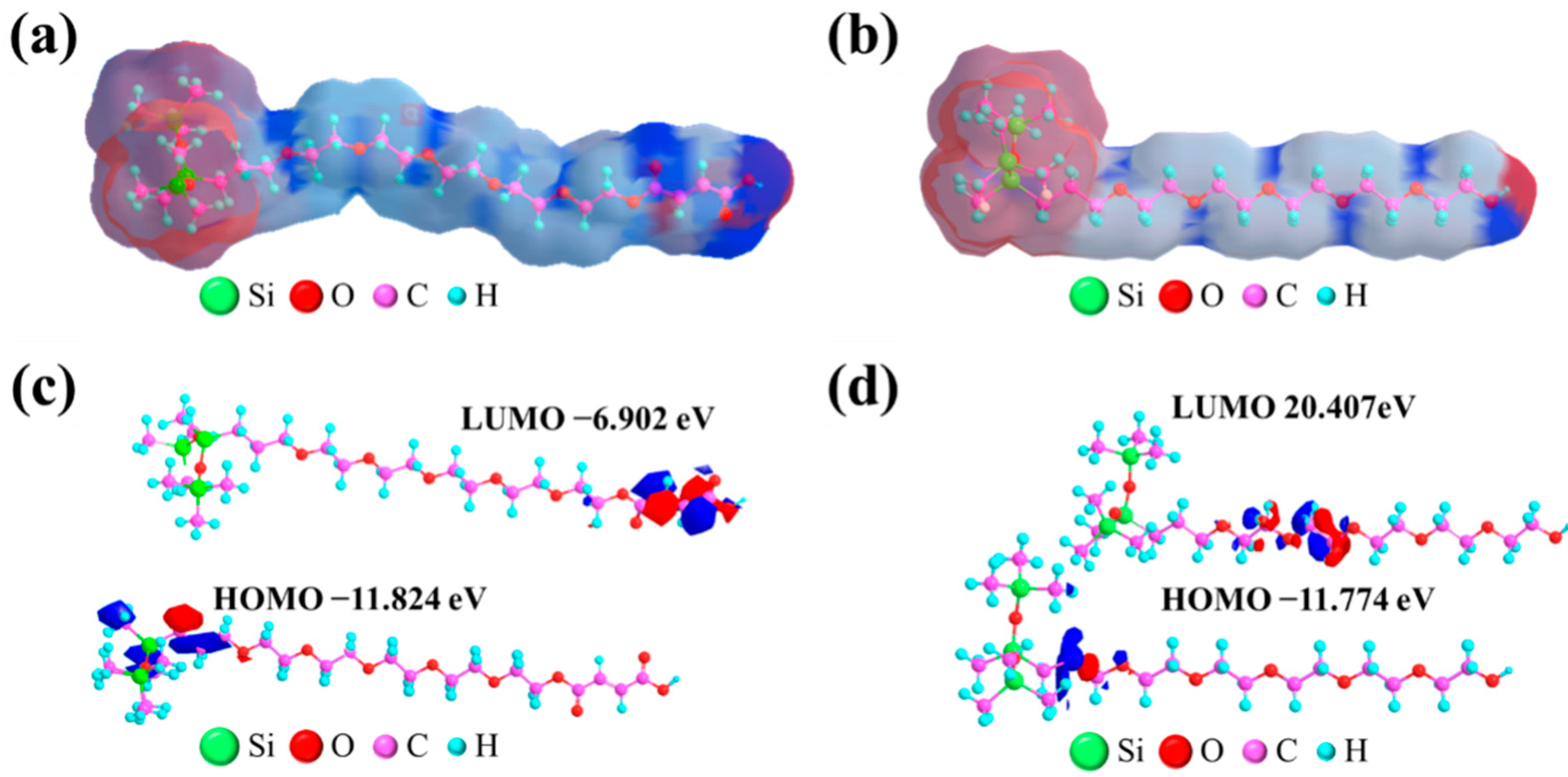
2.9. Electron Distribution Study
2.10. Fire Extinguishing Performance and Biodegradability of Foam Fire Extinguishing Agent
3. Materials and Methods
3.1. Materials
3.2. Sample Synthesis
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nickerson, A.; Maizel, A.C.; Kulkarni, P.R.; Adamson, D.T.; Kornuc, J.J.; Higgins, C.P. Enhanced Extraction of AFFF-Associated PFASs from Source Zone Soils. Environ. Sci. Technol. 2020, 54, 4952–4962. [Google Scholar] [CrossRef] [PubMed]
- Houtz, E.F.; Sutton, R.; Park, J.S.; Sedlak, M. Poly- and Perfluoroalkyl Substances in Wastewater: Significance of Unknown Precursors, Manufacturing Shifts, and Likely AFFF Impacts. Water Res. 2016, 95, 142–149. [Google Scholar] [CrossRef] [PubMed]
- McDonough, C.A.; Choyke, S.; Barton, K.E.; Mass, S.; Starling, A.P.; Adgate, J.L.; Higgins, C.P. Unsaturated PFOS and Other PFASs in Human Serum and Drinking Water from an AFFF-Impacted Community. Environ. Sci. Technol. 2021, 55, 8139–8148. [Google Scholar] [CrossRef]
- Barzen-Hanson, K.A.; Roberts, S.C.; Choyke, S.; Oetjen, K.; McAlees, A.; Riddell, N.; McCrindle, R.; Ferguson, P.L.; Higgins, C.P.; Field, J.A. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol. 2017, 51, 2047–2057. [Google Scholar] [CrossRef]
- Bräunig, J.; Baduel, C.; Heffernan, A.; Rotander, A.; Donaldson, E.; Mueller, J.F. Fate and Redistribution of Perfluoroalkyl Acids through AFFF-impacted Groundwater. Sci. Total Environ. 2017, 596, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Application of Green Surfactants Developing Environment Friendly Foam Extinguishing Agent. Fire Technol. 2015, 51, 503–511. [Google Scholar] [CrossRef]
- Wu, M.Y.; Liang, Y.T.; Zhao, Y.Y.; Wang, W.; Hu, X.M.; Tian, F.C.; He, Z.L.; Li, Y.S.; Liu, T.Y. Preparation of New Gel Foam and Evaluation of its Fire Extinguishing Performance. Colloids Surf. 2021, 629, 127443. [Google Scholar] [CrossRef]
- Sontake, A.R.; Wagh, S.M. The Phase-out of Perfluorooctane Sulfonate (PFOS) and the Global Future of Aqueous Film Forming Foam (AFFF), Innovations in Fire Fighting Foam. Fire Engineer 2014, 2, 11–14. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, N.; Lu, S.; Wang, Q.; Zhao, Y.; Liu, X. Study of Environmental-Friendly Firefighting Foam Based on the m-Mixture of Hydrocarbon and Silicone Surfactants. Fire Technol. 2020, 56, 1059–1075. [Google Scholar] [CrossRef]
- Sheng, Y.; Wu, X.; Lu, S.; Li, C. Experimental Study on Foam Properties of Mixed Systems of Silicone and Hydrocarbon Surfactants. J. Surfactants Deterg. 2016, 19, 823–831. [Google Scholar] [CrossRef]
- Hinnant, K.M.; Giles, S.L.; Smith, E.P.; Snow, A.W.; Ananth, R. Characterizing the Role of Fluorocarbon and Hydrocarbon Surfactants in Firefighting-Foam Formulations for Fire-Suppression. Fire Technol. 2020, 56, 1413–1441. [Google Scholar] [CrossRef]
- He, Y.-H.; Sun, Q.; Xing, H.; Wu, Y.; Xiao, J.-X. Cationic–anionic Fluorinated Surfactant Mixtures based on Short Fluorocarbon Chains as Potential Aqueous Film-Forming Foam. J. Disper. Sci. Technol. 2019, 40, 319–331. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B. Properties of Some Nonionic Fluorocarbon Surfactants and Their Mixtures with Hydrocarbon Ones. Adv. Colloid Interface Sci. 2021, 292, 102421. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Xue, M.; Zhang, S.; Wang, Y.; Zhai, X.; Ma, L.; Liu, X. Role of Nanoparticles in the Performance of Foam Stabilized by a Mixture of Hydrocarbon and Fluorocarbon Surfactants. Chem. EngSci. 2020, 228, 115977. [Google Scholar] [CrossRef]
- Fainerman, V.; Aksenenko, E.; Kovalchuk, V.; Mucic, N.; Javadi, A.; Liggieri, L.; Ravera, F.; Loglio, G.; Makievski, A.; Schneck, E. New View of the Adsorption of Surfactants at Water/Alkane Interfaces–Competitive and Cooperative Effects of Surfactant and Alkane Molecules. Adv. Colloid Interface Sci. 2020, 279, 102143. [Google Scholar] [CrossRef] [PubMed]
- Lusterio, A.; Brook, M.A. Naturally Derived Silicone Surfactants Based on Saccharides and Cysteamine. Molecules 2021, 26, 4802. [Google Scholar] [CrossRef]
- Fainerman, V.B.; Aksenenko, E.V.; Makievski, A.V.; Nikolenko, M.V.; Javadi, A.; Schneck, E.; Miller, R. Particular Behavior of Surface Tension at the Interface Between Aqueous Solution of Surfactant and Alkane. Langmuir 2019, 35, 15214–15220. [Google Scholar] [CrossRef]
- Ezhova, A.; Gritskova, I.; Gusev, S.; Milenin, S.; Gorodov, V.; Muzafarov, A.; Lazov, M.; Chvalun, S. Organosilicon Comb-Shaped Surfactants for the Synthesis of Polymer Suspensions with a Narrow Particle Size Distribution. Polym. Sci. Ser. B+ 2021, 63, 209–217. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, J.; Zhou, J.; Zhao, Y.; Huang, Z.; Zhu, Y.; Yu, D. Adsorption Characteristics and Conformational Transition of Polyethylene Glycol–Maleated Rosin Polyesters on the Water–Air Surface. Adv. Compos. Mater. 2021, 5, 1233–1240. [Google Scholar] [CrossRef]
- Yao, F.; Zhang, W.; Hu, D.; Li, S.; Kong, X.; Uemura, S.; Kusunose, T.; Feng, Q. Ultra-hydrophilic Nanofiltration Membranes Fabricated Via Punching in the HTO Nanosheets. Adv. Compos. Mater. 2022, 6, 1–16. [Google Scholar] [CrossRef]
- Liu, M.J.; Li, P.; Meng, Q.W.; Ge, Q. Membranes Constructed by Metal–Ligand Complexation for Efficient Phosphorus Removal and Fouling Resistance in Forward Osmosis. Adv. Compos. Mater. 2021, 5, 159–172. [Google Scholar] [CrossRef]
- Moncayo-Riascos, I.; Hoyos, B.A. Fluorocarbon Versus Hydrocarbon Organosilicon Surfactants for Wettability Alteration: A Molecular Dynamics Approach. J. Ind. Eng. Chem. 2020, 88, 224–232. [Google Scholar] [CrossRef]
- Gostenin, V.B.; Zubov, V.P.; Gritskova, I.A.; Shikhovtseva, I.S. One-step Synthesis of Polymer Dispersions with Large Narrow-Size-Distribution Particles Via Heterophase Polymerization in the Presence of Surface-Active Water-Insoluble Organosilicon Macromers. Polym. Int. 2022, 71, 656–661. [Google Scholar] [CrossRef]
- Gritskova, I.; Ezhova, A.; Chalikh, A.; Levachev, S.; Chvalun, S. Effect of the Composition and Structure of Carbofunctional Oligodimethylsiloxanes on their Colloidal-Chemical Properties. Russ. Chem. Bull. 2019, 68, 132–136. [Google Scholar] [CrossRef]
- Nowak, T.; Mazela, B.; Olejnik, K.; Peplińska, B.; Perdoch, W. Starch-Silane Structure and Its Influence on the Hydrophobic Properties of Paper. Molecules 2022, 27, 3136. [Google Scholar] [CrossRef] [PubMed]
- Istratov, V.V.; Vasnev, V.A.; Markova, G.D. Biodegradable and Biocompatible Silatrane Polymers. Molecules 2021, 26, 1893. [Google Scholar] [CrossRef]
- Czajka, A.; Hazell, G.; Eastoe, J. Surfactants at the Design Limit. Langmuir 2015, 31, 8205–8217. [Google Scholar] [CrossRef]
- Meng, L.N.; Chen, Z.X.; Feng, S.Y. Synthesis and Properties of Sodium Carboxylate Silicone Surfactant Via Thiol-Ene“Click”Reaction. Colloids Interface Sci. Commun. 2022, 49, 100642. [Google Scholar] [CrossRef]
- Fang, L.; Tan, J.; Zheng, Y.; Yang, G.; Yu, J.; Feng, S. Synthesis, Aggregation Behavior of Novel Cationic Silicone Surfactants in Aqueous Solution and their Application in Metal Extraction. J. Mol. Liq. 2017, 231, 134–141. [Google Scholar] [CrossRef]
- Vo, T.-V.; Chou, Y.-Y.; Chen, B.-H. Preparation of Microemulsion from an Alkyl Polyglycoside Surfactant and Tea Tree Oil. Molecules 2021, 26, 1971. [Google Scholar] [CrossRef]
- Milliasseau, D.; Jeftić, J.; Pessel, F.; Plusquellec, D.; Benvegnu, T. Transformation of Pectins into Non-Ionic or Anionic Surfactants Using a One-Pot and Cascade Mode Process. Molecules 2021, 26, 1956. [Google Scholar] [CrossRef]
- Al-Anssari, S.; Wang, S.; Barifcani, A.; Iglauer, S. Oil-Water Interfacial Tensions of Silica Nanoparticle-Surfactant Formulations. Tenside Surfact. Det. 2017, 54, 334–341. [Google Scholar] [CrossRef]
- Nowak, E.; Kovalchuk, N.M.; Che, Z.; Simmons, M.J. Effect of Surfactant Concentration and Viscosity of Outer Phase during the Coalescence of a Surfactant-Laden Drop with a Surfactant-Free Drop. Colloids Surf. 2016, 505, 124–131. [Google Scholar] [CrossRef]
- Czajka, A.; Hill, C.; Peach, J.; Pegg, J.C.; Grillo, I.; Guittard, F.; Rogers, S.E.; Sagisaka, M.; Eastoe, J. Trimethylsilyl Hedgehogs–A Novel Class of Super-Efficient Hydrocarbon Surfactants. Phys. Chem. Chem. Phys. 2017, 19, 23869–23877. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Xiong, X.; He, Z.; Cao, F.; Sun, D. Aggregation Behavior of Polyether Based Siloxane Surfactants in Aqueous Ssolutions: Effect of Alkyl Groups and Steric Hindrance. J. Phys. Chem. B 2019, 123, 1390–1399. [Google Scholar] [CrossRef]
- Meng, L.; Wang, W.; Li, L.; Feng, S. Syntheses, Properties and Aggregation Behaviors of Novel Carboxylate-Based Silicone Surfactants. ChemPlusChem 2022, 87, e202200337. [Google Scholar] [CrossRef]
- Ma, Z.Z.; Zhang, X.W.; Zhang, X.J.; Ahmed, N.; Fan, H.; Wan, J.T.; Bittencourt, C.; Li, B.G. Synthesis of CO2-Derived, Siloxane-Functionalized Poly(ether carbonate)s and Waterborne Polyurethanes. Ind. Eng. Chem. Res. 2020, 59, 3044–3051. [Google Scholar] [CrossRef]
- Jia, W.; Qi, Y.; Hu, Z.; Xiong, Z.; Luo, Z.; Xiang, Z.; Hu, J.; Lu, W. Facile Fabrication of Monodisperse CoFe2O4 Nanocrystals@dopamine@DOX Hybrids for Magnetic-responsive on-demand Cancer Theranostic Applications. Adv. Compos. Mater. 2021, 4, 989–1001. [Google Scholar] [CrossRef]
- Ding, Z.; Tian, Z.; Ji, X.; Dai, H.; Si, C. Bio-inspired Catalytic one-step Prepared R-siloxane Cellulose Composite Membranes with Highly Efficient Oil Separation. Adv. Compos. Mater. 2022, 5, 2138–2153. [Google Scholar] [CrossRef]
- Kamatham, N.; Ibraikulov, O.A.; Durand, P.; Wang, J.; Boyron, O.; Heinrich, B.; Heiser, T.; Lévêque, P.; Leclerc, N.; Méry, S. On the Impact of Linear Siloxanated Side Chains on the Molecular Self-Assembling and Charge Transport Properties of Conjugated Polymers. Adv. Funct. 2021, 31, 2007734. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, L.; Niu, C.; Du, T.; Yang, Y.; Feng, Z. Synthesis and Properties of a Novel Asymmetric Gemini Surfactant Based on Siloxane Skeleton. J. Mol. Liq. 2019, 296, 112073. [Google Scholar] [CrossRef]
- Kino, D.; Okada, K.; Tokudome, Y.; Takahashi, M.; Malfatti, L.; Innocenzi, P. Reactivity of Silanol Group on Siloxane Oligomers for Designing Molecular Structure and Surface Wettability. J. Sol. Gel. Techn. 2021, 97, 734–742. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, D.; Liu, T.; Ni, L.; Quan, H.; Sun, Q. Emulsion Stability and Water Tolerance of Cationic Waterborne Polyurethane with Different Soft Segment Ratios between Trifunctional Polyether and Bifunctional Polyester. Mater. Res. Express 2019, 6, 065303. [Google Scholar] [CrossRef]
- Kim, M.; Cho, Y.; Kang, S.W. Interactions of Ag Particles Stabilized by 7, 7, 8, 8-Tetracyanoquinodimethane with Olefin Molecules in Poly (ether-block-amide). Molecules 2022, 27, 4122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhou, H.; Chen, L.; Hao, L.; Chen, H.; Zhou, X. Carboxymethyl Chitosan Grafted Trisiloxane Surfactant Nanoparticles with Ph Sensitivity for Sustained Release of Pesticide. Carbohydr. Polym. 2020, 243, 116433. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Zhang, Y.-F. Synthesis and Micellization of Cationic Trisiloxane Surfactants with Poly (Ethylene Glycol). Colloids Surf. 2022, 634, 127946. [Google Scholar] [CrossRef]
- Delgado-Sánchez, C.; Cuadri, A.A.; Navarro, F.J.; Partal, P. Formulation and Processing of Novel Non-Aqueous Polyethylene Glycol-In-Silicone Oil (O/O) Phase Change Emulsions. Sol. Energy Mater Sol. Cells 2021, 221, 110898. [Google Scholar] [CrossRef]
- Sounouvou, H.T.; Lechanteur, A.; Piel, G.; Evrard, B. Silicones in Dermatological Topical Drug Formulation: Overview and advances. Int. J. Pharm. 2022, 625, 122111. [Google Scholar] [CrossRef]
- Dai, Z.; Yang, K.; Dong, Q. Synthesis and Characterization of Hydroxy-Terminated Polyether-Polydimethylsiloxane-Polyether (Pe-Pdms-Pe) Triblock Oligomers and their use in the Preparation of Thermoplastic Polyurethanes. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Liu, J.; He, N.; Shen, J.; Gong, Z.; Fan, Y.; Li, M.; Song, Y.; Yang, X. UV Cured Silicon-Containing Polyurethane-Acrylate Coatings with Non-Traditional Fluorescence and Temperature-Sensitive Transparency. Prog. Org. Coat. 2021, 161, 106513. [Google Scholar] [CrossRef]
- Kim, E.-N.; Kaygusuz, O.; Lee, H.-S.; Jeong, G.-S. Simultaneous Quantitative Analysis of Ginsenosides Isolated from the Fruit of Panax ginseng C.A. Meyer and Regulation of HO-1 Expression through EGFR Signaling Has Anti-Inflammatory and Osteogenic Induction Effects in HPDL Cells. Molecules 2021, 26, 2092. [Google Scholar] [CrossRef] [PubMed]
- Kellert, M.; Friedrichs, J.-S.J.; Ullrich, N.A.; Feinhals, A.; Tepper, J.; Lönnecke, P.; Hey-Hawkins, E. Modular Synthetic Approach to Carboranyl–Biomolecules Conjugates. Molecules 2021, 26, 2057. [Google Scholar] [CrossRef] [PubMed]
- Suhaimi, A.; Mahmoudi, E.; Latif, R.; Siow, K.S.; Zaid, M.H.M.; Mohammad, A.W.; Wee, M.M.R. Superhydrophilic Organosilicon Plasma Modification on PES Membrane for Organic Dyes Filtration. J. Water Process 2021, 44, 102352. [Google Scholar] [CrossRef]
- Petrunin, M.; Rybkina, A.; Yurasova, T.; Maksaeva, L. Effect of Organosilicon Self-Assembled Polymeric Nanolayers Formed during Surface Modification by Compositions Based on Organosilanes on the Atmospheric Corrosion of Metals. Polymers 2022, 14, 4428. [Google Scholar] [CrossRef]
- Ryan, K.M.; Drumm, A.D.; Martin, C.E.; Krumpfer, A.K.; Krumpfer, J.W. Synthetic Methods and Applications of Functional and Reactive Silicone Polymers. In Reactive and Functional Polymers Volume One: Biopolymers, Polyesters, Polyurethanes, Resins and Silicones; Springer: Berlin/Heidelberg, Germany, 2020; pp. 301–328. [Google Scholar]
- Jiang, N.; Sheng, Y.J.; Li, C.H.; Lu, S.X. Surface Activity, Foam Properties and Aggregation Behavior of Mixtures of Short-chain Fluorocarbon and Hydrocarbon Surfactants. J. Mol. Liq 2018, 268, 249–255. [Google Scholar] [CrossRef]
- Tan, J.; He, Z.; Miao, Y.; Zhou, D. Effect of Steric Hindrance on the Aggregation Behavior of Cationic Silicone Surfactants in Aqueous Solutions. J Solution Chem. 2019, 48, 891–904. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, M.; Tan, J.; Feng, S. Impact of Molecular Architecture on Surface Properties and Aqueous Stabilities of Silicone-Based Carboxylate Surfactants. Langmuir 2020, 36, 2023–2029. [Google Scholar] [CrossRef]
- Tan, J.; Lin, M.; Ye, Z. Synthesis, Micellar and Surface Properties of Cationic Trisiloxane Surfactants with Different Siloxane Hydrophobic Groups. J Solution Chem. 2018, 47, 2082–2093. [Google Scholar] [CrossRef]
- Chen, H.; Tan, J. Aggregation Behavior and Intermolecular Interaction of Cationic Trisiloxane Surfactants: Effects of Unsaturation. Langmuir 2020, 36, 14582–14588. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W.; et al. Adsorption of Trisiloxane Surfactant for Selective Flotation of Scheelite from Calcite at Room Temperature. Langmuir 2022, 38, 9010–9020. [Google Scholar] [CrossRef]
- Bourgeois, A.; Bergendahl, J.; Rangwala, A. Biodegradability of Fluorinated Fire-fighting Foams in Water. Chemosphere 2015, 131, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Bao, Z.M.; Hu, C.; Shuai, L.J.; Chen, Y. Organic Pollutant Loading and Biodegradability of Firefighting Foam. Earth Environ. Sci. 2017, 94, 12137. [Google Scholar] [CrossRef]
- Król, B.; Prochaska, K.; Chrzanowski, Ł. Biodegradability of Firefighting Foams. Fire Technol. 2012, 48, 173–181. [Google Scholar] [CrossRef]




| Experiment Number | Temperature (°C) | Reaction Time (h) | IPA Content (wt%) | n(HPMS):n(MA) | Surface Tension (mN/m) |
|---|---|---|---|---|---|
| 1 | 95 | 4.5 | 20% | 1:2 | 19.92 |
| 2 | 75 | 2.5 | 40% | 1:1 | 19.24 |
| 3 | 85 | 1.5 | 40% | 1:3 | 19.40 |
| 4 | 85 | 3.5 | 0% | 2:1 | 19.28 |
| 5 | 65 | 1.5 | 10% | 1:3 | 20.08 |
| 6 | 95 | 2.5 | 0% | 2:1 | 20.54 |
| 7 | 75 | 4.5 | 0% | 1:2 | 20.74 |
| 8 | 75 | 1.5 | 20% | 2:1 | 19.78 |
| 9 | 65 | 4.5 | 40% | 2:1 | 19.87 |
| 10 | 95 | 3.5 | 40% | 1:3 | 21.10 |
| 11 | 75 | 3.5 | 10% | 1:3 | 20.63 |
| 12 | 65 | 2.5 | 0% | 1:2 | 19.30 |
| 13 | 95 | 1.5 | 10% | 1:1 | 20.44 |
| 14 | 65 | 3.5 | 20% | 1:1 | 19.43 |
| 15 | 85 | 2.5 | 20% | 1:2 | 19.24 |
| 16 | 85 | 4.5 | 10% | 1:1 | 19.35 |
| Levels | A | B | C | D | |
|---|---|---|---|---|---|
| Surface tension (mN/m) | K1 | 19.67 | 19.93 | 19.97 | 19.87 |
| K2 | 20.10 | 19.61 | 20.13 | 19.62 | |
| K3 | 19.34 | 20.11 | 19.62 | 19.83 | |
| K4 | 20.50 | 19.97 | 19.90 | 20.30 | |
| R | 1.16 | 0.50 | 0.51 | 0.69 | |
| Primary and secondary analysis | A > D > C > B | ||||
| Best combination | A3B2C3D2 | ||||
| Levels (%) | Temperature (°C) | Reaction Time (h) | Solvent Content (wt%) | Feeding Ratio (a:b) |
|---|---|---|---|---|
| 1 | 65 | 1.5 | 0 | 2:1 |
| 2 | 75 | 2.5 | 10 | 1:1 |
| 3 | 85 | 3.5 | 20 | 1:2 |
| 4 | 95 | 4.5 | 40 | 1:3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, J.; Qi, L.; Wu, J.; Lang, X.; Wei, Y.; Zhang, G.; Cui, P.; Shang, Z.; Mu, X.; Mu, S.; et al. Synthesis of Carboxyl Modified Polyether Polysiloxane Surfactant for the Biodegradable Foam Fire Extinguishing Agents. Molecules 2023, 28, 3546. https://doi.org/10.3390/molecules28083546
Jiao J, Qi L, Wu J, Lang X, Wei Y, Zhang G, Cui P, Shang Z, Mu X, Mu S, et al. Synthesis of Carboxyl Modified Polyether Polysiloxane Surfactant for the Biodegradable Foam Fire Extinguishing Agents. Molecules. 2023; 28(8):3546. https://doi.org/10.3390/molecules28083546
Chicago/Turabian StyleJiao, Jinqing, Lei Qi, Jingfeng Wu, Xuqing Lang, Yuechang Wei, Guangwen Zhang, Pengyu Cui, Zuzheng Shang, Xiaodong Mu, Shanjun Mu, and et al. 2023. "Synthesis of Carboxyl Modified Polyether Polysiloxane Surfactant for the Biodegradable Foam Fire Extinguishing Agents" Molecules 28, no. 8: 3546. https://doi.org/10.3390/molecules28083546






