The Mosquito Larvicidal Activity of Lignans from Branches of Cinnamomum camphora chvar. Borneol
Abstract
:1. Introduction
2. Results
2.1. Structure Elucidation of the Isolated Compound
2.2. Mosquito Larvicidal Activity of Lignans(1–14)
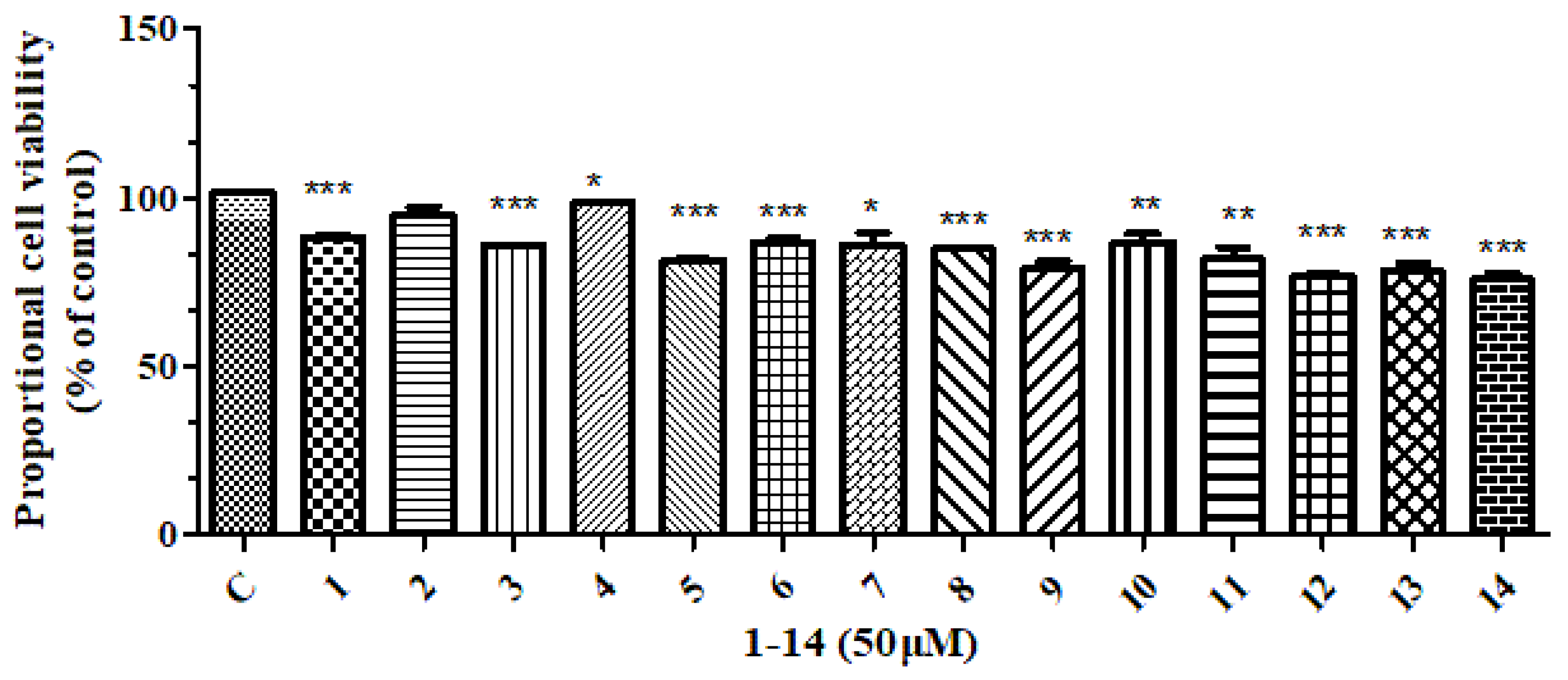
2.3. Anti-Inflammatory Activity, Cytotoxic Activity and Evaluation of Lignans(1–14)
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material and Mosquitoes
4.3. Extraction and Isolation
4.4. Biological Assays
4.5. Statistic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Zhao, T.; Lu, B. Biosystematics of Culex pipiens complex in China. Entomol. Sin. 1995, 2, 1–8. [Google Scholar] [CrossRef]
- Bhatt, S.; Weiss, D.J.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.E.; Moyes, C.L.; Henry, A.; Eckhoff, P.A.; et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 7572, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Tantely, M.L.; Tortosa, P.; Alout, H.; Berticat, C.; Berthomieu, A.; Rutee, A.; Dehecq, J.S.; Makoundou, P.; Labbe, P.; Pasteur, N.; et al. Insecticide resistance in Culex pipiens quinquefasciatus and Aedes albopictus mosquitoes from La Réunion Island. Insect Biochem. Mol. 2010, 40, 317–324. [Google Scholar] [CrossRef]
- Nakasone, Y.; Takara, K.; Wada, K.; Tanaka, J.; Yogi, S.; Nakatani, N. Antioxidative compounds isolated from Kokuto, non-centrifugal cane sugar. Biosci. Biotechnol. Biochem. 1996, 60, 1714–1716. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhou, J.; Ding, Z. Phenolic constituents from Manglietia crassipes. Acta Bot. Yunnanica 2000, 22, 365–367. [Google Scholar]
- Chang, C.I.; Hsu, C.M.; Li, T.S.; Huang, S.D.; Lin, C.C.; Yen, C.M.; Chou, C.H.; Cheng, H.L. Constituents of the stem of Cucurbita moschata exhibit antidiabetic activities through multiple mechanisms. J. Funct. Foods 2014, 10, 260–273. [Google Scholar] [CrossRef]
- Li, D.; Liu, M.; Zhou, X. A new dimeric lignan from Zanthoxylum simulans. China J. Chin. Mater. Med. 2015, 40, 2843–2848. [Google Scholar]
- Ina, H.; Ono, M.; Sashida, Y.; Iida, H. (+)-Piperitol from Paulownia tomentosa. Planta Med. 1987, 53, 504. [Google Scholar] [CrossRef]
- Pava, A.; Zamilpa, A.; Trejo-Espino, J.L.; Domínguez-Mendoza, B.E.; Jiménez-Ferrer, E.; Pérez-Martínez, L.; Trejo-Tapia, G. Synergism and subadditivity of verbascoside-lignans and -iridoids binary mixtures isolated from Castilleja tenuiflora Benth. on NF-κB/AP-1 inhibition activity. Molecules 2021, 26, 547. [Google Scholar] [CrossRef]
- Ye, M.; Yan, Y.; Qiao, L.; Ni, X. Studies on chemical constituents of Cuscuta chinensis. China J. Chin. Mater. Med. 2002, 27, 38–40. [Google Scholar]
- Lin, R.W.; Tsai, I.L.; Duh, C.Y.; Lee, K.H.; Chen, I.S. New lignans and cytotoxic constituents from Wikstroemia lanceolata. Planta Med. 2004, 70, 234–238. [Google Scholar] [PubMed]
- Zhang, W.; Wang, Y.; Geng, Z.; Guo, S.; Cao, J.; Zhang, Z.; Pang, X.; Chen, Z.; Du, S.; Deng, Z. Antifeedant activities of lignans from stem bark of Zanthoxylum armatum DC. against Tribolium castaneum. Molecules 2018, 23, 617. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.M.X.; Yoshida, M.; Gottlieb, O.R. Dibenzylbutyrolactone lignans from virola sebifera. Phytochemistry 1983, 22, 1516–1518. [Google Scholar] [CrossRef]
- Kato, M.J.; Yoshida, M.; Gottlieb, O.R. Flavones and lignans in flowers, fruits and seedlings of Virola Venosa. Phytochemistry 1992, 31, 283–287. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Wang, J. Chemical constituents of Nux Prinsepiae Uniflorae. J. Shenyang Pharm. Univ. 2006, 23, 209–211. [Google Scholar]
- Cui, Y.; Mu, Q.; Hu, C. Studies on the phenylpropanoids from Caragana rosea. Nat. Prod. Res. Dev. 2003, 15, 277–283. [Google Scholar]
- Xia, Z.; Zhang, H.; Xu, T.; Chen, Y.; Zhou, G. Phenylpropanoids from Fruits of Xanthium sibiricum. Chin. Pharm. J. 2021, 56, 13–22. [Google Scholar]
- Chen, X.; Ren, Q.; Liu, Y.; Zou, Z.; Xu, K.; Tan, G. Circular dichroism in the determination of absolute configuration of lignans and neolignans. Cent. South Pharm. 2020, 18, 1–10. [Google Scholar]
- Wang, L.X.; Wang, H.L.; Huang, J.; Chu, T.Z.; Peng, C.; Zhang, H.; Chen, H.L.; Xiong, Y.A.; Tan, Y.Z. Review of lignans from 2019 to 2021: Newly reported compounds, diverse activities, structure-activity relationships and clinical applications. Phytochemistry 2022, 202, 113326. [Google Scholar] [CrossRef]
- Barre, D.E.; Mizier-Barre, K.A. Lignans’ potential in pre and post-onset type 2 diabetes management. Curr. Diabetes Rev. 2020, 16, 2–11. [Google Scholar] [CrossRef]
- Haruna, M.; Koube, T.; Ito, K.; Murata, H. Balanophonin, a new neo-lignan from balanophora japonica makino. Chem. Pharm. Bull. 1982, 30, 1525–1527. [Google Scholar] [CrossRef]
- Park, I.K.; Shin, S.C.; Kim, C.S.; Lee, H.J.; Choi, W.S.; Ahn, Y.J. Larvicidal activity of lignans identified in Phryma leptostachya Var. asiatica roots against three mosquito species. J. Agric. Food Chem. 2005, 53, 969–972. [Google Scholar] [CrossRef]
- Qie, X.; Sun, A.; Hao, H.; Lv, B.; Wu, W.; Hu, Z. A potential lignan botanical insecticide from Phryma leptostachya against Aedes aegypti: Laboratory selection, metabolic mechanism, and resistance risk assessment. J. Pest. Sci. 2021, 95, 397–408. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, Z.; Shi, B.; Wei, S.; Wu, W. Larvicidal activity of lignans from Phryma leptostachya L. against Culex pipiens pallens. Parasitol. Res. 2012, 110, 1079–1084. [Google Scholar] [CrossRef]
- Kim, S.I.; Ahn, Y.J. Larvicidal activity of lignans and alkaloid identified in Zanthoxylum piperitum bark toward insecticide-susceptible and wild Culex pipiens pallens and Aedes aegypti. Parasites Vectors 2017, 10, 221. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Xu, Z.; Xiong, B.; Xu, J. Chemical investigation of secondary metabolites produced by mangrove endophytic fungus Phyllosticta capitalensis. Nat. Prod. Res. 2019, 35, 1561–1565. [Google Scholar] [CrossRef]
- Xie, C.; Wang, S.; Cao, M.; Xiong, W.; Wu, L. (E)-9-Octadecenoic acid ethyl ester derived from lotus seedpod ameliorates inflammatory responses by regulating MAPKs and NF-κB Signalling Pathways in LPS-Induced RAW264.7 Macrophages. Evid.-Based Complement. Altern. Med. 2022, 2022, 6731360. [Google Scholar] [CrossRef]


| Chemical | Regression Equation | x2 Value | p | LC50 | 95%CL |
|---|---|---|---|---|---|
| the CH2Cl2 fraction | y = 5.898 + 3.938x | 75.112 | 0 | 0.032 | 0.027~0.037 |
| Medioresinol (1) | y = 4.247 + 2.506x | 13.579 | 0.916 | 0.02 | 0.018~0.023 |
| Syringaresinol (2) | y = 4.129 + 2.455x | 13.049 | 0.9.32 | 0.021 | 0.018~0.023 |
| Pinoresinol (3) | y = 3.807 + 2.260x | 26.18 | 0.244 | 0.021 | 0.018~0.023 |
| Kobusin (4) | y = 5.057 + 2.529x | 8.936 | 0.994 | 0.01 | 0.008~0.012 |
| piperitol (5) | y = 3.824 + 1.869x | 15.116 | 0.857 | 0.009 | 0.007~0.011 |
| sesamin (6) | y = 3.951 + 1.972x | 15.797 | 0.826 | 0.01 | 0.008~0.012 |
| 9(R)-hydroxy-d-sesamin (7) | y = 3.645 + 2.032x | 43.046 | 0.005 | 0.016 | 0.012~0.020 |
| aptosimon (8) | y = 3.468 + 1.761x | 11.037 | 0.974 | 0.011 | 0.008~0.013 |
| acuminatolide (9) | y = 2.791 + 2.108x | 30.048 | 0.117 | 0.047 | 0.041~0.055 |
| (2R, 3R)-2,3-di-(3, 4- dimethoxybenzyl)- butyrolactone (10) | y = 1.125 + 1.153x | 36.884 | 0.024 | 0.106 | 0.079~0.161 |
| (−)-Dihydro-3′,4′- dimethoxy-3′,4′-demethylenedloxycubebin (11) | y = 0.919 + 1.483x | 21.332 | 0.500 | 0.240 | 0.183~0.355 |
| balanophonin (12) | y = 1.294 + 1.767x | 26.693 | 0.223 | 0.185 | 0.151–0.243 |
| buddlenol D (13) | y = 4.520 + 3.204x | 26.321 | 0.238 | 0.039 | 0.036~0.042 |
| (7R, 7′R, 7″S, 7‴S, 8S, 8′S, 8″S, 8‴S)-4″,4‴-dihydroxy-3, 3′, 3″, 3‴, 5, 5′-hexamethoxy-7, 9′; 7′, 9-diepoxy-4, 8″; 4’, 8‴-bisoxy-8, 8’-dineolignan-7″, 7‴, 9″, 9‴-tetraol (14) | y = 4.285 + 3.050x | 41.695 | 0.007 | 0.039 | 0.034~0.045 |
| Permethrin (positive control) | y = 4.105 + 1.908x | 10.023 | 0.986 | 0.007 | 0.005~0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Chen, J.; Shang, R.; Yang, F.; Xie, C.; Liu, Y.; Wen, X.; Fu, J.; Xiong, W.; Wu, L. The Mosquito Larvicidal Activity of Lignans from Branches of Cinnamomum camphora chvar. Borneol. Molecules 2023, 28, 3769. https://doi.org/10.3390/molecules28093769
Xu Z, Chen J, Shang R, Yang F, Xie C, Liu Y, Wen X, Fu J, Xiong W, Wu L. The Mosquito Larvicidal Activity of Lignans from Branches of Cinnamomum camphora chvar. Borneol. Molecules. 2023; 28(9):3769. https://doi.org/10.3390/molecules28093769
Chicago/Turabian StyleXu, Zhiyong, Junhui Chen, Ruifeng Shang, Fan Yang, Chuanqi Xie, Yunfei Liu, Xuefang Wen, Jianping Fu, Wei Xiong, and Lei Wu. 2023. "The Mosquito Larvicidal Activity of Lignans from Branches of Cinnamomum camphora chvar. Borneol" Molecules 28, no. 9: 3769. https://doi.org/10.3390/molecules28093769





