Indium-Based Silica Materials: Sustainable Syntheses Combined with a Challenging Insertion in SiO2 Mesoporous Structures
Abstract
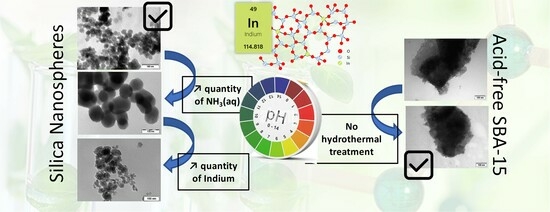
:1. Introduction
2. Results and Discussion
2.1. Extra-Small Nanospheres (NS)
2.2. Acid-Free (AF) In-SBA-15
3. Materials and Methods
3.1. Materials
3.2. Catalyst Synthesis
3.2.1. Extra-Small Silica Nanospheres
3.2.2. Acid-Free SBA-15 Particles
3.2.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Jammaer, J.; Aerts, A.; D’Haen, J.; Seo, J.W.; Martens, J.A. Convenient synthesis of ordered mesoporous silica at room temperature and quasi-neutral pH. J. Mater. Chem. 2009, 19, 8290–8293. [Google Scholar] [CrossRef]
- Feng, G.; Wang, J.; Boronat, M.; Li, Y.; Su, J.-H.; Huang, J.; Ma, Y.; Yu, J. Radical-Facilitated Green Synthesis of Highly Ordered Mesoporous Silica Materials. J. Am. Chem. Soc. 2018, 140, 4770–4773. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Principles of green chemistry. Green Chem. Theory Pract. 1998, 29, 14821–14842. [Google Scholar]
- Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host–guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. [Google Scholar] [CrossRef]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef]
- Burke, A.M.; Hanrahan, J.P.; Healy, D.A.; Sodeau, J.R.; Holmes, J.D.; Morris, M.A. Large pore bi-functionalised mesoporous silica for metal ion pollution treatment. J. Hazard. Mater. 2009, 164, 229–234. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Godard, N.; Collard, X.; Vivian, A.; Bivona, L.A.; Fiorilli, S.; Fusaro, L.; Aprile, C. Rapid room temperature synthesis of tin-based mesoporous solids: Influence of the particle size on the production of ethyl lactate. Appl. Catal. A Gen. 2018, 556, 73–80. [Google Scholar] [CrossRef]
- Cai, Q.; Luo, Z.-S.; Pang, W.-Q.; Fan, Y.-W.; Chen, X.-H.; Cui, F.-Z. Dilute Solution Routes to Various Controllable Morphologies of MCM-41 Silica with a Basic Medium. Chem. Mater. 2001, 13, 258–263. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Jang, L.-Y.; Cheng, S. Synthesis of Zr-Incorporated SBA-15 Mesoporous Materials in a Self-generated Acidic Environment. Chem. Mater. 2004, 16, 4174–4180. [Google Scholar] [CrossRef]
- Lin, S.; Shi, L.; Ribeiro Carrott, M.M.L.; Carrott, P.J.M.; Rocha, J.; Li, M.R.; Zou, X.D. Direct synthesis without addition of acid of Al-SBA-15 with controllable porosity and high hydrothermal stability. Microporous Mesoporous Mater. 2011, 142, 526–534. [Google Scholar] [CrossRef]
- Cai, W.; Ni, X.; Meng, F.; Sun, L.; Wang, R.; Lin, S. From Al-SBA-15 to Ga-SBA-15: Morphology and acidity evolution in the acid-free synthesis. Microporous Mesoporous Mater. 2022, 335, 111823. [Google Scholar] [CrossRef]
- Vivian, A.; Soumoy, L.; Fusaro, L.; Louette, P.; Felten, A.; Fiorilli, S.; Debecker, D.P.; Aprile, C. The high activity of mesoporous Ga-SiO2 catalysts in the upgrading of glycerol to solketal explained by in-depth characterization. J. Catal. 2021, 400, 83–92. [Google Scholar] [CrossRef]
- Hussein, H.; Vivian, A.; Fusaro, L.; Devillers, M.; Aprile, C. Synthesis of Highly Accessible Gallosilicates via Impregnation Procedure: Enhanced Catalytic Performances in the Conversion of Glycerol into Solketal. ChemCatChem. 2020, 12, 5966–5976. [Google Scholar] [CrossRef]
- Collard, X.; Li, L.; Lueangchaichaweng, W.; Bertrand, A.; Aprile, C.; Pescarmona, P.P. Ga-MCM-41 nanoparticles: Synthesis and application of versatile heterogeneous catalysts. Catal. Today 2014, 235, 184–192. [Google Scholar] [CrossRef]
- Gracia, M.J.; Losada, E.; Luque, R.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Activity of Gallium and Aluminum SBA-15 materials in the Friedel–Crafts alkylation of toluene with benzyl chloride and benzyl alcohol. Appl. Catal. A Gen. 2008, 349, 148–155. [Google Scholar] [CrossRef]
- El Berrichi, Z.; Cherif, L.; Tessonnier, J.P.; Louis, B.; Fraissard, J.; Ledoux, M.J.; Pham-Huu, C. GaSBA-15: A new and active Friedel-Crafts acylation catalyst. In Studies in Surface Science and Catalysis; Čejka, J., Žilková, N., Nachtigall, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 158, pp. 1413–1420. [Google Scholar]
- Satsuma, A.; Segawa, Y.; Yoshida, H.; Hattori, T. Involvement of solid acid on Al- and Ga-doped porous silica in the Diels–Alder reaction. Appl. Catal. A Gen. 2004, 264, 229–236. [Google Scholar] [CrossRef]
- Kugita, T.; Jana, S.K.; Owada, T.; Hashimoto, N.; Onaka, M.; Namba, S. Mesoporous Al-containing MCM-41 molecular sieves: Highly active catalysts for Diels–Alder reaction of cyclopentadiene with α,β-unsaturated aldehydes. Appl. Catal. A Gen. 2003, 245, 353–362. [Google Scholar] [CrossRef]
- Maertens, A.; Vivian, A.; Fusaro, L.; Felten, A.; Louette, P.; Armandi, M.; Fiorilli, S.; Aprile, C. Low-impact synthesis of mesostructured acidic catalysts: Toward the efficient conversion of crude glycerol. Sustain. Energy Fuels 2022, 6, 3818–3829. [Google Scholar] [CrossRef]
- Loh, T.-P.; Chua, G.-L. Discovery of indium complexes as water-tolerant Lewis acids. Chem. Commun. 2006, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Maunula, T.; Ahola, J.; Hamada, H. Reaction mechanism and kinetics of NOx reduction by methane on In/ZSM-5 under lean conditions. Appl. Catal. B Environ. 2006, 64, 13–24. [Google Scholar] [CrossRef]
- Gabrienko, A.A.; Arzumanov, S.S.; Moroz, I.B.; Prosvirin, I.P.; Toktarev, A.V.; Wang, W.; Stepanov, A.G. Methane Activation on In-Modified ZSM-5: The State of Indium in the Zeolite and Pathways of Methane Transformation to Surface Species. J. Phys. Chem. C 2014, 118, 8034–8043. [Google Scholar] [CrossRef]
- Uysal, B.; Oksal, B.S. Catalytic activity of SBA-15-grafted indium tri-isopropoxide in chemoselective MPV reductions. J. Porous Mater. 2015, 22, 1053–1064. [Google Scholar] [CrossRef]
- Kumar, R.; Shah, S.; Bahadur, J.; Melnichenko, Y.B.; Sen, D.; Mazumder, S.; Vinod, C.P.; Chowdhury, B. Highly stable In-SBA-15 catalyst for vapor phase Beckmann rearrangement reaction. Microporous Mesoporous Mater. 2016, 234, 293–302. [Google Scholar] [CrossRef]
- Böhlmann, W.; Klepel, O.; Michel, D.; Papp, H. Synthesis and characterization of In-MCM-41 mesoporous molecular sieves with different Si/In ratios. Stud. Surf. Sci. Catal. 2002, 142, 1355–1362. [Google Scholar] [CrossRef]
- de la Iglesia, Ó.; Sarango, M.; Munárriz, M.; Malankowska, M.; Navajas, A.; Gandía, L.M.; Coronas, J.; Téllez, C. Mesoporous Sn-In-MCM-41 Catalysts for the Selective Sugar Conversion to Methyl Lactate and Comparative Life Cycle Assessment with the Biochemical Process. ACS Sustain. Chem. Eng. 2022, 10, 2868–2880. [Google Scholar] [CrossRef] [PubMed]
- Khaled, R.K.; Wahba, M.A.; Badry, M.D.; Zawrah, M.F.; Heikal, E.A. Highly ordered pure and indium-incorporated MCM-41 mesoporous adsorbents: Synthesis, characterization and evaluation for dye removal. J. Mater. Sci. 2022, 57, 4504–4527. [Google Scholar] [CrossRef]
- He, N.-Y.; Bao, S.-L.; Xu, Q.-H. Synthesis and characterization of FeSiMCM-41 and LaSiMCM-41. In Studies in Surface Science and Catalysis; Chon, H., Ihm, S.-K., Uh, Y.S., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; Volume 105, pp. 85–92. [Google Scholar]
- Shamzhy, M.; Opanasenko, M.; Concepcion, P.; Martinez, A. New trends in tailoring active sites in zeolite-based catalysts. Chem. Soc. Rev. 2019, 48, 1095–1149. [Google Scholar] [CrossRef]
- Li, L.; Cani, D.; Pescarmona, P.P. Metal-containing TUD-1 mesoporous silicates as versatile solid acid catalysts for the conversion of bio-based compounds into valuable chemicals. Inorganica Chim. Acta 2015, 431, 289–296. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Soumoy, L.; Célis, C.; Debecker, D.P.; Armandi, M.; Fiorilli, S.; Aprile, C. Hafnium-doped silica nanotubes for the upgrading of glycerol into solketal: Enhanced performances and in-depth structure-activity correlation. J. Catal. 2022, 411, 41–53. [Google Scholar] [CrossRef]
- Motokura, K.; Ito, Y.; Noda, H.; Miyaji, A.; Yamaguchi, S.; Baba, T. Surface Functionalization for Synergistic Catalysis: Silica–Alumina-Supported Cationic Indium and Organic Base for Cyanoethoxycarbonylation. ChemPlusChem 2014, 79, 1053–1058. [Google Scholar] [CrossRef]








| Entry | Sample | Amount of Ammonia | Medium of Precursor Solubilization | Si/In Ratio (ICP-OES) | SBET (m2/g) | Pore Diameter (nm) | Total Pore Volume (cm3/g) | Particle Size (nm) | |
|---|---|---|---|---|---|---|---|---|---|
| Targeted | Exp. | ||||||||
| 1 | In-NS-Nit | 1.0 g | Acidic | 74 | 71 | 1033 | 3.5 | 1.8 | n.a. |
| 2 | In-NS-Cl-a | 1.0 g | Acidic | 74 | 73 | 1073 | 3.5 | 2.1 | n.a. |
| 3 | In-NS-Cl-b | 1.0 g | Alcohol | 74 | 77 | 1033 | 3.7 | 1.5 | 29 |
| 4 | In-NS-Cl-c | 1.0 g | Alcohol | 37 | 33 | 1256 | 3.7 | 1.7 | n.a. |
| 5 | In-NS-Cl-d | 5.0 g | Alcohol | 74 | 55 | 1250 | 3.0 | 0.9 | 97 |
| 6 | In-NS-Cl-e | 1.0 g | Alcohol | 74 | 55 | 1123 | 3.4 | 1.9 | n.a. |
| Entry | Sample | Precursor | Hydrothermal Treatment | pH Adjustement | Si/In Ratio (ICP-OES) | SBET (m2/g) | BJH Pore Diameter (nm) | Pore Volume * (cm3/g) |
|---|---|---|---|---|---|---|---|---|
| 1 | In-AF-Cl-HT | InCl3 | ✓ | ✗ | 505 | 873 | 9 | 1.0 (0.02) |
| 2 | In-AF-Cl-RT | InCl3 | ✗ | ✗ | 586 | 565 | 4 | 0.3 (0.1) |
| 3 | In-AF-Cl-pH-HT | InCl3 | ✓ | ✓ | 72 | 678 | 9 | 0.7 (0.02) |
| 4 | In-AF-Cl-pH-RT | InCl3 | ✗ | ✓ | 85 | 734 | 8 | 0.9 (0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maertens, A.; Aprile, C. Indium-Based Silica Materials: Sustainable Syntheses Combined with a Challenging Insertion in SiO2 Mesoporous Structures. Molecules 2024, 29, 102. https://doi.org/10.3390/molecules29010102
Maertens A, Aprile C. Indium-Based Silica Materials: Sustainable Syntheses Combined with a Challenging Insertion in SiO2 Mesoporous Structures. Molecules. 2024; 29(1):102. https://doi.org/10.3390/molecules29010102
Chicago/Turabian StyleMaertens, Amélie, and Carmela Aprile. 2024. "Indium-Based Silica Materials: Sustainable Syntheses Combined with a Challenging Insertion in SiO2 Mesoporous Structures" Molecules 29, no. 1: 102. https://doi.org/10.3390/molecules29010102