Studies of the Immunomodulatory Activity of Polysaccharides from the Stem of Cynomorium songaricum Based on Intestinal Microbial Analysis
Abstract
1. Introduction
2. Results
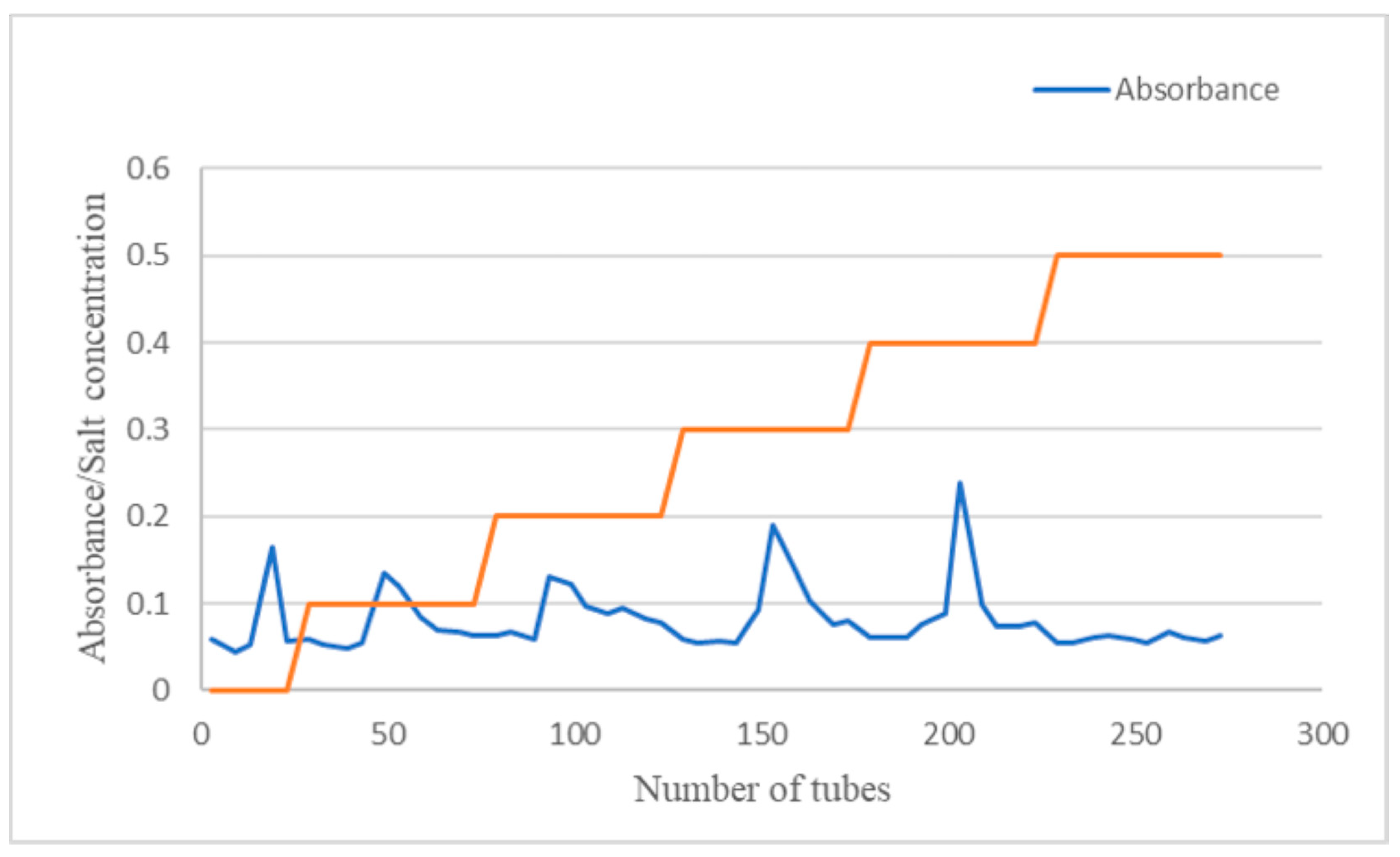
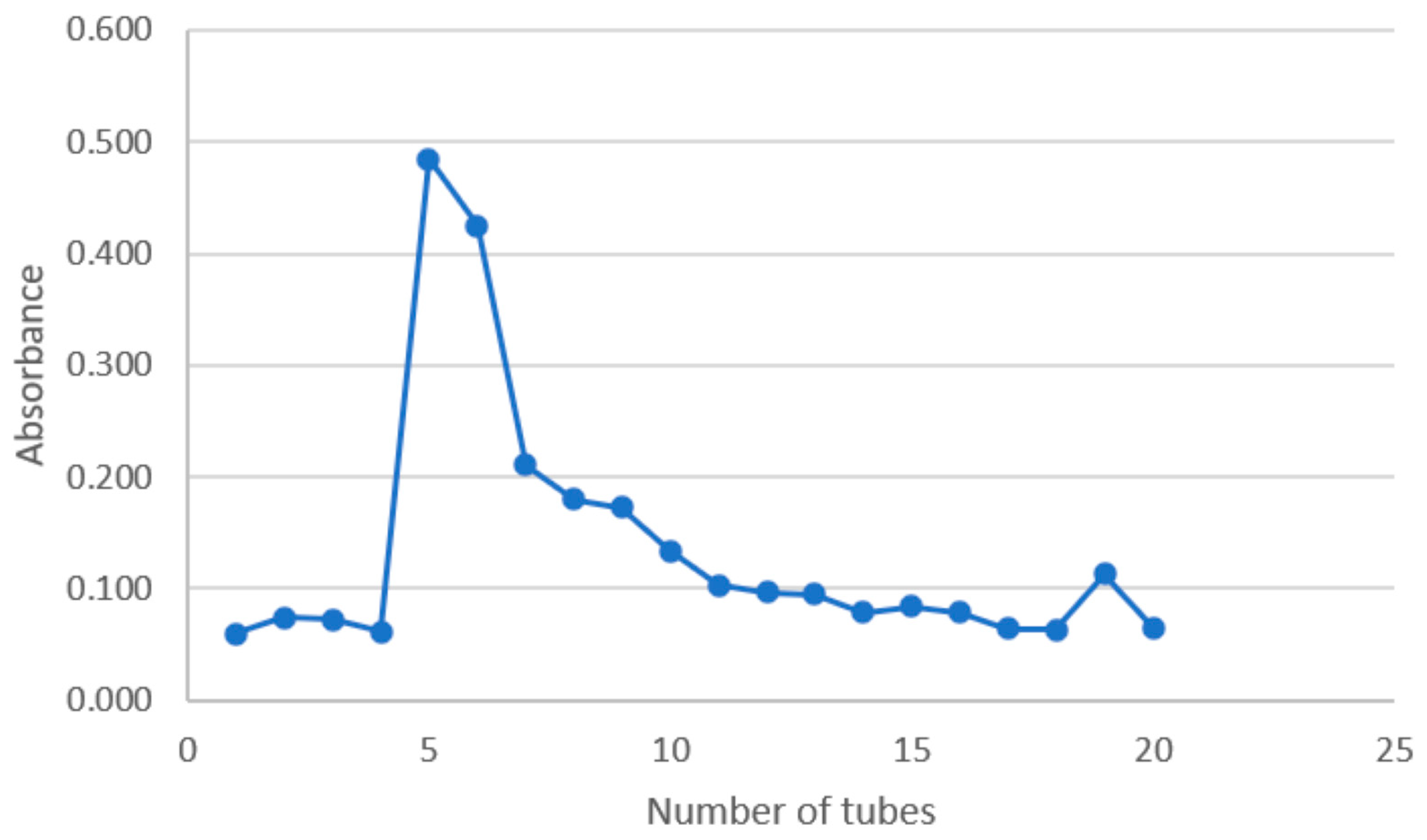
2.1. Isolation and Purification of Polysaccharides
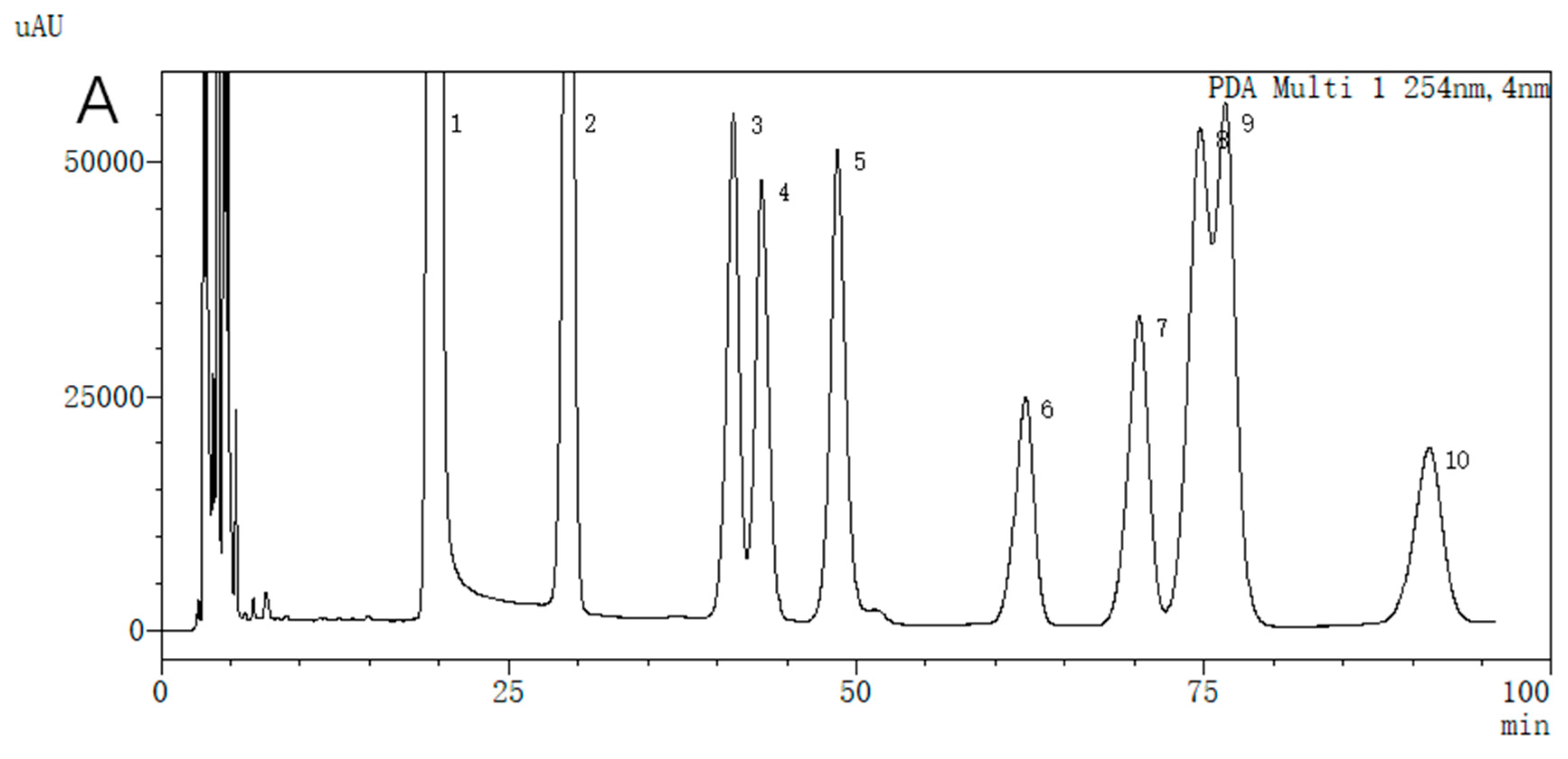
2.2. Monosaccharide Composition
2.3. Molecular Weight Determination Results
2.4. In Vitro Experiments
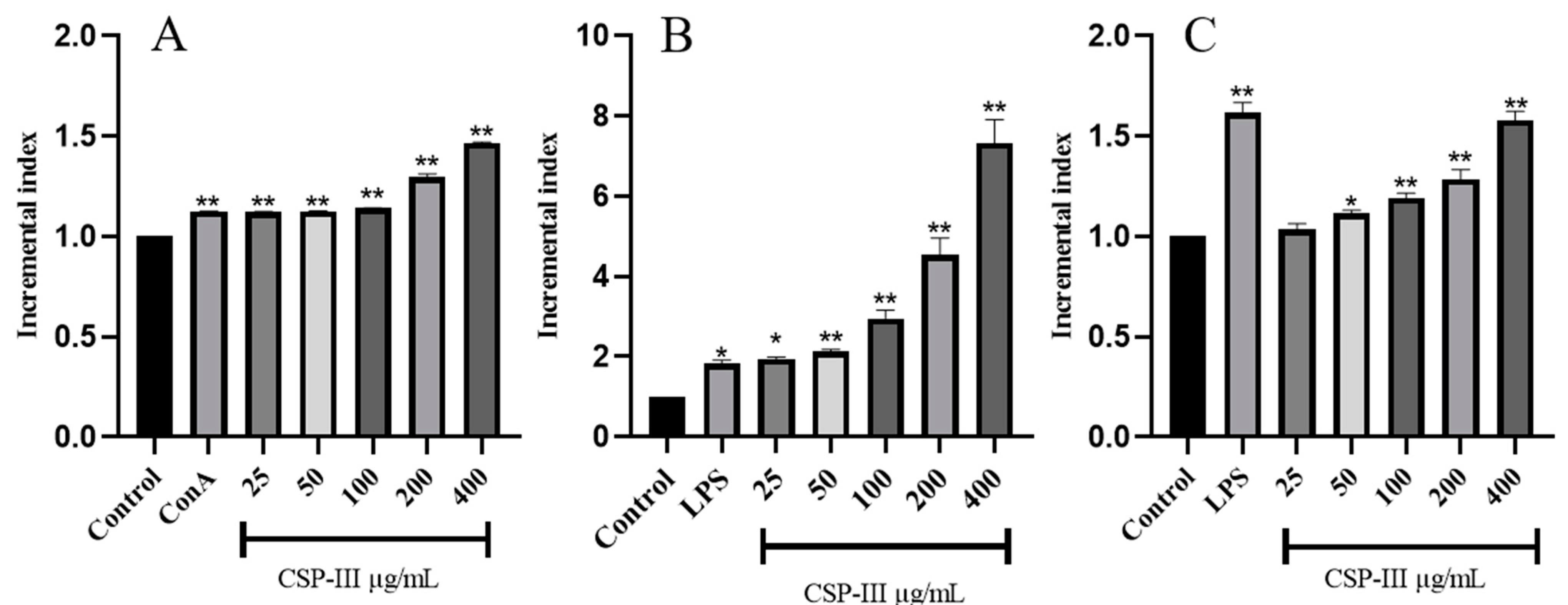
2.4.1. Spleen Lymphocyte Proliferation Assay Results
2.4.2. RAW264.7 Macrophage Proliferation Assay Results
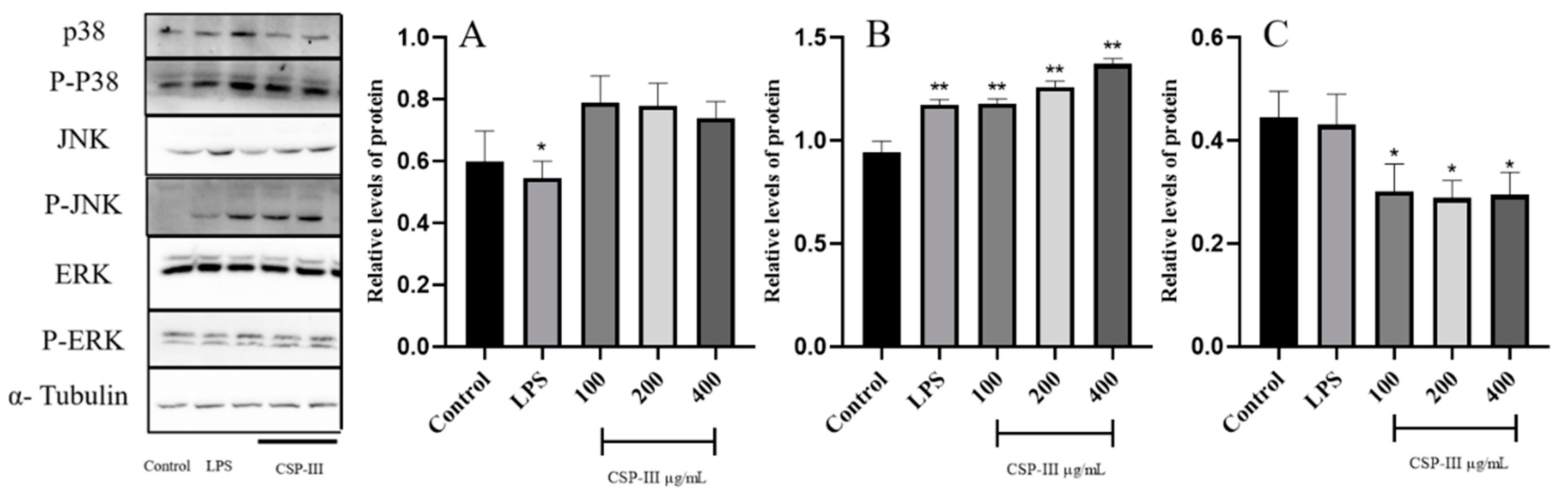
2.4.3. Impact of CSP-III on the Protein Expression of the MAPK Signaling Pathway
2.5. In Vivo Experiments
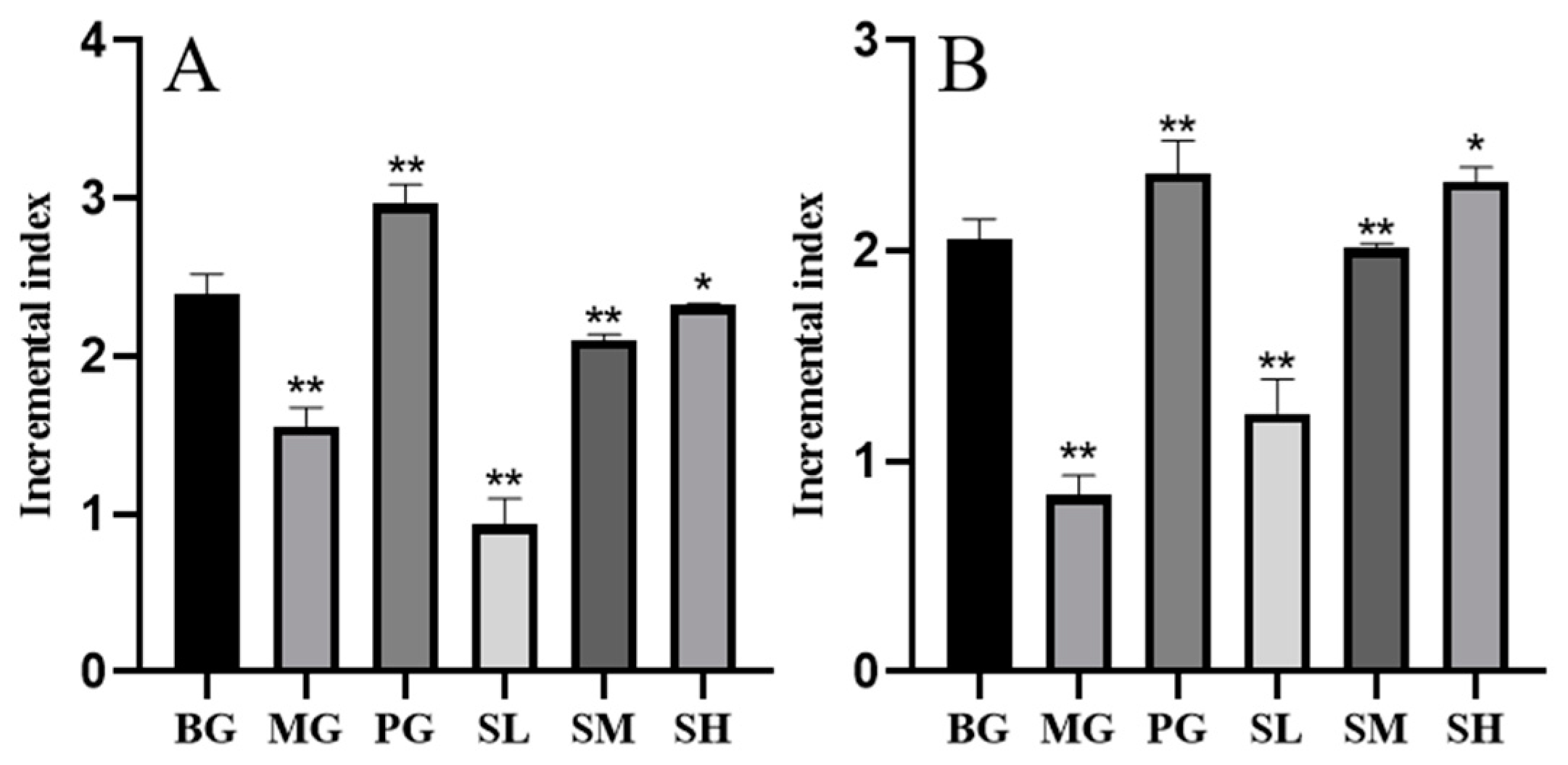
2.5.1. Spleen Lymphocyte Proliferation Assay Results
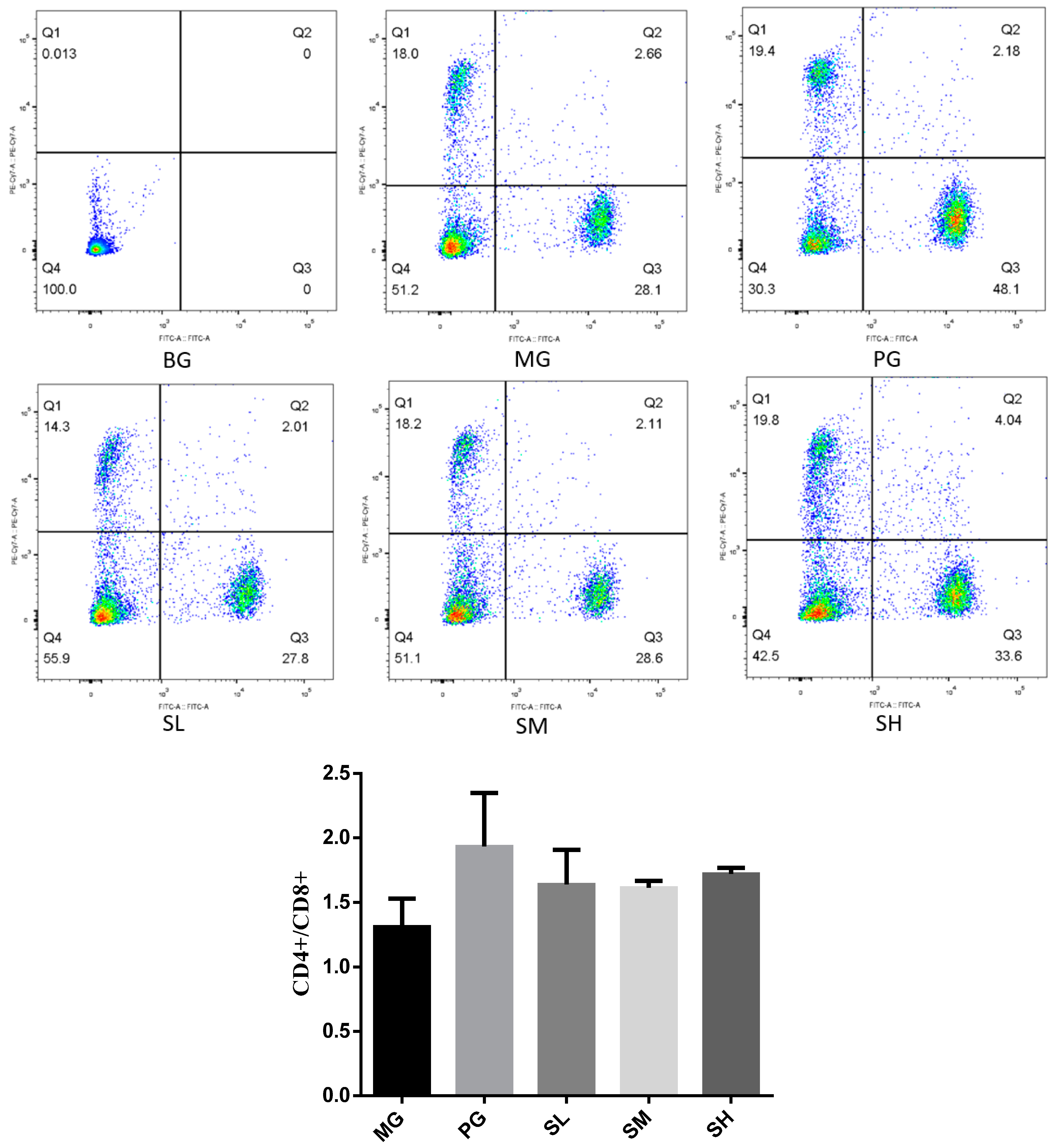
2.5.2. T-Lymphocyte Subsets Detected by Flow Cytometry
2.5.3. Impact of CSP-III on the IgG and IgM Serum Levels in Mice
2.5.4. Effect of CSP III on the Morphological Structure of Mouse Mesenteric Lymph Nodes and Spleen
2.5.5. Effect of CSP III on the Expression of MAPK Signaling Pathway-Related Proteins in the Spleen of Mice
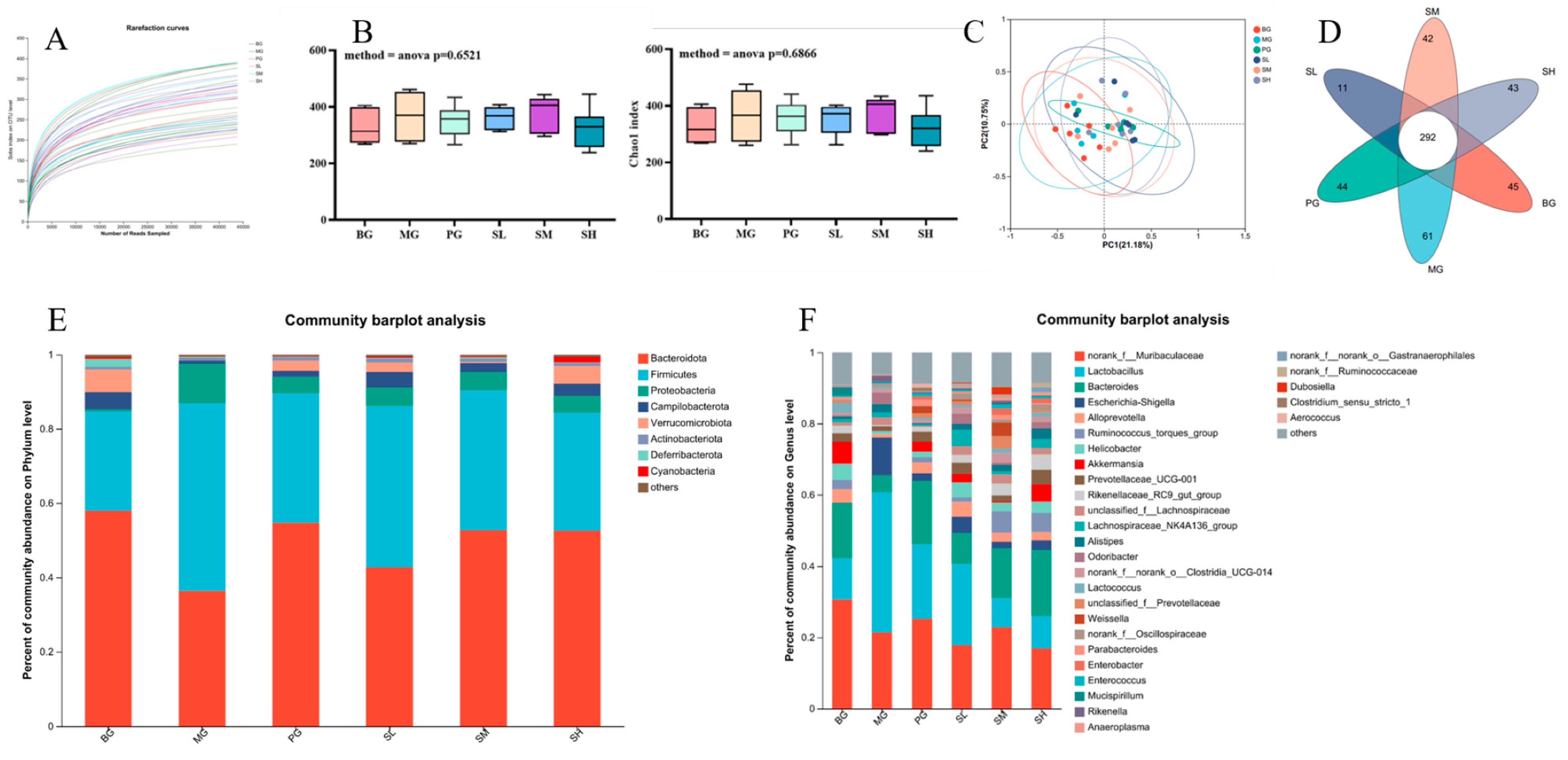
2.5.6. Analysis of Intestinal Flora Data in Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Reagents
4.3. Instruments
4.4. Isolation and Purification of Polysaccharides
4.5. Analysis of Monosaccharide Composition
Preparation of Test Solutions
4.6. Molecular Weight Determination via High-Performance Gel Molecular Exclusion Chromatography
4.6.1. Standard Curve Preparation and Sample Determination
4.6.2. Preparation of Test Solutions
4.6.3. Chromatographic Conditions
4.7. In Vitro Experiments
4.7.1. Splenocyte Proliferation Assay
Culture of Mouse Splenic Lymphocytes
T-Lymphocyte Proliferation Assays
B-Lymphocyte Proliferation Assays
4.7.2. RAW264.7 Macrophage Proliferation
4.7.3. Impact of CSP-III on the Expression of MAPK Signaling Pathway-Related Proteins
4.8. In Vivo Experiments
4.8.1. Animals
4.8.2. Splenic Lymphocyte Proliferation Assay
4.8.3. Identification of the T-Lymphocyte Subset by Flow Cytometry
4.8.4. ELISA Technique for Detecting Serum IgG and IgM Levels in Mice
4.8.5. HE Staining
4.8.6. Western Blotting Assays
4.8.7. Intestinal Flora
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CTX | Cyclophosphamide |
| CSP-I | Purification of polysaccharides using macroporous adsorption resin D101 |
| CSP-II | Purification of polysaccharides using DEAE-52 cellulose column |
| CSP-III | Purification of polysaccharide by Sephadex G-100 gel column chromatography |
| PMP | 1-phenyl-3-methyl-5-pyrazolone |
| Mw | Mass average molar mass |
| PDI | Polymer dispersity index |
| HPLC | High Performance Liquid Chromatography |
| MAPK | Mitogen-activated protein kinase |
| P38 | p38 MAPK |
| ERK | Extracellular regulated protein kinases |
| JNK | c-Jun N-terminal kinase |
| CCK-8 | Cell Counting Kit-8 |
| LPS | Lipopolysaccharide |
| Ig | Immunoglobulin |
| IL | Interleukin |
| ConA | Concanavalin A |
| PBS | Phosphate Buffer |
| TNF | Tumor necrosis factor |
| SCFAs | Short chain fatty acid |
References
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; The Medicine Science and Technology Press of China: Beijing, China, 2020; Volume 1, pp. 57–58. [Google Scholar]
- Mao, X.W.; Gu, Z.R.; Lv, X.; Qi, M.; Ge, B. Research Progress on Resource Chemistry, Pharmacological Effects and Development of Cynomorium cynomorium. Chin. Wild Plant Resour. 2022, 41, 50–54. [Google Scholar]
- Cui, J.-L.; Zhang, Y.Y.; Vijayakumar, V.; Zhang, G.; Wang, M.-L.; Wang, J.-H. Secondary metabolite accumulation associates with ecological succession of endophytic fungi in Cynomorium songaricum Rupr. J. Agric. Food Chem. 2018, 66, 5499–5509. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Guo, Z.; Miao, J.; Wang, Z.; Li, Q.; Chai, X.; Li, M. The genus Cynomorium in China: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2013, 147, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.-J.; Choi, H.-D.; Ha, S.K.; Choi, I.-W.; Park, H.-Y. Immunomodulatory effects of polysaccharide fraction isolated from Fagopyrum esculentum on innate immune systemBiochem. Biophys. Res. Commun. 2018, 496, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ni, H.; Yu, L.; Xu, S.; Bo, R.; Qiu, T.; Gu, P.; Zhu, T.; He, J.; Wusiman, A.; et al. Adjuvant activities of CTAB-modified Polygonatum sibiricum polysaccharide cubosomes on immune responses to ovalbumin in mice. Int. J. Biol. Macromol. 2020, 148, 793–801. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wang, W.-Q.; Wu, J.-Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Foods 2014, 6, 33–47. [Google Scholar] [CrossRef]
- Zhou, Q.Y.; Fang, B.H.; Gao, L.Y.; Zhong, Y.H.; Ling, C.J. Interaction between gut microbiota and natural plant extract. Chin. J. Vet. Sci. 2022, 42, 836–840. [Google Scholar] [CrossRef]
- Yadav, M.; Verma, M.K.; Chauhan, N.S. A review of metabolic potential of human gut microbiome in human nutrition. Arch. Microbiol. 2017, 200, 203–217. [Google Scholar] [CrossRef]
- Edwards, C.A.; Parrett, A.M. Intestinal flora during the first months of life: New perspectives. Brit. J. Nutr. 2002, 88, S11–S18. [Google Scholar] [CrossRef]
- Shen, J.; Obin, M.S.; Zhao, L.P. The gut microbiota, obesity and insulin resistance. Mol. Asp. Med. 2013, 34, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, Y.M.; Zhang, J.; Wang, G.M.; Zhao, Y.P.; Xiu, J.Y. Research progress on interaction of seaweed polysaccharides with intestinal flor. J. Food Saf. Qual. 2022, 13, 5723–5729. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef] [PubMed]
- El Kaoutari, A.; Armougom, F.; Gordon, J.I.; Raoult, D.; Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 2013, 11, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhou, F.; Zheng, X.; Lv, J.; Yu, X.; Zhou, Y.; Li, Q. In vitro fermentation of cereal dietary fibre carbohydrates by probiotic and intestinal bacteria. J. Sci. Food Agric. 2002, 82, 781–789. [Google Scholar] [CrossRef]
- Wang, F.X. Study on Separation, Purification, Structure, Hypoglycemic Activity and Pd Nanoparticle Synthetic Application of Cynomorium Polysaccharide. Ph.D. Thesis, Northwest Normal University, Lanzhou, China, 2016. [Google Scholar]
- Ma, L.J. Study on Anti-Senescence Effects of Cynomorium Songaricum Polysaccharide and Telomerase Regulation Mechanism. Ph.D. Thesis, Inner Mongolia University, Hohhot, Mongolia, 2009. [Google Scholar]
- He, Y.; Li, L.; Chang, H.; Cai, B.; Gao, H.; Chen, G.; Hou, W.; Jappar, Z.; Yan, Y. Research progress onextraction, purification, structure and bio-logical activity of Dendrobium officinale polysaccharides. Front. Nutr. 2022, 9, 965073. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Sun, Y. Antioxidant properties of different molecular weight polysaccharides from Athyrium multidentatum (Doll.) Ching. Carbohydr Polym. 2014, 108, 41–45. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Zhao, B.; Wu, Y.; Wang, C.; Wang, Y. Structural features and hypoglycaemic effects of Cynomorium songaricum polysaccharides on STZ-induced rats. Food Chem. 2010, 120, 443–451. [Google Scholar] [CrossRef]
- Tuvaanjav, S.; Shuqin, H.; Komata, M.; Ma, C.; Kanamoto, T.; Nakashima, H.; Yoshida, T. Isolation and antiviral activity of water-soluble Cynomorium songaricum Rupr. polysaccharides. J. Asian Nat. Prod. Res. 2016, 18, 159–171. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.C. Inflammatory response of macrophages in infection. Hepatobiliary Pancreat Dis Int. 2014, 13, 138–152. [Google Scholar] [CrossRef]
- Vivier, E.; Raulet, D.H.; Moretta, A.; Caligiuri, M.A.; Zitvogel, L.; Lanier, L.L.; Yokoyama, W.M.; Ugolini, S. Innate or Adaptive Immunity? The Example of Natural Killer Cells. Am. Assoc. Adv. Sci. 2011, 331, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M. Lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. Immunol. Rev. 2010, 238, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Richardson, B.C. The MAPK signalling pathways and colorectal cancer. Lancet Oncol. 2005, 6, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Safa, A.; Abak, A.; Shoorei, H.; Taheri, M.; Ghafouri-Fard, S. MicroRNAs as regulators of ERK/MAPK pathway: A comprehensive review. Biomed. Pharmacother. 2020, 132, 110853. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.-Y.; Koh, M.-S.; Moon, A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin. Investig. Drugs 2009, 18, 1893–1905. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Nebreda, Á.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef]
- Kong, X.F.; Hu, Y.L.; Song, D.L. Research progress in immunopharmacology of Astragalus polysaccharides. Chin. J. Tradit. Vet. Sci. 2023, 3, 34–37. [Google Scholar] [CrossRef]
- Xiu, T.; Zuo, A.X.; Lin, W.Z.; Liu, P.H. Isolation, Purification, Chemical Composition and Antioxidant Activities of Polysaccharides from Dictyosphaerium sp. 1A10. Food Res. Dev. 2023, 44, 57–65. [Google Scholar]
- Shao, X. Inflammatory Activity Small Molecule Garlic Polysaccharide and Its Anti-Isolation, Purification, Structural Identification of a Novel. Ph.D. Thesis, South China University of Technology, Guangzhou, China, 2021. [Google Scholar] [CrossRef]
- Yu, Y.T.; Pi, W.X.; Xie, H.; Cao, L.; Li, X.; Lu, T.; Yan, G. Content Determination of 6 Kinds of Monosaccharide in Astragalus membranaceus with Different GrowthYears by Pre-column Derivatization-HPLC. China Pharm. 2021, 32, 1448–1452. [Google Scholar] [CrossRef]
- Zheng, R. Extraction, Isolation, Biological Activity and Structure Analysis of Polysaccharides from Acanthopanax sessiliflorus. Master’s Thesis, Changchun University of Chinese Medicine, Changchun, China, 2022. [Google Scholar] [CrossRef]
- Liu, Y. Study on Molecular Epidemiology and PCV2/PCV3 Cap Genetically Engineered Candidate Subunit Vaccines of PCV2 and PCV3. Ph.D. Thesis, Jilin Agricultural University, Changchun, China, 2022. [Google Scholar] [CrossRef]
- Qiao, S.; Min, S.M.; Li, R.N.; Yu, C.S.; Zhang, Y.D.; Huang, Y.F.; Ma, Y.F. Study on Radix Pseudostellaria Fibrous Root Polysaccharide Promoting Proliferation and Cytokine Secretion of Mouse Spleen Lymphocytes in vitro. Prog. Vet. Med. 2022, 43, 73–77. [Google Scholar] [CrossRef]
- Yang, Y. Effects of Sika Deer Antler Protein on Intestinal Flora and Immune Regulation Mechanism of Kidney-Yang Deficiency Mice. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2022. [Google Scholar] [CrossRef]
- Li, H.Y.; Ji, P.; Wei, Y.M.; Mai, Z.H.; Zhang, X.S.; Zhou, K.; Wu, F.L.; Zhao, N.S.; Hua, Y.L.; Yao, W.L.; et al. Effects of Jiawei Yupingfeng and Its Polysaccharides on Immune Function in Mice. Prog. Vet. Med. 2022, 43, 73–79. [Google Scholar] [CrossRef]





| Sample | Total Sugar Content (%) |
|---|---|
| CSP-II-1 | 7.28 |
| CSP-II-2 | 20.07 |
| CSP-II-3 | 79.50 |
| CSP-II-4 | 36.22 |
| CSP-II-5 | 5.87 |
| Condition | |
|---|---|
| Column temperature | 40 °C |
| Detection wavelength | 250 nm |
| Velocity of flow | 0.8 mL/min |
| Sample injection volume | 20 μL |
| Mobile phase A | Phosphate buffer solution (pH 6.8)—acetonitrile (85:15) |
| Mobile phase B | Phosphate buffer solution (pH 6.8)—acetonitrile (60:40) |
| Condition | |
|---|---|
| Chromatographic column | SRT SEC-100 (7.8 mm × 300 mm) gel column |
| Detector | Oscillometric refractive detector |
| Column temperature | 35 °C |
| Velocity of flow | 0.5 mL/min |
| Sample injection volume | 20 μL |
| Mobile phase | Ultrapure water |
| Antibody Name | Dilution Ratio |
|---|---|
| Mouse Anti-p38 monoclonal antibody | 1:1000 |
| Rabbit Anti-P-p38 monoclonal antibody | 1:1000 |
| Mouse Anti-JNK monoclonal antibody | 1:1000 |
| Rabbit Anti-P-JNK polyclonal antibody | 1:1000 |
| Rabbit Anti-ERK monoclonal antibody | 1:1000 |
| Rabbit Anti-P-ERK monoclonal antibody | 1:1000 |
| Mouse Anti-α-Tubulin monoclonal antibody | 1:5000 |
| Goat Anti-Rabbit IgG | 1:6000 |
| Goat Anti-Mouse IgG | 1:6000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, T.; Chen, J.; He, Z.; Chen, W.; Zong, Y.; Du, R. Studies of the Immunomodulatory Activity of Polysaccharides from the Stem of Cynomorium songaricum Based on Intestinal Microbial Analysis. Molecules 2024, 29, 143. https://doi.org/10.3390/molecules29010143
Lv T, Chen J, He Z, Chen W, Zong Y, Du R. Studies of the Immunomodulatory Activity of Polysaccharides from the Stem of Cynomorium songaricum Based on Intestinal Microbial Analysis. Molecules. 2024; 29(1):143. https://doi.org/10.3390/molecules29010143
Chicago/Turabian StyleLv, Tong, Jiarong Chen, Zhongmei He, Weijia Chen, Ying Zong, and Rui Du. 2024. "Studies of the Immunomodulatory Activity of Polysaccharides from the Stem of Cynomorium songaricum Based on Intestinal Microbial Analysis" Molecules 29, no. 1: 143. https://doi.org/10.3390/molecules29010143
APA StyleLv, T., Chen, J., He, Z., Chen, W., Zong, Y., & Du, R. (2024). Studies of the Immunomodulatory Activity of Polysaccharides from the Stem of Cynomorium songaricum Based on Intestinal Microbial Analysis. Molecules, 29(1), 143. https://doi.org/10.3390/molecules29010143






