Bioprospecting of Selected Species of Polypore Fungi from the Western Balkans
Abstract
:1. Introduction
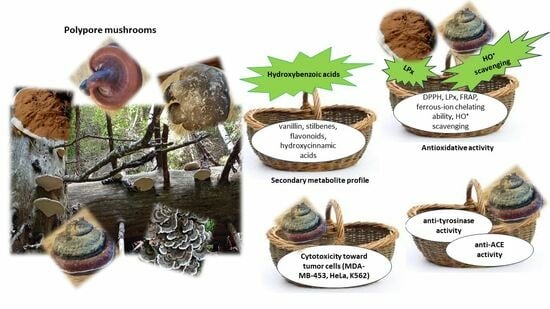
2. Results
2.1. Extraction Yields and Secondary Metabolite Content
Phenolic Profile of the Methanol Extracts
2.2. Antioxidant Potential
2.2.1. Scavenging Ability
2.2.2. LPx Inhibition
2.2.3. FRAP Assay
2.2.4. Capability to Chelate Fe2+
2.2.5. Correlation between Antioxidant Activity and Components of Methanol Extracts
2.3. Enzyme Inhibition
2.3.1. Tyrosinase Inhibitory Potential
2.3.2. Anti-ACE Potential
2.4. In Vitro Cytotoxic Activity
Selectivity Coefficient in the Cytotoxicity against Tumor Cells
3. Discussion
3.1. Polyphenols
3.2. Lycopene and β-Carotene
3.3. Vitamin C
4. Materials and Methods
4.1. Collection, Identification, and Drying of Mushroom Fruiting Bodies
4.2. Preparation of Extracts
4.3. Secondary Metabolites Analysis
4.3.1. Total Polyphenol Content (TPC)
4.3.2. Vitamin C Content
4.3.3. Determination of β-Carotene and Lycopene Content
4.3.4. LC-MS/MS of Phenolics
4.4. Determination of Antioxidant Potential
4.5. Enzyme Inhibition Activities
4.6. In Vitro Cytotoxic Activity
Selectivity Coefficient in the Cytotoxic/Anti-Tumor Action
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giusti, A.; Tinacci, L.; Verdigi, F.; Narducci, R.; Gasperetti, L.; Armani, A. Safety and Commercial Issues in Fresh Mushrooms and Mushroom-Based Products Sold at Retail in Tuscany Region. Ital. J. Food Saf. 2022, 11, 10044. [Google Scholar] [CrossRef] [PubMed]
- European Parliament. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliam. Off. J. Eur. Union 2015, L327, 1–22. [Google Scholar]
- Vunduk, J.; Tura, D.; Biketova, A.Y. Medicinal Mushroom Nutraceutical Commercialization: Two Sides of a Coin. In Wild Mushrooms Characteristics, Nutrition, and Processing; Dhull, S.B., Bains, A., Chawla, P., Sadh, P.K., Eds.; CRC Press: Boca Raton, FL, USA, 2022; p. 43. ISBN 9781003152583. [Google Scholar]
- Almádi, B. The Producer and Consumer Aspects of the Mushroom Sector in Hungary; Szent István University: Gödöllő, Hungary, 2021. [Google Scholar]
- Bringye, B.; Fekete-Farkas, M.; Vinogradov, S. An Analysis of Mushroom Consumption in Hungary in the International Context. Agriculture 2021, 11, 677. [Google Scholar] [CrossRef]
- Hola, B.; Murshed, R.; Jbour, M. Chemical Composition and Antioxidant Activity of Some Syrian Wild Mushroom (Agaricus spp.) Strains. Sci. Rep. 2023, 13, 15896. [Google Scholar] [CrossRef]
- Campi, M.; Mancuello, C.; Ferreira, F.; Maubet, Y.; Cristaldo, E.; Gayoso, E.; Robledo, G. Does the Source Matter? Phenolic Compounds and Antioxidant Activity from Mycelium in Liquid Medium, Wild and Cultivated Fruiting Bodies of the Neotropical Species Ganoderma Tuberculosum. J. Microbiol. Biotechnol. Food Sci. 2023, 13, e6148. [Google Scholar] [CrossRef]
- Molitorisová, A.; Monaco, A.; Purnhagen, K.P. An Analysis of the Regulatory Framework Applicable to Products Obtained from Mushroom and Mycelium. SSRN Electron. J. 2021, 3955899. [Google Scholar] [CrossRef]
- Vunduk, J.; Klaus, A.; Kozarski, M.; Petrovic, P.; Zizak, Z.; Niksic, M.; Van Griensven, L.J.L.D. Did the Iceman Know Better? Screening of the Medicinal Properties of the Birch Polypore Medicinal Mushroom, Piptoporus betulinus (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 1113–1125. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Szewczyk, A.; Galanty, A.; Gdula-Argasińska, J.; Muszyńska, B. Chemical Composition and Biological Activity of Extracts from Fruiting Bodies and Mycelial Cultures of Fomitopsis Betulina. Mol. Biol. Rep. 2018, 45, 2535–2544. [Google Scholar] [CrossRef]
- Bishop, K.S. Characterisation of Extracts and Anti-Cancer Activities of Fomitopsis pinicola. Nutrients 2020, 12, 609. [Google Scholar] [CrossRef]
- Darkal, A.K.; Zuraik, M.M.; Ney, Y.; Nasim, M.J.; Jacob, C. Unleashing the Biological Potential of Fomes fomentarius via Dry and Wet Milling. Antioxidants 2021, 10, 303. [Google Scholar] [CrossRef]
- Hossain, M.S.; Barua, A.; Tanim, M.A.H.; Hasan, M.S.; Islam, M.J.; Hossain, M.R.; Emon, N.U.; Hossen, S.M.M. Ganoderma Applanatum Mushroom Provides New Insights into the Management of Diabetes Mellitus, Hyperlipidemia, and Hepatic Degeneration: A Comprehensive Analysis. Food Sci. Nutr. 2021, 9, 4364–4374. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Lazarevic, N.; Novakovic, J.; Jeremic, N.; Jakovljevic, V.; Zivkovic, V.; Bradic, J.; Pecarski, D.; Tel-Çayan, G.; Glamocija, J.; et al. Characterization, In Vitro Biological Activity and In Vivo Cardioprotective Properties of Trametes Versicolor (L.:Fr.) Quél. Heteropolysaccharides in a Rat Model of Metabolic Syndrome. Pharmaceuticals 2023, 16, 787. [Google Scholar] [CrossRef] [PubMed]
- Vunduk, J.; Klaus, A.; Lazić, V.; Kozarski, M.; Radić, D.; Šovljanski, O.; Pezo, L. Artificial Neural Network Prediction of Antiadhesion and Antibiofilm-Forming Effects of Antimicrobial Active Mushroom Extracts on Food-Borne Pathogens. Antibiotics 2023, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Luyten, W. Medicinal Mushrooms: Clinical Perspective and Challenges. Drug Discov. Today 2022, 27, 636–651. [Google Scholar] [CrossRef] [PubMed]
- PDQ Integrative—Alternative and Complementary Therapies Editorial Board. Medicinal Mushrooms (PDQ®): Health Professional Version; National Cancer Institute: Bethesda, MD, USA, 2002. [Google Scholar]
- Pleszczyńska, M.; Wiater, A.; Siwulski, M.; Lemieszek, M.K.; Kunaszewska, J.; Kaczor, J.; Rzeski, W.; Janusz, G.; Szczodrak, J. Cultivation and Utility of Piptoporus betulinus Fruiting Bodies as a Source of Anticancer Agents. World J. Microbiol. Biotechnol. 2016, 32, 151. [Google Scholar] [CrossRef] [PubMed]
- Den Herder, M. Chaga Cultivation. Available online: https://efi.int/sites/default/files/files/knowledge/projects/EFI_Chaga_Cultivation_EN.pdf (accessed on 25 June 2023).
- Macáková, K.; Opletal, L.; Polásek, M.; Samková, V. Free-Radical Scavenging Activity of Some European Polyporales. Nat. Prod. Commun. 2010, 5, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Onar, O.; Akata, I.; Celep, G.S.; Yildirim, O. Antioxidant Activity of Extracts from the Red-Belt Conk Medicinal Mushroom, Fomitopsis Pinicola (Agaricomycetes), and Its Modulatory Effects on Antioxidant Enzymes. Int. J. Med. Mushrooms 2016, 18, 501–508. [Google Scholar] [CrossRef]
- Reis, F.S.; Pereira, E.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Biomolecule Profiles in Inedible Wild Mushrooms with Antioxidant Value. Molecules 2011, 16, 4328–4338. [Google Scholar] [CrossRef]
- Janjušević, L.; Karaman, M.; Šibul, F.; Tommonaro, G.; Iodice, C.; Jakovljević, D.; Pejin, B. The Lignicolous fungus Trametes Versicolor (L.) Lloyd (1920): A Promising Natural Source of Antiradical and AChE Inhibitory Agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 355–362. [Google Scholar] [CrossRef]
- Matijašević, D.; Pantić, M.; Rašković, B.; Pavlović, V.; Duvnjak, D.; Sknepnek, A.; Nikšić, M. The Antibacterial Activity of Coriolus Versicolor Methanol Extract and Its Effect on Ultrastructural Changes of Staphylococcus aureus and Salmonella enteritidis. Front. Microbiol. 2016, 7, 1226. [Google Scholar] [CrossRef]
- Adhikari, M.; Bhusal, S.; Pandey, M.; Raut, J.; Bhatt, L. Mycochemical and Nutritional Analysis of Selected Wild Mushrooms from Gaurishankar Conservation Area. Int. J. Pharmacogn. Chin. Med. 2019, 3, 000169. [Google Scholar]
- Davey, M.W.; Van Montagu, M.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.; Strain, J.J.; Favell, D.; Fletcher, J. PlantL-Ascorbic Acid: Chemistry, Function, Metabolism, Bioavailability and Effects of Processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic Acid (SA)—A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K.; et al. Discovering Multifaceted Role of Vanillic Acid beyond Flavours: Nutraceutical and Therapeutic Potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Antibacterial, Antiradical Potential and Phenolic Compounds of Thirty-One Polish Mushrooms. PLoS ONE 2015, 10, e0140355. [Google Scholar] [CrossRef] [PubMed]
- Çayan, F.; Deveci, E.; Tel-Çayan, G.; Duru, M.E. Identification and Quantification of Phenolic Acid Compounds of Twenty-Six Mushrooms by HPLC–DAD. J. Food Meas. Charact. 2020, 14, 1690–1698. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; van Griensven, L.; Jakovljevic, D.; Todorovic, N.; Wan-Mohtar, W.A.A.Q.I.; Vunduk, J. Mushroom β-Glucan and Polyphenol Formulations as Natural Immunity Boosters and Balancers: Nature of the Application. Food Sci. Hum. Wellness 2023, 12, 378–396. [Google Scholar] [CrossRef]
- Erbiai, E.H.; Amina, B.; Kaoutar, A.; Saidi, R.; Lamrani, Z.; Pinto, E.; Esteves da Silva, J.C.G.; Maouni, A.; Pinto da Silva, L. Chemical Characterization and Evaluation of Antimicrobial Properties of the Wild Medicinal Mushroom Ganoderma Lucidum Growing in Northern Moroccan Forests. Life 2023, 13, 1217. [Google Scholar] [CrossRef]
- Siddeeg, A.; AlKehayez, N.M.; Abu-Hiamed, H.A.; Al-Sanea, E.A.; AL-Farga, A.M. Mode of Action and Determination of Antioxidant Activity in the Dietary Sources: An Overview. Saudi J. Biol. Sci. 2021, 28, 1633–1644. [Google Scholar] [CrossRef]
- Karaman, M.; Jovin, E.; Malbasa, R.; Matavuly, M.; Popović, M. Medicinal and Edible Lignicolous Fungi as Natural Sources of Antioxidative and Antibacterial Agents. Phytother. Res. 2010, 24, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Tumbas Šaponjac, V.; Čanadanović-Brunet, J.; Ćetković, G.; Djilas, S.; Četojević-Simin, D. Dried Bilberry (Vaccinium myrtillus L.) Extract Fractions as Antioxidants and Cancer Cell Growth Inhibitors. LWT Food Sci. Technol. 2015, 61, 615–621. [Google Scholar] [CrossRef]
- Poli, A.; Agostoni, C.; Visioli, F. Dietary Fatty Acids and Inflammation: Focus on the n-6 Series. Int. J. Mol. Sci. 2023, 24, 4567. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; van Griensven, L. Oxidative Stress Prevention by Edible Mushrooms and Their Role in Cellular Longevity. In Wild Mushrooms Characteristics, Nutrition, and Processing; Dhull, S.B., Bains, A., Chawla, P., Sadh, P.K., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 319–348. ISBN 9781003152583. [Google Scholar]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Niksic, M.; Jakovljevic, D.; Helsper, J.P.F.G.; Van Griensven, L.J.L.D. Antioxidative and Immunomodulating Activities of Polysaccharide Extracts of the Medicinal Mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011, 129, 1667–1675. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Zengin, G.; Gunia-Krzyżak, A.; Popiół, J.; Szewczyk, A.; Jaszek, M.; Rogalski, J.; Muszyńska, B. Bioactivity and Mycochemical Profile of Extracts from Mycelial Cultures of Ganoderma Spp. Molecules 2022, 27, 275. [Google Scholar] [CrossRef] [PubMed]
- Mau, J.-L.; Lin, H.-C.; Chen, C.-C. Antioxidant Properties of Several Medicinal Mushrooms. J. Agric. Food Chem. 2002, 50, 6072–6077. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Baek, N.; Nam, T. Natural, Semisynthetic and Synthetic Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2016, 31, 1–13. [Google Scholar] [CrossRef]
- Itharat, A.; Houghton, P.J.; Eno-Amooquaye, E.; Burke, P.; Sampson, J.H.; Raman, A. In Vitro Cytotoxic Activity of Thai Medicinal Plants Used Traditionally to Treat Cancer. J. Ethnopharmacol. 2004, 90, 33–38. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.; van Griensven, L. Antioxidants of Edible Mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. Carotenoid Action on the Immune Response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, S.; Klicic Badoux, J.; Scott-Boyer, M.-P.; Parolo, S.; Matone, A.; Priami, C.; Morine, M.J.; Kaput, J.; Moco, S. A Computationally Driven Analysis of the Polyphenol-Protein Interactome. Sci. Rep. 2018, 8, 2232. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Zöll, M.; Peintner, U.; Rollinger, J.M. European Medicinal Polypores—A Modern View on Traditional Uses. J. Ethnopharmacol. 2014, 154, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, M.; Huang, Y.; Peng, Z. Synthesis and Biological Evaluation of New Kojic Acid-1,3,4-Oxadiazole Hybrids as Tyrosinase Inhibitors and Their Application in the Anti-Browning of Fresh-Cut Mushrooms. Food Chem. 2023, 409, 135275. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.H.A.; Salgado, H.R.N. Gallic Acid: Review of the Methods of Determination and Quantification. Crit. Rev. Anal. Chem. 2016, 46, 257–265. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Olasehinde, T.A.; Koorbanally, N.A.; Islam, M.S. Vanillin and Vanillic Acid Modulate Antioxidant Defense System via Amelioration of Metabolic Complications Linked to Fe2+-Induced Brain Tissues Damage. Metab. Brain Dis. 2020, 35, 727–738. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of Polyphenols with Carbohydrates, Lipids and Proteins. Food Chem. 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Theivendren, P.; Kunjiappan, S.; Mariappa Hegde, Y.; Vellaichamy, S.; Gopal, M.; Rajan Dhramalingam, S.; Kumar, S. Importance of Protein Kinase and Its Inhibitor: A Review. In Protein Kinases—Promising Targets for Anticancer Drug Research; Kumar Singh, R., Ed.; IntechOpen: London, UK, 2021. [Google Scholar]
- Kciuk, M.; Alam, M.; Ali, N.; Rashid, S.; Głowacka, P.; Sundaraj, R.; Celik, I.; Yahya, E.B.; Dubey, A.; Zerroug, E.; et al. Epigallocatechin-3-Gallate Therapeutic Potential in Cancer: Mechanism of Action and Clinical Implications. Molecules 2023, 28, 5246. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Wan-Mohtar, W.; Niksic, M. Ganoderma Lucidumas a Cosmeceutical: Antiradical Potential and Inhibitory Effect on Hyperpigmentation and Skin Extracellular Matrix Degradation Enzymes. Arch. Biol. Sci. 2019, 71, 253–264. [Google Scholar] [CrossRef]
- Srisuksomwong, P.; Kaenhin, L.; Mungmai, L. Collagenase and Tyrosinase Inhibitory Activities and Stability of Facial Cream Formulation Containing Cashew Leaf Extract. Cosmetics 2023, 10, 17. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Hyoung Lee, D.; Ho Kim, J.; Sik Park, J.; Jun Choi, Y.; Soo Lee, J. Isolation and Characterization of a Novel Angiotensin I-Converting Enzyme Inhibitory Peptide Derived from the Edible Mushroom Tricholoma Giganteum. Peptides 2004, 25, 621–627. [Google Scholar] [CrossRef]
- Choi, H.; Cho, H.; Yang, H.; Ra, K.; Suh, H. Angiotensin I-Converting Enzyme Inhibitor from Grifola Frondosa. Food Res. Int. 2001, 34, 177–182. [Google Scholar] [CrossRef]
- Tran, H.-B.; Yamamoto, A.; Matsumoto, S.; Ito, H.; Igami, K.; Miyazaki, T.; Kondo, R.; Shimizu, K. Hypotensive Effects and Angiotensin-Converting Enzyme Inhibitory Peptides of Reishi (Ganoderma Lingzhi) Auto-Digested Extract. Molecules 2014, 19, 13473–13485. [Google Scholar] [CrossRef] [PubMed]
- Cateni, F.; Gargano, M.L.; Procida, G.; Venturella, G.; Cirlincione, F.; Ferraro, V. Mycochemicals in Wild and Cultivated Mushrooms: Nutrition and Health. Phytochem. Rev. 2022, 21, 339–383. [Google Scholar] [CrossRef]
- Mohanta, T.K. Fungi Contain Genes Associated with Flavonoid Biosynthesis Pathway. J. Funct. Foods 2020, 68, 103910. [Google Scholar] [CrossRef]
- Shao, Y.; Guo, H.; Zhang, J.; Liu, H.; Wang, K.; Zuo, S.; Xu, P.; Xia, Z.; Zhou, Q.; Zhang, H.; et al. The Genome of the Medicinal Macrofungus Sanghuang Provides Insights into the Synthesis of Diverse Secondary Metabolites. Front. Microbiol. 2020, 10, 3035. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal Secondary Metabolism: Regulation, Function and Drug Discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and Human Health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Beta Carotene (Oral Route) Proper Use—Mayo Clinic. Available online: https://www.mayoclinic.org/drugs-supplements/beta-carotene-oral-route/proper-use/drg-20066795 (accessed on 15 November 2023).
- Nutrients: Carotene, Beta (Μg). Available online: https://ods.od.nih.gov/pubs/usdandb/VitA-betaCarotene-Content.pdf (accessed on 15 November 2023).
- Einafshar, S.; Poorazrang, H.; Farhoosh, R.; Seiedi, S.M. Antioxidant Activity of the Essential Oil and Methanolic Extract of Cumin Seed (Cuminum cyminum). Eur. J. Lipid Sci. Technol. 2012, 114, 168–174. [Google Scholar] [CrossRef]
- Anantharaman, A.; Hemachandran, H.; Priya, R.R.; Sankari, M.; Gopalakrishnan, M.; Palanisami, N.; Siva, R. Inhibitory Effect of Apocarotenoids on the Activity of Tyrosinase: Multi-Spectroscopic and Docking Studies. J. Biosci. Bioeng. 2016, 121, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Gloria, N.F.; Soares, N.; Brand, C.; Oliveira, F.L.; Borojevic, R.; Teodoro, A.J. Lycopene and Beta-Carotene Induce Cell-Cycle Arrest and Apoptosis in Human Breast Cancer Cell Lines. Anticancer Res. 2014, 34, 1377–1386. [Google Scholar]
- Rowles, J.L.; Erdman, J.W. Carotenoids and Their Role in Cancer Prevention. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158613. [Google Scholar] [CrossRef]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kader, A.A. Preharvest and Postharvest Factors Influencing Vitamin C Content of Horticultural Crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Gonzalez, M.J.; Miranda-Massari, J.R.; Olalde, J. Vitamin C and Mitochondrial Function in Health and Exercise. In Molecular Nutrition and Mitochondria; Elsevier: Amsterdam, The Netherlands, 2023; pp. 225–242. [Google Scholar]
- Shterman, N.; Kupfer, B.; Moroz, C. Comparison of Transferrin Receptors, Iron Content and Isoferritin Profile in Normal and Malignant Human Breast Cell Lines. Pathobiology 1991, 59, 19–25. [Google Scholar] [CrossRef]
- Lui, G.Y.L.; Kovacevic, Z.; Richardson, V.; Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Targeting Cancer by Binding Iron: Dissecting Cellular Signaling Pathways. Oncotarget 2015, 6, 18748–18779. [Google Scholar] [CrossRef]
- Lim, J.Y.; Kim, D.; Kim, B.R.; Jun, J.S.; Yeom, J.S.; Park, J.S.; Seo, J.-H.; Park, C.H.; Woo, H.O.; Youn, H.-S.; et al. Vitamin C Induces Apoptosis in AGS Cells via Production of ROS of Mitochondria. Oncol. Lett. 2016, 12, 4270–4276. [Google Scholar] [CrossRef]
- Uzelac, B. Gljive Srbije i Zapadnog Balkana; BGV Logik: Belgrade, Serbia, 2009; ISBN 9788691267704. (In Serbian) [Google Scholar]
- Chen, B.; Ke, B.; Ye, L.; Jin, S.; Jie, F.; Zhao, L.; Wu, X. Isolation and Varietal Characterization of Ganoderma resinaceum from Areas of Ganoderma lucidum Production in China. Sci. Hortic. 2017, 224, 109–114. [Google Scholar] [CrossRef]
- Vunduk, J.; Kozarski, M.; Djekic, I.; Tomašević, I.; Klaus, A. Effect of Modified Atmosphere Packaging on Selected Functional Characteristics of Agaricus bisporus. Eur. Food Res. Technol. 2021, 247, 829–838. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Vunduk, J.; Zizak, Z.; Niksic, M.; Jakovljevic, D.; Vrvic, M.M.; Van Griensven, L.J.L.D. Nutraceutical Properties of the Methanolic Extract of Edible Mushroom Cantharellus cibarius (Fries): Primary Mechanisms. Food Funct. 2015, 6, 1875–1886. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Vunduk, J.; Nikšić, M. The Influence of Mushroom Coriolus Versicolor and Hazelnuts Enrichment on Antioxidant Activities and Bioactive Content of Dark Chocolate. Food Feed Res. 2020, 47, 23–32. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Špirović-Trifunović, B.; Vuković, G.; Ćurić, A.; Bursić, V.; Petrović, A.; Marinković, D. Influence of Climate Change on Fumonizine Production. Biljn. Lek. 2022, 50, 127–139. [Google Scholar] [CrossRef]
- Šćepanović, M.; Sarić-Krsmanović, M.; Šoštarčić, V.; Brijačak, E.; Lakić, J.; Špirović Trifunović, B.; Gajić Umiljendić, J.; Radivojević, L. Inhibitory Effects of Brassicaceae Cover Crop on Ambrosia Artemisiifolia Germination and Early Growth. Plants 2021, 10, 794. [Google Scholar] [CrossRef]






| Extract | Yield (mg g−1) α | Total Content (mg g−1) β | Total Content (μg g−1) β | ||
|---|---|---|---|---|---|
| Phenols δ | Vitamin C | β-Carotene | Lycopene | ||
| F. betulina | 260.5 ± 15.3 Bγ | 32.6 ± 2.1 C | 1.9 ± 0.1 B | 51.7 ± 6.2 E | 23.8 ± 2.6 E |
| F. pinicola | 360.3 ± 20.1 A | 133.1 ± 6.2 A | 2.0 ± 0.2 B | 541.5 ± 21.1 A | 265.7 ± 13.1 A |
| G. applanatum | 120.2 ± 10.3 C | 39.4 ± 1.8 B | 1.2 ± 0.1 C | 188.6 ± 13.5 C | 57.5 ± 4.7 D |
| G. lucidum | 100.8 ± 7.9 D | 38.6 ± 2.6 B | 3.0 ± 0.1 A | 372.2 ± 18.9 B | 224.6 ± 17.3 B |
| C. versicolor | 60.7 ± 5.8 E | 10.5 ± 0.9 D | 3.1 ± 0.2 A | 158.7 ± 10.0 D | 109.2 ± 8.2 C |
| Compounds | F. betulina | F.pinicola | G. applanatum | G.lucidum | C.versicolor |
|---|---|---|---|---|---|
| Simple phenols/ derivative (μg g−1) | |||||
| Vanillin | n.d. | 1.06 ± 0.02 Bγ | 1.81 ± 0.07 A | 0.81 ± 0.01 C | 0.83 ± 0.02 C |
| Hydroxybenzoic acids (μg g−1) | |||||
| Gallic acid | 1.48 ± 0.05 C | 951.12 ± 6.30 A | 1.93 ± 0.06 B | 0.94 ± 0.01 D | 0.77 ± 0.01 E |
| Ellagic acid | n.d. | 31.23 ± 1.22 A | 22.03 ± 1.01 B | n.d. | n.d. |
| Protocatechuic acid | 29.94 ± 0.09 A | 11.62 ± 0.62 B | 8.92 ± 0.31 C | 5.26 ± 0.11 E | 6.72 ± 0.13 D |
| p-Hydroxybenzoic acid | 94.27 ± 1.11 A | 7.59 ± 0.13 D | 12.76 ± 0.08 B | 11.42 ± 0.07 C | 0.24 ± 0.01 E |
| Vanillic acid | 11.20 ± 0.07 B | n.d. | 19.80 ± 0.10 A | n.d. | n.d. |
| Syringic acid | 6.37 ± 0.22 B | 3.07 ± 0.09 C | 9.34 ± 0.12 A | n.d. | n.d. |
| Hydroxycinnamic acids (μg g−1) | |||||
| Trans-cinnamic acid | n.d. | n.d. | n.d. | n.d. | n.d. |
| Chlorogenic acid | 1.33 ± 0.02 A | 0.76 ± 0.01 B | 0.75 ± 0.02 B | 0.36 ± 0.01 C | n.d. |
| Caffeic acid | n.d. | n.d. | n.d. | 3.73 ± 0.10 | n.d. |
| p-coumaric acid | 0.49 ± 0.01 C | n.d. | 0.79 ± 0.01 B | 18.24 ± 0.92 A | n.d. |
| Ferulic acid | LOD | LOD | LOD | LOD | LOD |
| Sinapic acid | LOD | LOD | LOD | LOD | LOD |
| Property | F. betulina | F. pinicola | G. applanatum | G. lucidum | C. versicolor |
|---|---|---|---|---|---|
| Antioxidant potential EC50 (mg mL−1) | |||||
| Inhibition of LPx | 0.091 ± 0.003 C γ | 0.012 ± 0.001 A | 0.018 ± 0.001 A | 0.036 ± 0.004 B | 0.096 ± 0.002 C |
| SA•DPPH | 1.51 ± 0.11 E | 0.49 ± 0.05 A | 0.048 ± 0.006 B | 0.88 ± 0.08 C | 0.68 ± 0.09 D |
| SA•OH | 0.23 ± 0.01 B | 0.05 ± 0.005 A | 0.06 ± 0.005 A | 0.29 ± 0.02 C | 0.36 ± 0.03 C |
| FRAP | 3.51 ± 0.16 E | 2.37 ± 0.13 D | 0.30 ± 0.02 A | 1.36 ± 0.09 B | 1.92 ± 0.12 C |
| Fe2+ chelating ability | 4.78 ± 0.31 D | 0.99 ± 0.09 A | 4.13 ± 0.10 C | 1.17 ± 0.07 B | 1.02 ± 0.09 A,B |
| Enzyme inhibition IC50 (mg mL−1) | |||||
| Tyrosinase inhibitory activity | 0.69 ± 0.05 C | 0.10 ± 0.04 A | 0.51 ± 0.08 B | 0.81 ± 0.07 C | 1.16 ± 0.09 E |
| ACE inhibitory activity | 1.01 ± 0.12 B | 0.65 ± 0.04 A | 0.89 ± 0.09 B | 1.28 ± 0.11 C | 1.96 ± 0.18 D |
| Cytotoxicity IC50 (µg mL−1) | F. betulina | F. pinicola | G. applanatum | G.lucidum | C. versicolor |
|---|---|---|---|---|---|
| Human malignant cells | |||||
| HeLa | 42 ± 4 B γ | 24 ± 5 A | 60 ± 12 C | 164 ± 20 D | 232 ± 23 E |
| K562 | 40 ± 2 B | 29 ± 3 A | 76 ± 10 C | 148 ± 64 D | 220 ± 29 D |
| MDA-MB-453 | 42 ± 1 B | 23 ± 2 A | 66 ± 13 C | 170 ± 2 D | 251 ± 21 E |
| Non-cancer human cells | |||||
| MRC-5 | 43 ± 5 A | 32 ± 6 A | 83 ± 6 B | 360 ± 10 C | 402 ± 21 D |
| BEAS-2B | 40 ± 7 A | 29 ± 4 A | 79 ± 6 B | 230 ± 20 C | 280 ±13 D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozarski, M.; Klaus, A.; Špirović-Trifunović, B.; Miletić, S.; Lazić, V.; Žižak, Ž.; Vunduk, J. Bioprospecting of Selected Species of Polypore Fungi from the Western Balkans. Molecules 2024, 29, 314. https://doi.org/10.3390/molecules29020314
Kozarski M, Klaus A, Špirović-Trifunović B, Miletić S, Lazić V, Žižak Ž, Vunduk J. Bioprospecting of Selected Species of Polypore Fungi from the Western Balkans. Molecules. 2024; 29(2):314. https://doi.org/10.3390/molecules29020314
Chicago/Turabian StyleKozarski, Maja, Anita Klaus, Bojana Špirović-Trifunović, Srdjan Miletić, Vesna Lazić, Željko Žižak, and Jovana Vunduk. 2024. "Bioprospecting of Selected Species of Polypore Fungi from the Western Balkans" Molecules 29, no. 2: 314. https://doi.org/10.3390/molecules29020314