Rare Earths—The Answer to Everything
Abstract
:1. Introduction
2. Discussion
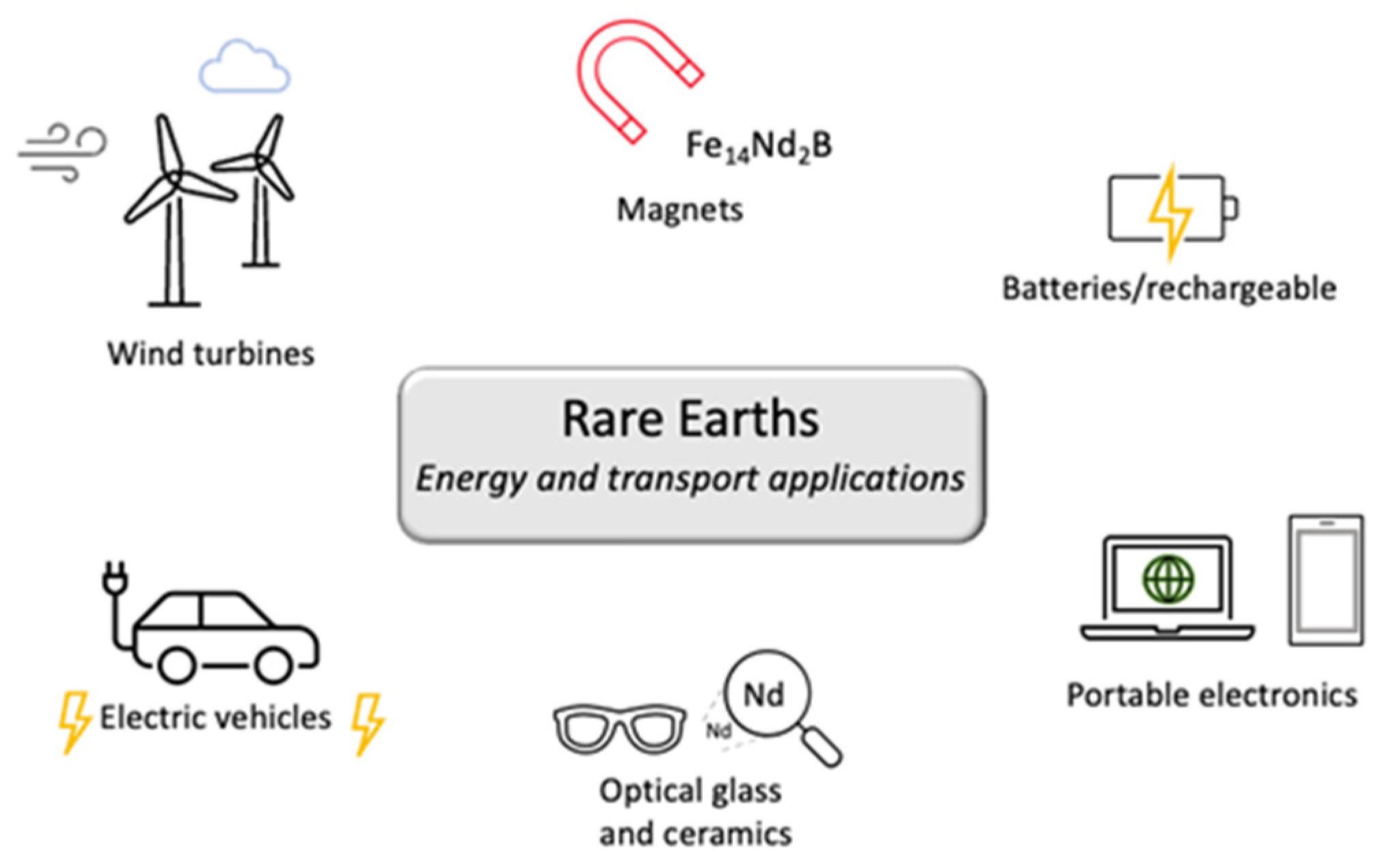
2.1. Energy Generation and Transport
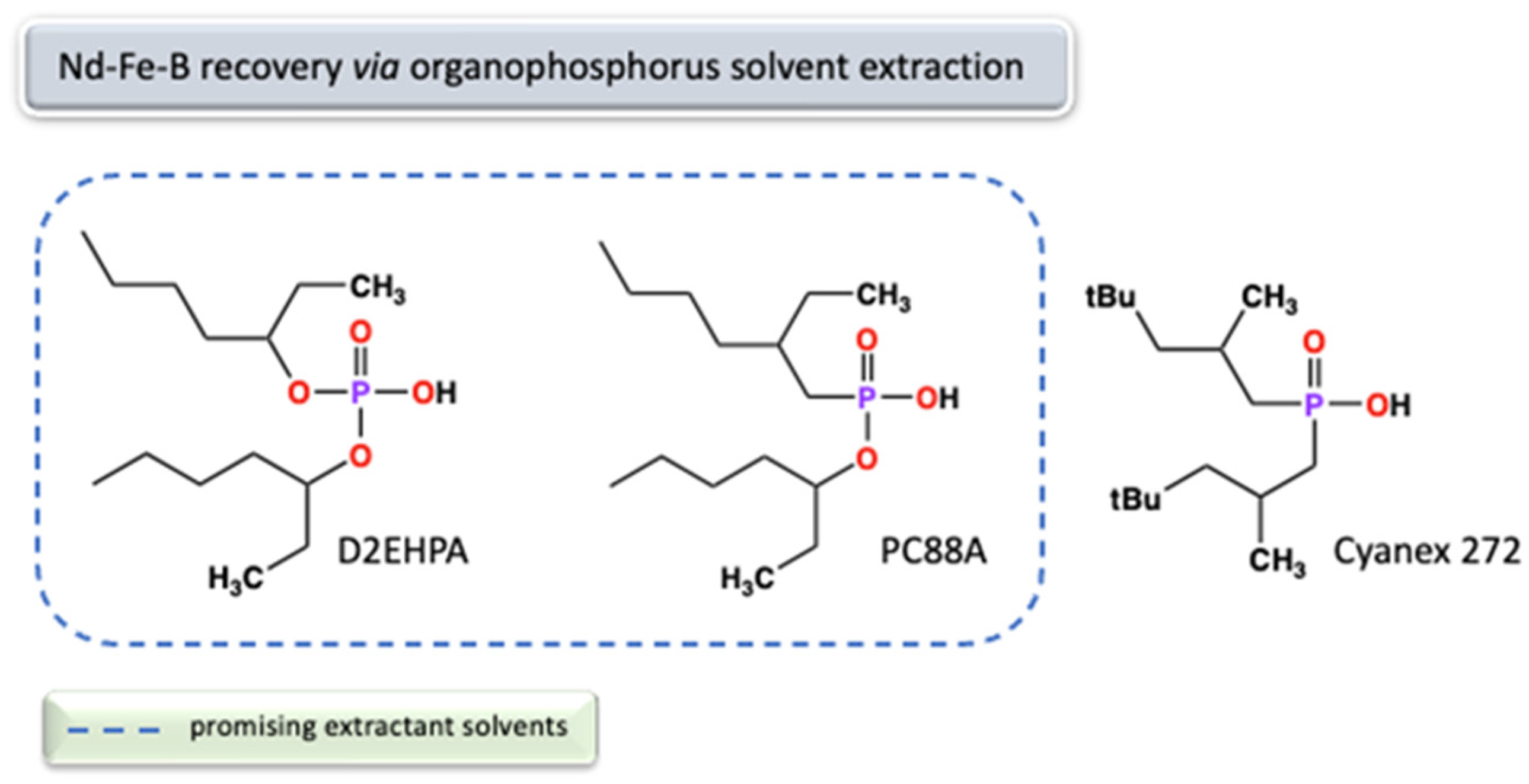
2.2. Supply, Processing, and Separation
2.2.1. Supply
2.2.2. Processing and Separation
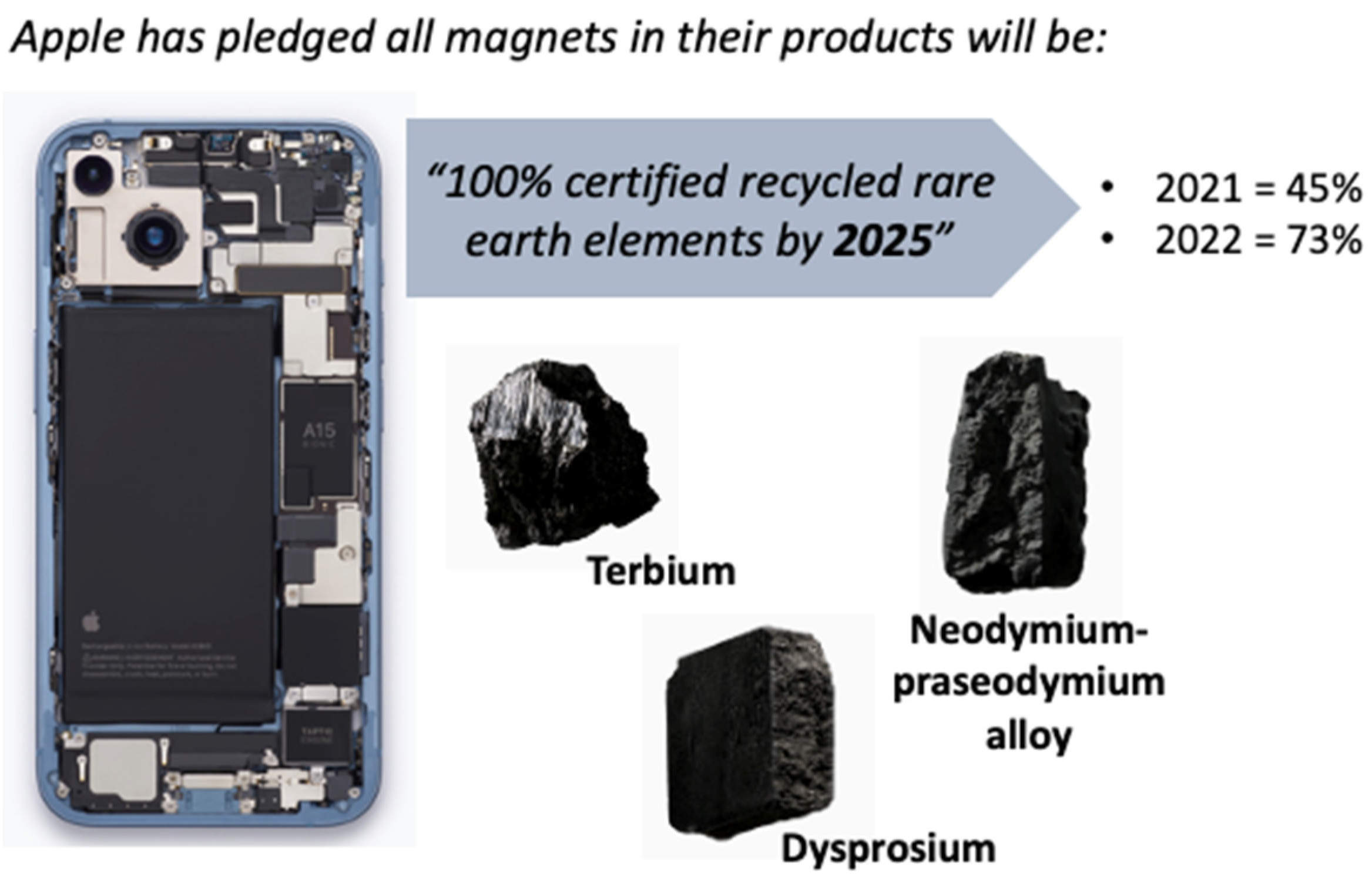
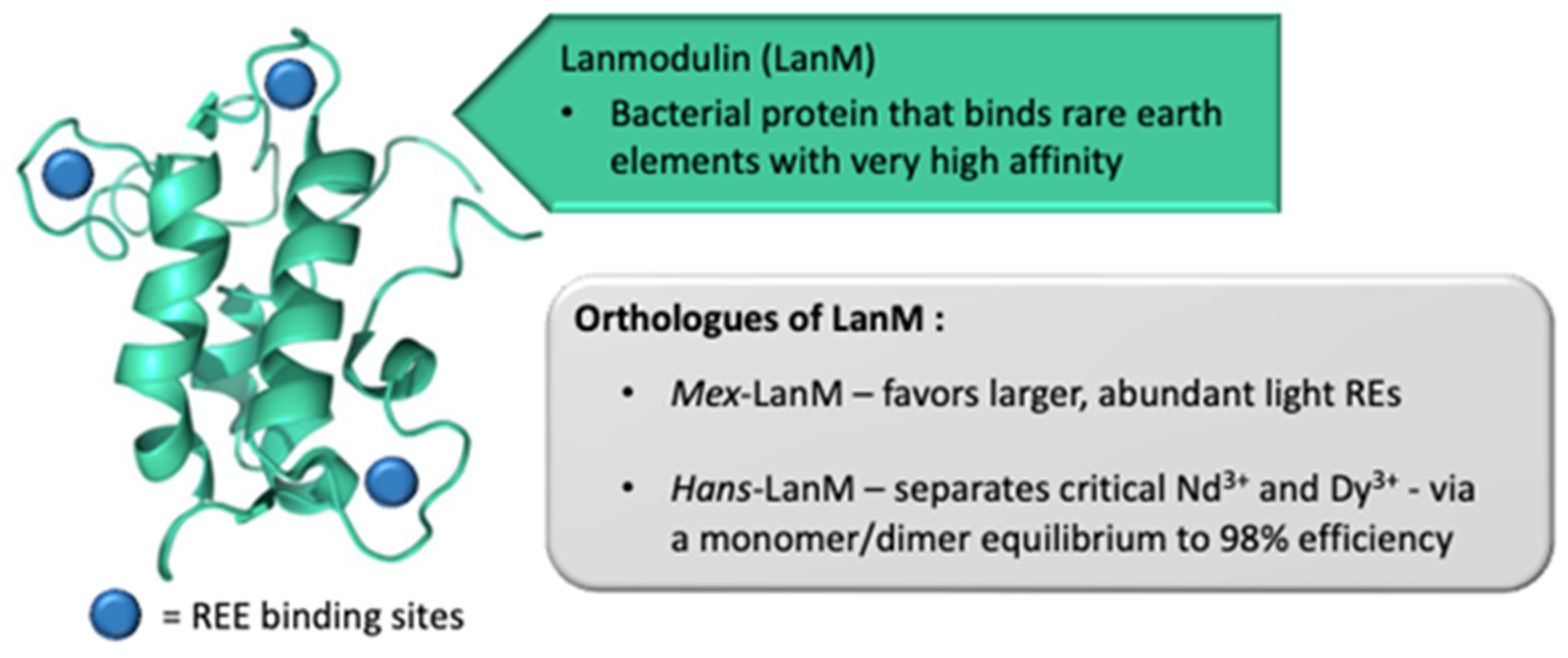
2.3. Rare Earth Recycling and Recovery
2.3.1. Introductory Comments
2.3.2. Disseminated Sources
2.3.3. Concentrated Sources
2.4. Rare Earth Corrosion Inhibitors—A Contribution to Sustainability
2.5. Rare Earths in Agriculture—Crop Production and Animal Feedstocks
2.6. Catalysis by Rare Earths
2.6.1. Industrial Catalysts
- (i)
- Petroleum Cracking Catalysts
- (ii)
- Exhaust Emission Catalysts
- (iii)
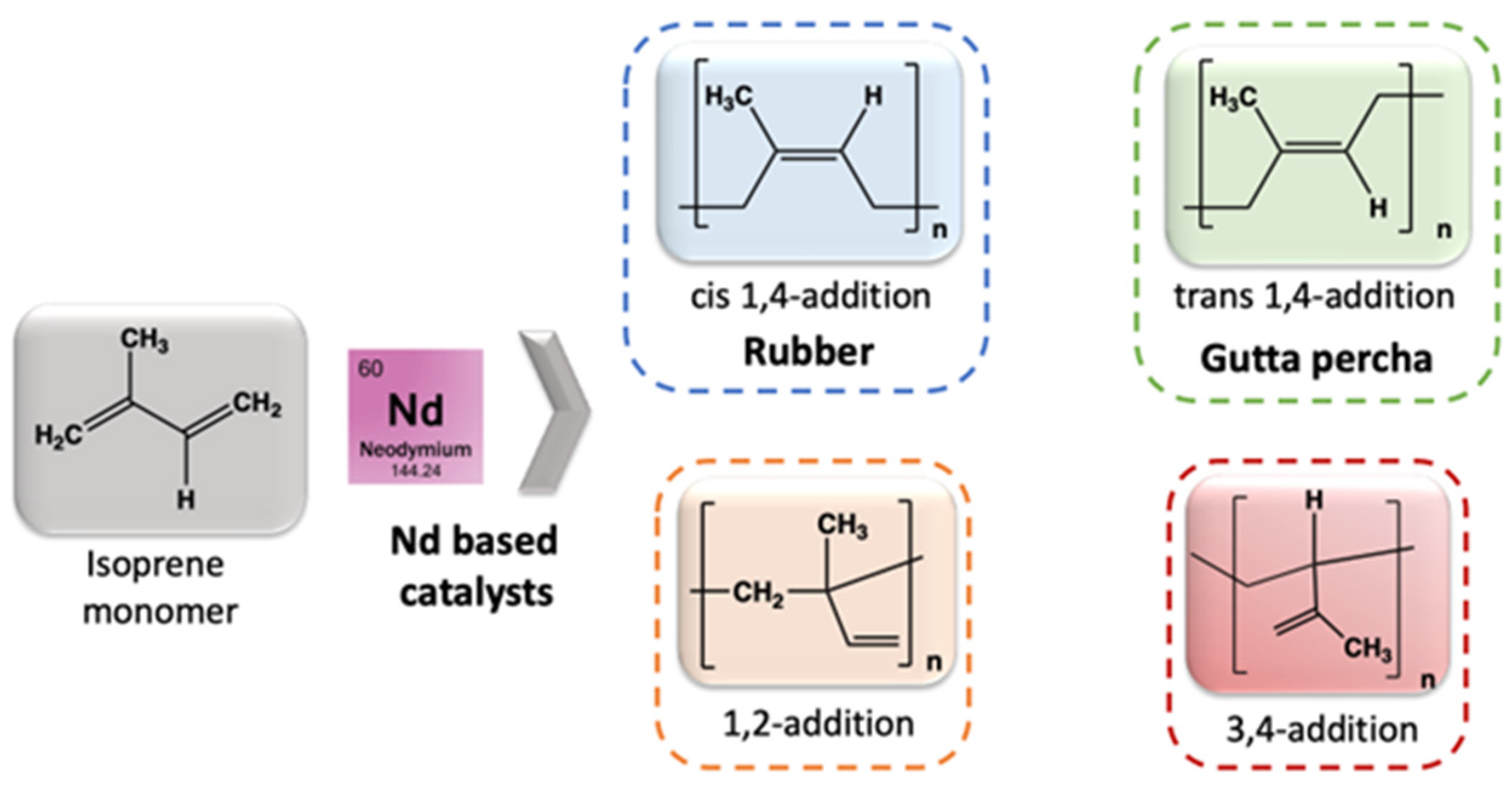
- In Artificial Rubber Production
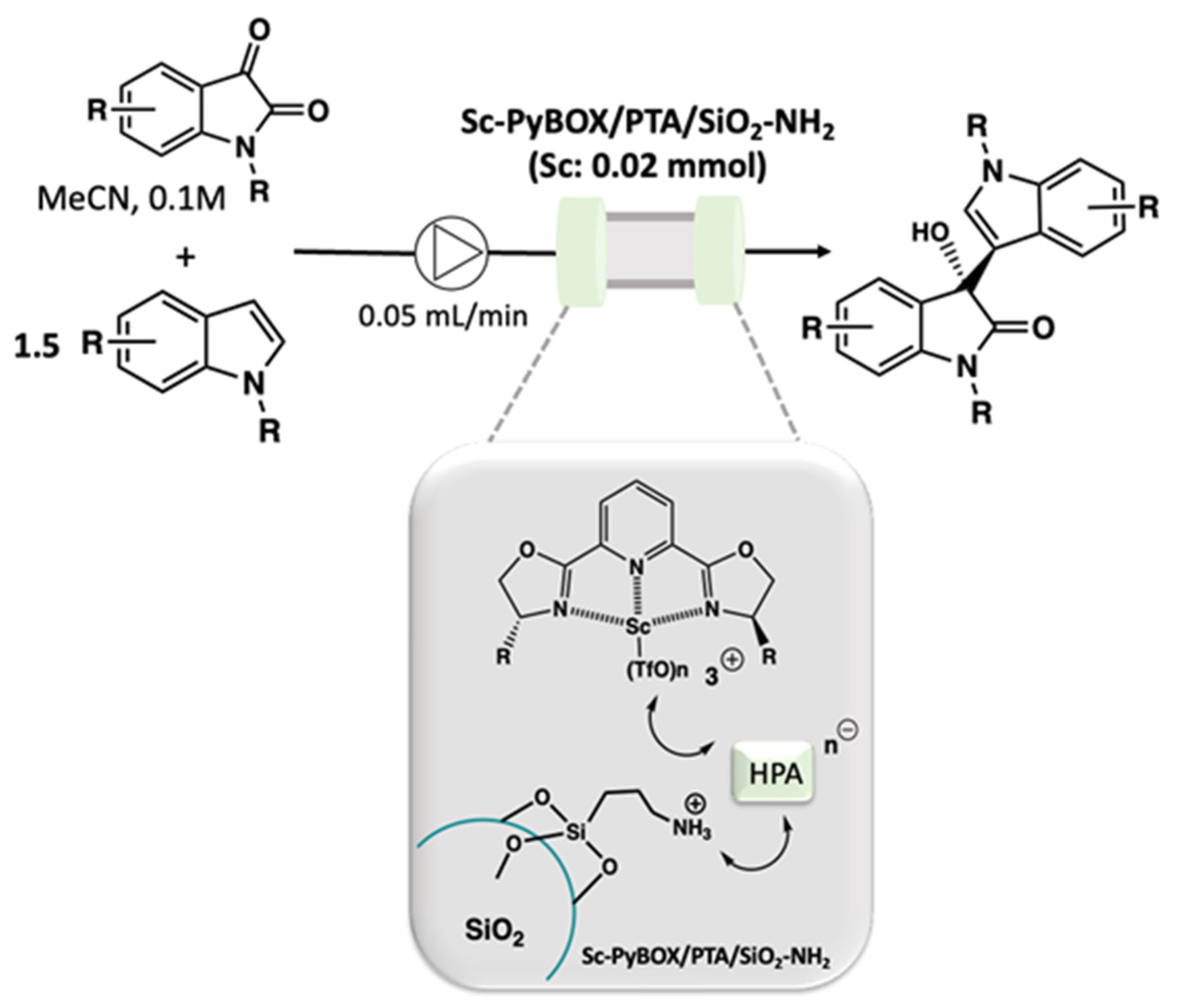
2.6.2. Lab-Scale Catalysis
2.7. Health
2.8. Material Science
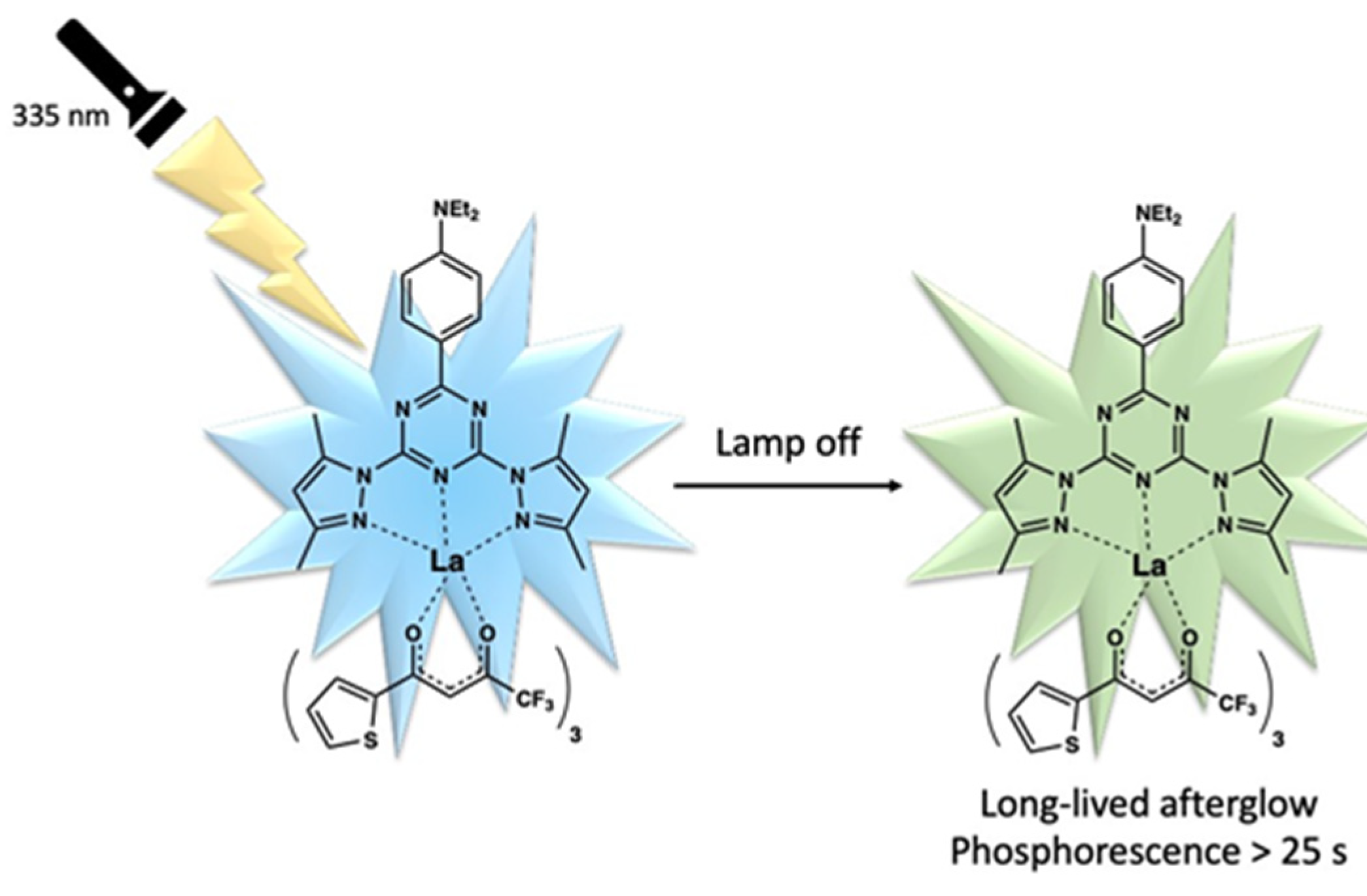
2.8.1. Luminescence
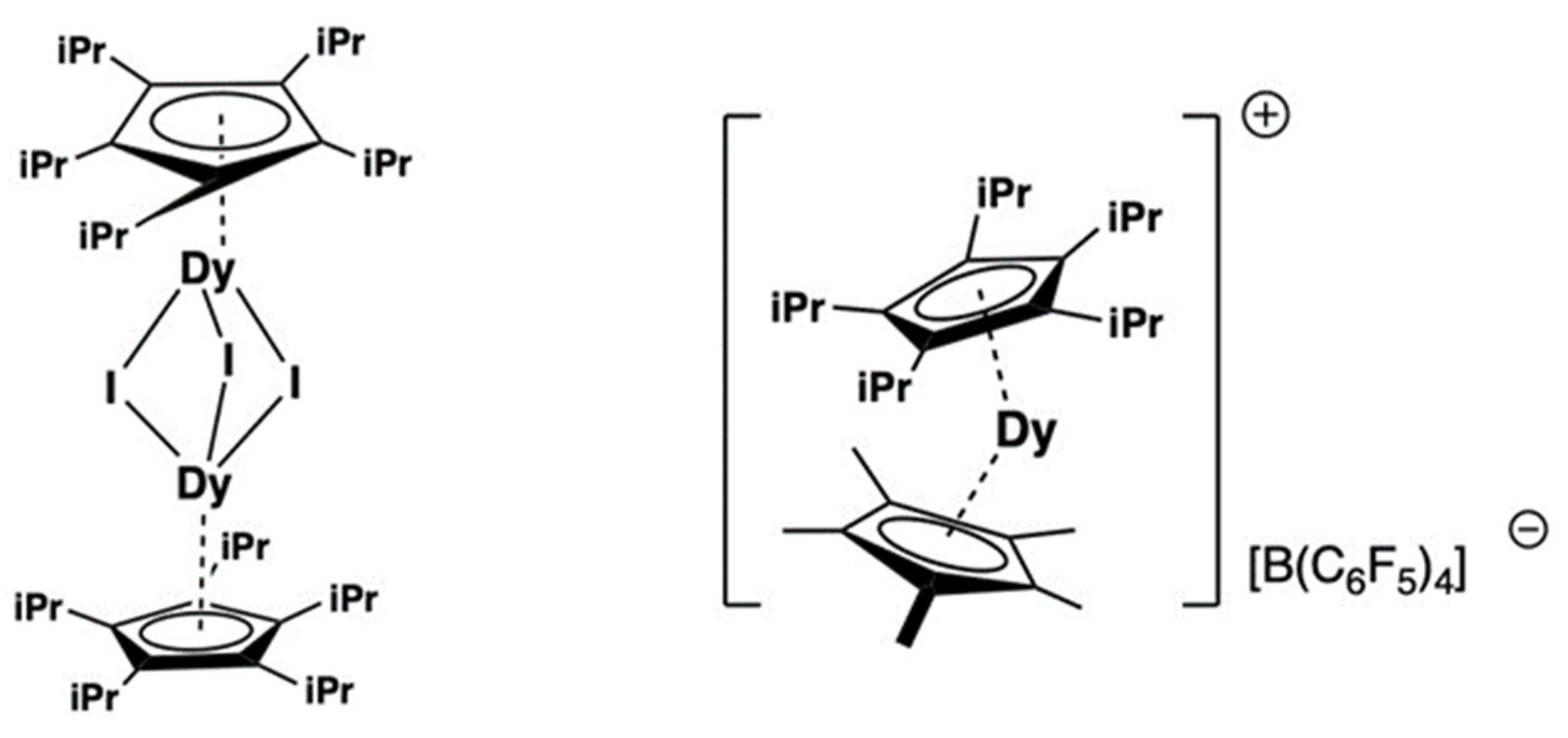
2.8.2. Magnetism
2.8.3. Redox
2.8.4. Various Applications
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Charalampides, G.; Vatalis, K.I.; Apostoplos, B.; Ploutarch-Nikolas, B. Rare Earth Elements: Industrial Applications and Economic Dependency of Europe. Procedia Econ. Financ. 2015, 24, 126–135. [Google Scholar] [CrossRef]
- Binnemans, K.; McGuiness, P.; Jones, P.T. Rare-earth recycling needs market intervention. Nat. Rev. Mater. 2021, 6, 459–461. [Google Scholar] [CrossRef]
- US Department of Energy. Available online: https://www.energy.gov/sites/prod/files/DOE_CMS2011_FINAL_Full.pdf (accessed on 27 December 2023).
- Committee on Assessing the Need for a Defense Stockpile; National Research Council; Latiff, R.H. Managing Materials for a Twenty-first Century; The National Academic Press: Washington, DC, USA, 2008; ISBN 0-309-11258-3. Available online: http://www.nap.edu/catalog/12028.html (accessed on 27 December 2023).
- Li, X.-Y.; Ge, J.-P.; Chen, W.-Q.; Wang, P. Scenarios of rare earth elements demand driven by automotive electrification in China: 2018–2030. Resour. Conserv. Recycl. 2019, 145, 322–333. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Wei, Y.; Liu, J.; Zhao, Z. Research advances of rare earth catalysts for catalytic purification of vehicle exhausts—Commemorating the 100th anniversary of the birth of Academician Guangxian Xu. J. Rare Earths. 2021, 39, 1151–1180. [Google Scholar] [CrossRef]
- Australia’s Identified Mineral Resources 2022; Australian Government—Geoscience Publication: Canberra, Australia, 2022; p. 47. Available online: https://d28rz98at9flks.cloudfront.net/147673/147673_00_1.pdf (accessed on 27 December 2023).
- Goodenough, K.M.; Schilling, J.; Jonsson, E.; Kalvig, P.; Charles, N.; Tuduri, J.; Deady, E.A.; Sadeghi, M.; Schiellerup, H.; Müller, A.; et al. Europe’s rare earth element resource potential: An overview of REE metallogenetic provinces and their geodynamic setting. Ore Geol. Rev. 2016, 72, 838–856. [Google Scholar] [CrossRef]
- Euronews. Available online: https://www.euronews.com/green/2023/01/13/swedish-mining-company-discovers-europes-largest-deposit-of-rare-earth-elements (accessed on 19 December 2023).
- Bomgardner, M.M. Lynas to build Texas Rare-earths separation plant. Chem. Eng. News 2021, 99, 10. [Google Scholar] [CrossRef]
- Available online: https://www.apple.com/au/environment/pdf/Apple_Environmental_Progress_Report_2022.pdf (accessed on 27 December 2023).
- Available online: https://www.apple.com/au/newsroom/2023/04/apple-will-use-100-percent-recycled-cobalt-in-batteries-by-2025/ (accessed on 19 December 2023).
- Lynasrareearths. Available online: http://lynasrareearths.com/wp-content/uploads/2019/12/191209-Lynas-Selects-Kalgoorlie.pdf (accessed on 27 December 2023).
- Zhu, Z.; Pranolo, Y.; Cheng, C.Y. Separation of uranium and thorium from rare earths for rare earth production—A review. Miner. Eng. 2015, 77, 185–196. [Google Scholar] [CrossRef]
- Behrsing, T.; Deacon, G.B.; Junk, P.C. Chapter 1: The Chemistry of Rare-Earth Metals. In The Lanthanides and Actinides—Synthesis, Reactivity, Properties and Applications; Liddle, S.T., Mills, D.P., Natrajan, L.S., Eds.; World Scientific: London, UK, 2022; pp. 1–36. [Google Scholar]
- Traore, M.; Gong, A.; Wang, Y.; Qiu, L.; Bai, Y.; Zhao, W.; Liu, Y.; Chen, Y.; Liu, Y.; Wu, H.; et al. Research progress of rare earth separation methods and technologies. J. Rare Earths. 2023, 41, 182–189. [Google Scholar] [CrossRef]
- Higgins, R.F.; Ruoff, K.P.; Kumar, A.; Schelter, E.J. Coordination Chemistry-Driven Approaches to Rare Earth Element Separations. Acc. Chem. Res. 2022, 55, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Scheibe, B.; Spirling, J.M.; Albrecht-Schoenzart, T.E. Syntheses and Characterization of Tetrazolate-Based Lanthanide Compounds and Selective Crystallization Separation of Neodymium and Dysprosium. Inorg. Chem. 2022, 61, 19193–19202. [Google Scholar] [CrossRef] [PubMed]
- Meihaus, K.R.; Corbey, J.F.; Fang, M.; Ziller, J.W.; Long, J.R.; Evans, W.J. Influence of an Inner-Sphere K+ Ion on the Magnetic Behavior of N23− Radical-Bridged Dilanthanide Complexes Isolated Using an External Magnetic Field. Inorg. Chem. 2014, 53, 3099–3107. [Google Scholar] [CrossRef]
- Kumar, A.; Geng, H.; Schelter, E.J. Harnessing magnetic fields for rare-earth complex crystallization–separations in aqueous solutions. RSC Adv. 2022, 12, 27895–27898. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Johnson, K.R.; Jansone-Popova, S.; Jiang, D. Advancing Rare-Earth Separation by Machine Learning. JACS Au 2022, 2, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.J.M.; Cheisson, T.; Rugh, H.J.; Gaul, M.R.; Carroll, P.J.; Schelter, E.J. High-throughput screening for discovery of benchtop separations systems for selected rare earth elements. Commun. Chem. 2020, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, M.; Breiter, R.; Becker, A.M. Towards rare earth element recovery from wastewaters: Biosorption using phototrophic organisms. Appl. Microbiol. Biotechnol. 2021, 105, 5229–5239. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, G.J.-P.; Mattocks, J.A.; Park, D.M.; Reed, D.W.; Cotruvo, J.A., Jr.; Jiao, Y. Selective and Efficient Biomacro-molecular Extraction of Rare-Earth Elements using Lanmodulin. Inorg. Chem. 2020, 59, 11855–11867. [Google Scholar] [CrossRef] [PubMed]
- Mattocks, J.A.; Jung, J.J.; Lin, C.-Y.; Dong, Z.; Yennawar, N.H.; Featherston, E.R.; Kang-Yun, C.S.; Hamilton, T.H.; Park, D.M.; Boal, A.K.; et al. Enhanced rare-earth separation with a metal-sensitive lanmodulin dimer. Nature 2023, 618, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Mattocks, J.A.; Cotruvo, J.A., Jr.; Deblonde, G.J.-P. Engineering lanmodulin’s selectivity for actinides over lanthanides by controlling solvent coordination and second-sphere interactions. Chem. Sci. 2022, 13, 6054–6066. [Google Scholar] [CrossRef] [PubMed]
- Baez, A.G.; Munoz, L.P.; Garelick, H.; Purchase, D. Characterization of industrially pre-treated waste printed circuit boards for the potential recovery of rare earth elements. Environ. Technol. Innov. 2022, 27, 102481. [Google Scholar] [CrossRef]
- Grimes, S.M.; Maguire, D. Assessment of priorities in critical material recovery from Waste Electrical and Electronic Equipment. Resour. Policy. 2020, 68, 101658. [Google Scholar] [CrossRef]
- Mir, S.; Dhawan, N. A comprehensive review on the recycling of discarded printed circuit boards for resource recovery. Resour. Conserv. Recycl. 2022, 178, 106027. [Google Scholar] [CrossRef]
- Khanna, R.; Ellamparuthy, G.; U.S. Department of Energy. Critical Materials Strategy; DIANE Publishing: Washington, DC, USA, 2011; pp. 1–196. [Google Scholar]
- Cayumil, R.; Mishra, S.K.; Mukherjee, P.S. Concentration of rare earth elements during high temperature pyrolysis of waste printed circuit boards. Waste Manag. 2018, 78, 602–610. [Google Scholar]
- Franus, W.; Wiatros-Motyka, M.M.; Wdowin, M. Coal fly ash as a resource for rare earth elements. Environ. Sci. Pollut. Res. 2015, 22, 9464–9474. [Google Scholar] [CrossRef] [PubMed]
- Ahmaruzzamann, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 327–363. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Kim, C.-J.; Chung, K.-W.; Kim, S.-D.; Lee, J.-Y.; Kumar, J.R. Solvent extraction, separation and recovery of dysprosium (Dy) and neodymium (Nd) from aqueous solutions: Waste recycling strategies for permanent magnet processing. Hydrometallurgy 2016, 165, 27–43. [Google Scholar] [CrossRef]
- Bogart, J.A.; Connor, A.; Lippincott, C.A.; Carroll, P.J.; Schelter, E.J. An Operationally Simple Method for Separating the Rare-Earth Elements Neodymium and Dysprosium. Angew. Chem. Int. Ed. 2015, 54, 8222–8225. [Google Scholar] [CrossRef] [PubMed]
- Riano, S.; Binnemans, K. Extraction and separation of neodymium and dysprosium from used NdFeB magnets: An application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem. 2015, 17, 2931–2942. [Google Scholar] [CrossRef]
- Kumari, A.; Sahu, S.K. A comprehensive review on recycling of critical raw materials from spent neodymium iron boron (NdFeB) magnets. Waste Manag. 2023, 317, 123527. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.-M.; Van Gerven, T.; Jones, P.T.; et al. REE recovery from end-of-life NdFeB permanent magnet scrap: A critical review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Kraikaew, J.; Srinuttrakul, W.; Chayavadhanakur, C. Solvent extraction study of rare earths from nitrate medium by the mixtures of TBP and D2EHPA in kerosene. J. Met. Mater. Miner. 2005, 15, 89–95. [Google Scholar]
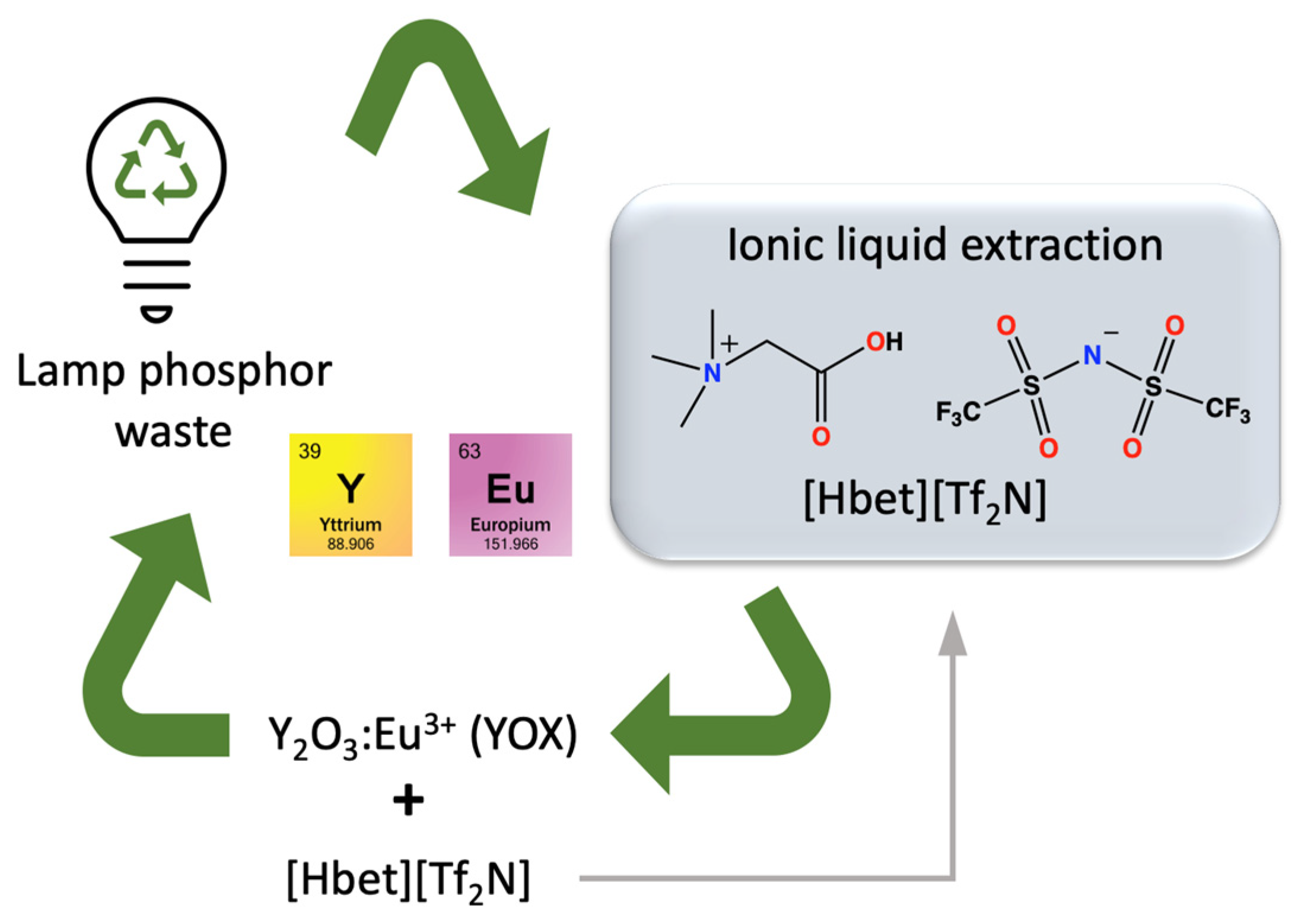
- Dupont, D.; Binnemans, K. Rare-earth recycling using a functionalized ionic liquid for the selective dissolution and revalorization of Y2O3:Eu3+ from lamp phosphor waste. Green Chem. 2015, 17, 856–868. [Google Scholar] [CrossRef]
- Shaeffer, N.; Grimes, S.; Cheeseman, C. Interactions between trivalent rare earth oxides and mixed [Hbet][Tf2N]:H2O sytems in the development of a one-step process for the separation of light from heavy rare earth elements. Inorg. Chim. Acta 2016, 439, 55–60. [Google Scholar] [CrossRef]
- Lu, G.; Lu, X.; Liu, P. Recovery of rare earth elements from spent fluid catalytic cracking catalyst using hydrogen peroxide as a reductant. Miner. Eng. 2020, 145, 106104. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Liu, Z.; Zhong, L.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Brongers, M.; Thompson, N.; Virmani, Y.; Payer, J. Corrosion Cost and Preventive Strategies in the United States; Report R315-01; Office of Infrastructure Research and Development, Federal Highway Administration: Ashburn, VA, USA; Lakewood, CO, USA; Vancouver, WA, USA, 2002. [Google Scholar]
- Schmitt, G. Global Needs for Knowledge Dissemination, Research, and Development in Materials Deterioration and Corrosion Control; The World Corrosion Organisation: New York, NY, USA, 2009. [Google Scholar]
- Behrsing, T.; Deacon, G.B.; Junk, P.C. The Chemistry of rare earth metals, compounds, and corrosion inhibitors. In Rare Earth-Based Corrosion Inhibitors; Forsyth, M., Hinton, B., Eds.; Woodhead Publishing: London, UK, 2014; pp. 1–37. [Google Scholar]
- Zhou, B.; Huang, D.; Wu, J.; Qihong Zhu, Q.; Zhu, H. Horizontal and Vertical Distributions of Chromium in a Chromate Production District of South Central China. Int. J. Environ. Res. Public Health 2018, 15, 571. [Google Scholar] [CrossRef] [PubMed]
- Costa, M. Toxicity and carcinogenicity of Cr(VI) in animal models and humans. Crit. Rev. Toxicol. 1997, 27, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.B.; Junk, P.C.; Forsyth, M.; Lee, W.W. From Chromates to Rare Earth Carboxylates: A Greener Take on Corrosion Inhibition. Chem. Aust. 2008, 75, 18–21. Available online: https://search.informit.org/doi/10.3316/ielapa.373920188232588 (accessed on 27 December 2023).
- Sinko, J. Challenges of chromate inhibitor pigments replacement in organic coatings. Prog. Org. Coat. 2001, 42, 267–282. [Google Scholar] [CrossRef]
- Somers, A.E.; Peng, Y.; Chong, A.L.; Forsyth, M.; MacFarlane, D.R.; Deacon, G.B.; Hughes, A.E.; Hinton, B.R.W.; Mardel, J.I.; Junk, P.C. Advances in the development of rare earth metal and carboxylate compounds as corrosion inhibitors for steel. Corros. Eng. Sci. Technol. 2020, 55, 311–321. [Google Scholar] [CrossRef]
- Hinton, B.R.W. Corrosion inhibition with rare earth metal salts. J. Alloys Compd. 1992, 180, 15–25. [Google Scholar] [CrossRef]
- Ryan, N.E.; Hinton, B.R.W.; Wilson, L.; Arnott, D.R. Corrosion Protection and Surface Pretreatment with Rare Earth Compounds. In Rare Earth Horizons; Department of Industry, Technology and Commerce: Washington, DC, USA, 1987; pp. 187–198. [Google Scholar]
- Hu, Z.; Richter, H.; Sparovek, G.; Schung, E. Physiological and Biochemical Effects of Rare Earth Elements on Plants and Their Agricultural Significance: A Review. J. Plant Nutr. 2004, 27, 183–220. [Google Scholar] [CrossRef]
- Tariq, H.; Sharma, A.; Sarkar, S.; Ojha, L.; Pal, R.P.; Mani, V. Perspectives for rare earth elements as feed additive in livestock – A review. Asian-Austr. J. Anim. Sci. 2020, 33, 373. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, M.; Seter, M.; Hinton, B.; Deacon, G.; Junk, P. New ‘Green’ Corrosion Inhibitors Based on Rare Earth Compounds. Aust. J. Chem. 2011, 64, 812. [Google Scholar] [CrossRef]
- Blin, F.; Leary, S.G.; Deacon, G.B.; Junk, P.C.; Forsyth, M. The nature of the surface film on steel treated with cerium and lanthanum cinnamate based corrosion inhibitors. Corros. Sci. 2006, 48, 404–419. [Google Scholar] [CrossRef]
- de Bruin-Dickason, C.N.; Deacon, G.B.; Forsyth, C.M.; Schirin, H.; Heilmann, O.B.; Hinton, B.R.W.; Junk, P.C.; Somers, A.E.; Qing, T.Y.; Turner, D.R. Synthesis and Structures of Rare Earth 3-(4′-Methylbenzoyl)-propanoate Complexes—New Corrosion Inhibitors. Aust. J. Chem. 2017, 70, 478–484. [Google Scholar] [CrossRef]
- Peng, Y.; Hughes, A.E.; Deacon, G.B.; Junk, P.C.; Hinton, B.R.W.; Forsyth, M.; Mardell, J.I.; Somers, A.E. The leaching behavior and inhibitive performance of rare earth carboxylate incorporated epoxy coating system. ACS Appl. Mater. Inter. 2019, 11, 36154–36168. [Google Scholar] [CrossRef]
- Somers, A.; Talbi, G.; deBruin-Dickason, C.; Forsyth, M.; Deacon, G.; Junk, P.; Hinton, B. Green Rare Earth compounds as Corrosion Inhibitors for Steel. In Proceedings of the Australasian Corrosion Association (ACA) Conference 2016: Corrosion and Prevention, Auckland, New Zealand, 13–16 November 2016. [Google Scholar]
- Somers, A.E.; Hinton, B.R.W.; de Bruin-Dickason, C.; Deacon, G.B.; Junk, P.C.; Forsyth, M. New, environmentally friendly, rare earth carboxylate corrosion inhibitors for mild steel. Corros. Sci. 2018, 139, 430–437. [Google Scholar] [CrossRef]
- Deacon, G.B.; Junk, P.C.; Somers, A.E. A study of rare-earth 3-(4-methylbenzoyl)-propanoate compounds as corrosion inhibitors for AS1020 mild steel in NaCl solutions. Corros. Sci. 2018, 145, 199–211. [Google Scholar]
- Ho, D.; Brack, N.; Scully, J.; Markley, T.; Forsyth, M.; Hinton, B. Cerium Dibutylphosphate as a Corrosion Inhibitor for AA2024-T3 Aluminum Alloys. J. Electrochem. Soc. 2006, 153, B392. [Google Scholar] [CrossRef]
- Ren, P.; Li, J.; Wang, L.; Guo, H.; Lei, B.; Feng, Z.; Meng, G. Organo-Cerium as a Quick Repair Agent for Coating Damage on Carbon Steel. J. Mater. Eng. Perform. 2023, 32, 14755–14764. [Google Scholar] [CrossRef]
- Nguyen, D.N.; Mathesh, M.; Hinton, B.; Tan, M.J.Y.; Forsyth, M. Rare Earth 4-Hydroxycinnamate Compounds as Carbon Dioxide Corrosion Inhibitors for Steel in Sodium Chloride Solution. J. Electrochem. Soc. 2014, 161, C527. [Google Scholar]
- Somers, A.E.; Deacon, G.B.; Hinton, B.R.W.; MacFarlane, D.R.; Junk, P.C.; Tan, M.Y.J.; Forsyth, M. Recent Developments in Environment-Friendly Corrosion Inhibitors for Mild Steel. J. Ind. Inst. Sci. 2016, 96, 285–292. [Google Scholar]
- Xu, G.; Xiao, J. (Eds.) New Frontiers in Rare Earth Science and Applications. In Proceedings of the International Conference on Rare Earth Development and Applications, Beijing, China, 10–14 September 1985; Elsevier: Amsterdam, The Netherlands, 1985; pp. 146–154.
- Bosheng, G. A new application field for rare earths—Agriculture. in Rare Earth Horizons. In Proceedings of the Conference, National Measurement Laboratory, Lindfield, Australia, 27–28 April 1987; Dept. of Industry, Technology and Commerce: Canberra, Australia, 1987; pp. 237–246. [Google Scholar]
- Brown, P.H.; Rathjen, A.H.; Graham, R.D.; Tribe, D.E. Rare Earth Elements. In Biological Systems, in the Handbook on The Physics and Chemistry of Rare Earths; Gschneidner, K.A., Eyring, L., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1990; Volume 13, Chapter 92; pp. 423–451. [Google Scholar]
- Bingkun, X. Application of rare earths in Chinese Agriculture and their prospective of development, Effects of rare earth elements (REEs) on the growth and mineral nutrition of plants. In Rare Earths in Agriculture Seminar; AATSE: Canberra, Australia, 1995; pp. 5–10. [Google Scholar]
- Diatloff, E.; Asher, C.J.; Smith, F.W. Effects of rare earth elements (REEs) on the growth and mineral nutrition of plants. In Rare Earths in Agriculture Seminar; AATSE: Canberra, Australia, 1995; pp. 11–23. [Google Scholar]
- Diatloff, E.; Smith, F.W.; Asher, C.J. Rare-earth elements and plantgrowth: I. Effects of lanthanum and cerium on root elongation of corn and mungbean. J. Plant Nutr. 1995, 18, 1963–1976. [Google Scholar] [CrossRef]
- Diatloff, E.; Smith, F.W.; Asher, C.J. Rare-earth elements and plant growth: II. Responses of corn and mungbean to low concentrations of lanthanum in dilute, continuously flowing nutrient solutions. J. Plant Nutr. 1995, 18, 1977–1989. [Google Scholar] [CrossRef]
- Diatloff, E.; Smith, F.W.; Asher, C.J. Rare-earth elements and plant growth: III. Responses of corn and mungbean to low concentrations of cerium in dilute, continuously flowing nutrient solutions. J. Plant Nutr. 1995, 18, 1991–2003. [Google Scholar] [CrossRef]
- Diatloff, E.; Smith, F.W.; Asher, C.J. Effects of lanthanum and cerium on the growth and mineral nutrition of corn and Mungbean. Ann. Bot. 2008, 101, 971–982. [Google Scholar] [CrossRef]
- Buckingham, S.; Meehan, B.; Peverill, K.; Skroce, A. Rare earth research at RMIT University and State Chemistry Laboratory (SCL). Part B Application of lanthanum to agricultural plants. In Rare Earths in Agriculture Seminar; AATSE: Canberra, Australia, 1995; pp. 29–35. [Google Scholar]
- Buckingham, S.; Maheswaran, J.; Meehan, B.; Peverill, K.; Stokes, J. The role of applications of rare earth elements in enhancement of crop and pasture production. Mater. Sci. Forum. 1999, 312–317, 339–347. [Google Scholar] [CrossRef]
- Maheswaran, J.; Meehan, B.; Reddy, N.; Peverill, K.; Buckingham, S. Impact of Rare Earth Elements on Plant Physiology and Productivity; A Report for the Rural Industries Research and Development Corporation, RIRDC Publication No 01/145RIRDC Project No DAV-122A; RIRDC: Barton, ACT, Australia, December 2001. [Google Scholar]
- Tyler, G. Rare earth elements in soil and plant systems—A review. Plant Soil 2004, 267, 191–206. [Google Scholar] [CrossRef]
- Redling, K. Rare Earth Elements in Agriculture with Emphasis on Animal Husbandry. Doctoral Thesis, Veterinary Faculty of the Ludwig-Maximillian University, Munich, Germany, 2006. [Google Scholar]
- Shen, Q.; Zhang, J.; Wang, C. Application of Rare Earth Elements on animal production. Feed Ind. 1991, 12, 20–22. [Google Scholar]
- Tomassi, F.; Thomas, P.J.; Pagano, G.; Perono, G.A.; Oral, R.; Lyons, D.M.; Toscanesi, M.; Trifuoggi, M. Review of Rare Earth Elements as Fertilizers and Feed Additives: A Knowledge Gap Analysis. Arch. Environ. Contam. Toxicol. 2021, 81, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Rambeck, W.A.; He, M.L.; Chang, J.; Arnold, R.; Henkelmann, R.; Süss, A. Possible role of rare earth elements as growth promoters. In Proceedings of the Vitamine und Zusatzstoffe in der Ernährung von Mensch und Tier, 7. Symposium, Jena, Germany, 22–23 September 1999. [Google Scholar]
- He, M.L.; Rambeck, W.A. Rare earth elements—A new generation of growth promoters for pigs? Arch. Anim. Nutr. 2000, 53, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Borger, C. Alternative Methoden in der Schweinemast. Untersuchungen zum leistungsstiegerndenPotential Seltener Erden und zur Jodanreicherung im Gewebe durch die Verfütterungvon Meeresalgen. Doctoral Thesis, Ludwig-Maximilians-Universität, München, Germany, 2003. [Google Scholar]
- Rambeck, W.A.; He, M.L.; Wehr, U. Influence of the alternative growth promoter “Rare Earth Elements” on meat quality in pigs. In Proceedings of the British Society of Animal Science Pig and Poultry Meat Quality—Genetic and Non-Genetic Factors, Krakow, Poland, 14–15 October 2004. [Google Scholar]
- Rambeck, W.A.; Wehr, U. Use of rare earth elements as feed additives in pig production. Pig News Inf. 2005, 26, 41 N–47 N. [Google Scholar]
- He, M.L.; Ranz, D.; Rambeck, W.A. Study on performance enhancing effect of rare earth elements in growing and fattening pigs. J. Anim. Physiol. Anim. Nutr. 2001, 85, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Eisele, N. Untersuchungen zum Einsatz Seltener Erden als Leistungsförderer beim Schwein. Doctoal Thesis, Ludwig-Maximilians-Universität, München, Germany, 2003. [Google Scholar]
- Richter, H. Hinweise zur Toxikologie von Seltenen Erden. In Proceedings of the XVI. Tage Der Seltenen Erden, Berlin, Germany, 4–6 December 2003; p. 18. [Google Scholar]
- He, R.; Xia, Z. Effect of rare earth compound added to diet on performance of growing-finishing pigs. In Proceedings of the 2nd International Symposium on Trace Elements and Food Chain, Wuhan, China, 12–15 November 1998. [Google Scholar]
- Rambeck, W.; Wehr, U. Seltene Erden, alternative Leistungsförderer der Zukunft: Anwendungenbeim Schwein. In Proceedings of the Verein engagierter Tierärzte e. V., Kassel, Germany, 25 September 2004; pp. 1–35. [Google Scholar]
- Wendel, F.A.W. Untersuchungen zur Abklärung einer Rückstandsproblematik sowiezu Nebeneffekten Seltener Erden beim Einsatz als Leistungsförderer bei Absatzferkeln. Doctoral Thesis, Veterinary Faculty of the Ludwig-Maximillian University, Munich, Germany, 2014. [Google Scholar]
- EFSA panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Safety of Lancer (Lanthanide citrate) as a zootechnical additive for weaned piglets. EFSA J. 2019, 17, 5912. [Google Scholar]
- He, M.L.; Wehr, U.; Rambeck, W.A. Effect of low doses of dietary rare earth elements on growth performance of broilers. J. Anim. Physiol. Anim. Nutr. 2010, 94, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Park, Y.S.; Seong, S.I.; Yoo, S.W.; Kim, I.H. Effects of rare earth elements-enriched yeast on growth performance, nutrient digestibility, meat quality, relative organ weight and excreta microflora in broiler chickens. Livest Sci. 2015, 172, 43–49. [Google Scholar] [CrossRef]
- Igbasan, F.A.; Adebayo, O.S. Growth response, carcass quality, some haematological and biochemical parameters of broiler chickens fed on diets supplemented with lanthanum salts. Int. J. Sci. Eng. Res. 2012, 3, 1–17. [Google Scholar]
- Zohravi, M. The Effect of Rare Earth Elements on Growth Performance, Tibia Mineralization and Blood Serum of Japanese Quails. Doctoral Thesis, Veterinary Faculty of the Ludwig-Maximillian University, Munich, Germany, 2006. [Google Scholar]
- Eleraky, A.W.; Rambeck, W. Study on performance enhancing effect of rare earth elements as alternatives to antibiotic feed additives for Japanese quails. J. Am. Sci. 2011, 7, 211–215. Available online: http://www.americanscience.org (accessed on 27 December 2023).
- Li, J.; Yan, L.; Cao, X.; Luo, Y.; Peng, X.; Wang, Z.; Zou, T.; Chen, J.; You, J. Effects of rare earth as feed additive on production performance, egg quality, serum biochemical parameters, antioxidant capacity, intestinal morphology, and gut microbiota in late-phase laying hens. Front. Sustain. Food Syst. 2023, 7, 1155543. [Google Scholar] [CrossRef]
- Bernd, R. Seltene Erden als Leistungsfördererin der FischzuchtUntersuchungen an Regenbogenforellen und Karpfen. Doctoral Thesis, Veterinary Faculty of the Ludwig-Maximillian University, Munich, Germany, 2005. [Google Scholar]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Noreldin, A.E.; Arif, M.; Chaudhry, M.T.; Losacco, C.; Abdeen, A.; Abdel-Daim, M.M. Impacts of rare earth elements on animal health and production: Highlights of cerium and lanthanum. Sci. Total Environ. 2019, 672, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Akah, A. Application of rare earths in fluid catalytic cracking: A review. J. Rare Earths 2017, 35, 941–956. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342. [Google Scholar] [CrossRef] [PubMed]
- Louwen, J.N.; Simko, S.; Stanciakova, K.; Bulo, R.E.; Weckhuysen, B.M.; Vogt, E.T.C. Role of Rare Earth Ions in the Prevention of Dealumination of Zeolite Y for Fluid Cracking Catalysts. J. Phys. Chem. C 2020, 124, 4626–4636E. [Google Scholar] [CrossRef]
- Shackleford, A.; Masak, T.; Fu, Q.; Smith, G.M.; Yilmaz, B.; Culp, R.D.; Gawecki, P. An Alternative to Rare Earth Elementsin FCC Catalysts—The Use of Phinesse at Shell Sarnia. Available online: www.digitalrefining.com/article/1001234 (accessed on 27 December 2023).
- Baillie, C.; Schiller, R. Zero and Low Rare Earth FCC Catalysts. Available online: www.digitalrefining.com/article/1000137,2011 (accessed on 27 December 2023).
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-Based Ziegler/Natta Catalysts and their Application in Diene Polymerization. Adv. Polym. Sci. 2006, 204, 1–154. [Google Scholar]
- Fischbach, A.; Klimpel, M.G.; Widenmeyer, M.; Herdtweck, E.; Scherer, W.; Anwander, R. Stereospecific Polymerization of Isoprene with Molecular and MCM-48-grafted Lanthanide(III)-Tetraalkylaluminates LnAl3R12. Angew. Chem. 2004, 116, 2284–2289. [Google Scholar] [CrossRef]
- Fischbach, A.; Perdih, F.; Herdtweck, E.; Anwander, R. Structure-Reactivity Relationships in Rare-Earth Metal Carboxylate-based Binary Ziegler-type Catalysts. Organometallics 2006, 25, 1626–1642. [Google Scholar] [CrossRef]
- Hollfelder, C.O.; Jende, L.N.; Diether, D.; Zelger, T.; Stauder, R.; Maichle-Mössmer, C.; Anwander, R. 1,3-Diene Polymer. Catalysts 2018, 8, 61. [Google Scholar] [CrossRef]
- Meermann, C.; Törnroos, K.W.; Nerdal, W.; Anwander, R. Rare-Earth Metal Mixed Chloro/Methyl Compounds: Heterogeneo-us–Homogeneous Borderline Catalysts in 1,3-Diene Polymerization. Angew. Chem. Int. Ed. 2007, 46, 6508–6513. [Google Scholar] [CrossRef]
- Zimmermann, M.; Törnroos, K.W.; Anwander, R. Cationic Rare-Earth Metal Complexes for the Living trans-1,4 Isoprene Polymerization. Angew. Chem. Int. Ed. 2008, 47, 775–778. [Google Scholar] [CrossRef]
- Fischbach, A.; Anwander, R. Rare-Earth Metals and Aluminum Getting Close in Ziegler-Type Organometallics. Adv. Polym. Sci. 2006, 240, 155–281. [Google Scholar]
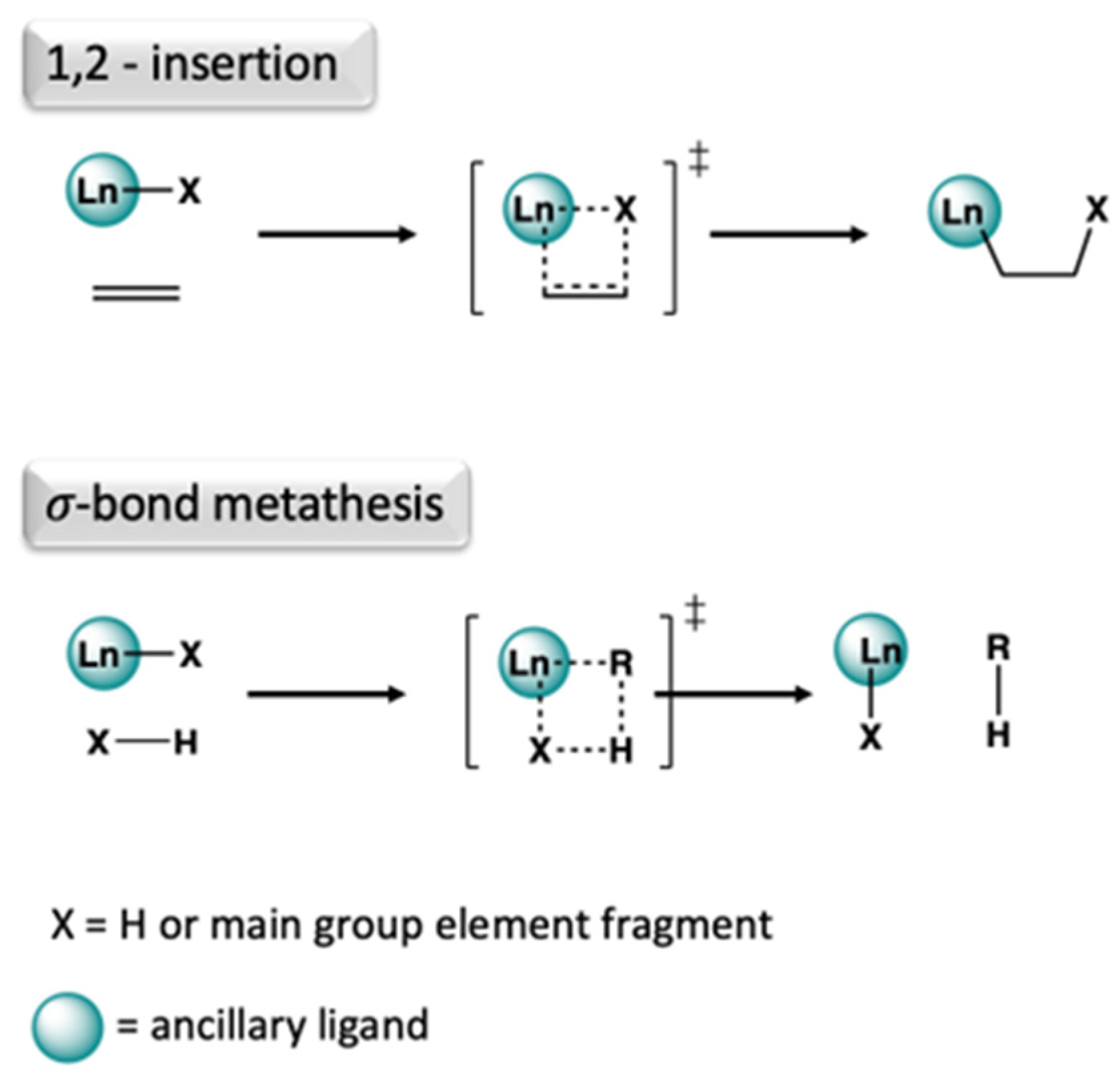
- Nishiura, M.; Guo, F.; Hou, Z. Half-Sandwich Rare-Earth-Catalyzed Olefin Polymerization, Carbometalation, and Hydroarylation. Acc. Chem. Res. 2015, 48, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Farnaby, J.H.; Chowdhury, T.; Horsewill, S.J.; Wilson, B.; Jaroschik, F. Lanthanides and actinides: Annual survey of their organometallic chemistry covering the year 2019. Coord. Chem. Rev. 2021, 437, 213830. [Google Scholar] [CrossRef]
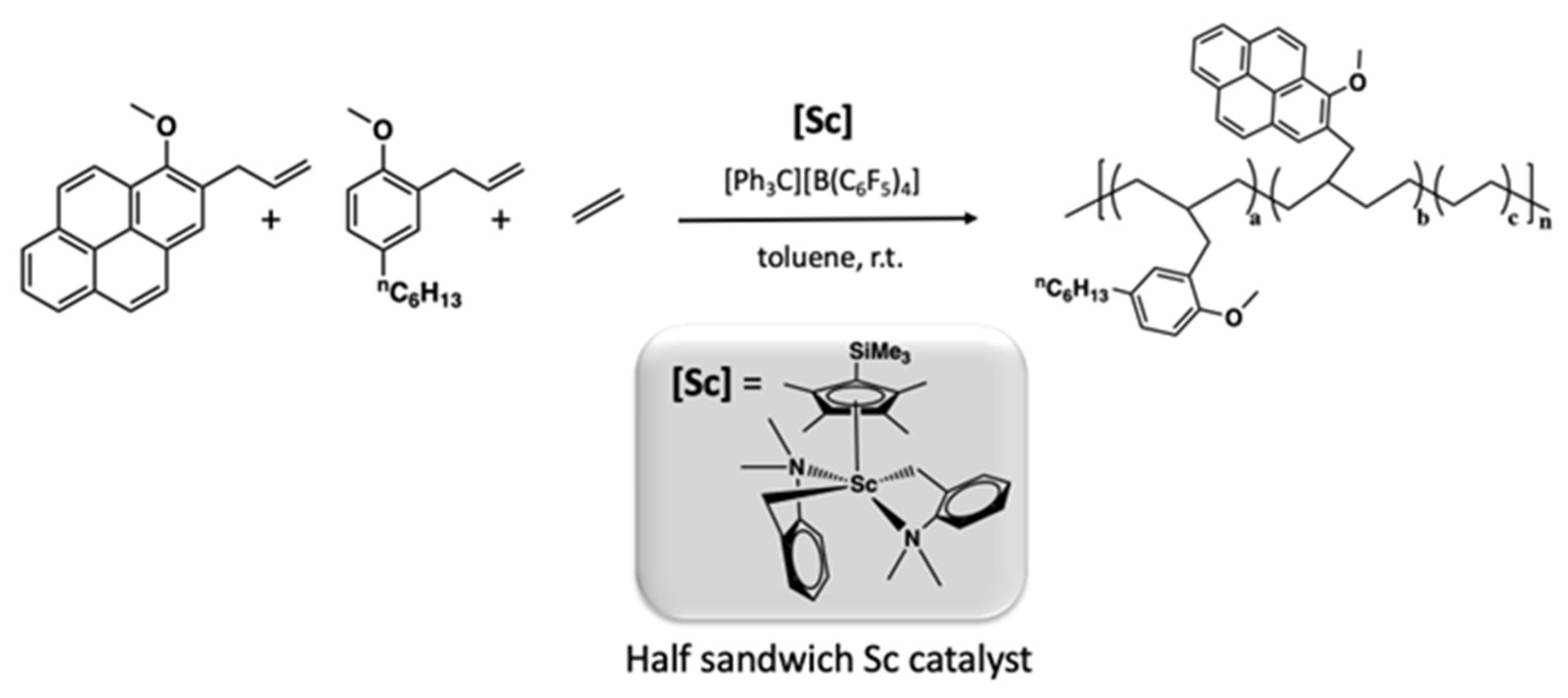
- Yang, Y.; Wang, H.; Huang, L.; Nishiura, M.; Higaki, Y.; Hou, Z. Terpolymerization of Ethylene and Two Different Methoxyaryl-Substituted Propylenes by Scandium Catalyst Makes Tough and Fast Self-Healing Elastomers. Agew. Chem. Int. Ed. 2021, 60, 26192–26198. [Google Scholar] [CrossRef] [PubMed]
- Molander, G.A. Application of Lanthanide Reagents in Organic Synthesis. Chem. Rev. 1992, 92, 29–68. [Google Scholar] [CrossRef]
- Hong, S.; Marks, T.J. Organolanthanide-Catalyzed Hydroamination. Acc. Chem. Res. 2004, 37, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Reznichenko, A.L.; Nawara-Hultzsch, A.J.; Hultzsch, K.C. Asymmetric Hydroamination. Top. Curr. Chem. 2014, 343, 191–260. [Google Scholar] [PubMed]
- Novas, B.T.; Waterman, R. Metal-Catalyzed Hydrophosphination. Chem. Cat. Chem. 2022, 14, e202200988. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Cui, C. Rare-Earth-Catalyzed Hydrosilylation and Dehydrogenative Coupling of Hydrosilanes. Synlett 2020, 31, 962–970. [Google Scholar]
- Liu, H.; Eisen, M.S. Organo-f-Complexes for Efficient and Selective Hydroborations. Synth 2020, 52, 629–644. [Google Scholar] [CrossRef]
- Dicken, R.D.; Motta, A.; Marks, T.J. Homoleptic Lanthanide Amide Catalysts for Organic Synthesis: Experiment and Theory. ACS Catal. 2021, 11, 2715–2734. [Google Scholar] [CrossRef]
- Sun, Q.; Xu, X.; Xu, X. Recent Advances in Rare-Earth Metal-Catalyzed C-H Functionalization Reactions. Chem. Cat. Chem. 2022, 14, e202201083. [Google Scholar] [CrossRef]
- Miao, Y.; von Jouanne, A.; Yokochi, A. Current Technologies in Depolymerization Process and the Road Ahead. Polymers 2021, 13, 449. [Google Scholar] [CrossRef] [PubMed]
- Kobylarski, M.; Berthet, J.-C.; Cantat, T. Reductive depolymerization of polyesters and polycarbonates with hydroboranes by using a lanthanum(III) tris(amide) catalyst. Chem. Commun. 2022, 58, 2830–2833. [Google Scholar] [CrossRef] [PubMed]
- Wursthorn, L.; Beckett, K.; Rothbaum, J.O.; Cywar, R.M.; Lincoln, C.; Kratish, Y.; Marks, T.J. Selective Lanthanide-Organic Catalyzed Depolymerization of Nylon-6 to ε-Caprolactam. Angew. Chem. Int. Ed. 2023, 62, e202212543. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Sugiura, M.; Kitagawa, H.; Lam, W.W.-L. Rare-Earth Metal Triflates in Organic Synthesis. Chem. Rev. 2002, 102, 2227–2302. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, N.; Kanai, M.; Sasai, H. A Career in Catalysis: Masakatsu Shibasaki. ACS Catal. 2016, 6, 4699–4709. [Google Scholar] [CrossRef]
- Pellissier, H. Recent developments in enantioselective scandium–catalyzed transformations. Coord. Chem. Rev. 2016, 313, 1–37. [Google Scholar] [CrossRef]
- Saito, Y.; Kobayashi, S. Chiral Heterogeneous Scandium Lewis Acid Catalysts for Continuous-Flow Enantioselective Friedel–Crafts Carbon–Carbon Bond-Forming Reactions. Angew. Chem. Int. Ed. 2021, 60, 26566–26570. [Google Scholar] [CrossRef]
- Qiao, Y.; Schelter, E.J. Lanthanide Photocatalysis. Acc. Chem. Res. 2018, 51, 2926–2936. [Google Scholar] [CrossRef]
- Prieto, A.; Jaroschik, F. Recent Applications of Rare Earth Complexes in Photoredox Catalysis for Organic Synthesis. Curr. Org. Chem. 2022, 26, 6–41. [Google Scholar] [CrossRef]
- Yoon, T.P. Photochemical stereocontrol using tandem photoredox-chiral lewis acid catalysis. Acc. Chem. Res. 2016, 49, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Xing, Y.-Y.; Pu, R.; Jia, M.; Chen, Y.; Hu, A.; Zhang, S.-Q.; Yu, N.; Du, J.; Zhang, Y.; et al. Identification of Alkoxy Radicals as Hydrogen Atom Transfer Agents in Ce-Catalyzed C–H Functionalization. J. Am. Chem. Soc. 2023, 145, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Kynman, A.E.; Christodoulou, S.; Ouellette, E.T.; Peterson, A.; Kelly, S.N.; Maron, L.; Arnold, P. Photocatalytic dechlorination of unactivated chlorocarbons including PVC using organolanthanide complexes. Chem. Commun. 2023, 59, 10924–10927. [Google Scholar] [CrossRef] [PubMed]
- Tomar, M.; Bhimpuria, R.; Kocsi, D.; Thapper, A.; Borbas, K.E. Photocatalytic Generation of Divalent Lanthanide Reducing Agents. J. Am. Chem. Soc. 2023, 145, 22555–22562. [Google Scholar] [CrossRef] [PubMed]
- Horn, E.J.; Rosen, B.R.; Baran, P.S. Synthetic Organic Electrochemistry: An Enabling Innately Sustainable Method. ACS Cent. Sci. 2016, 2, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Röckl, J.L.; Lundberg, H. Samarium and Ytterbium in Organic Electrosynthesis. Synth. 2023, 55, 1375–1384. [Google Scholar]
- Szostak, M.; Fazakerley, N.J.; Parmar, D.; Procter, D.J. Cross-Coupling Reactions Using Samarium(II) Iodide. Chem. Rev. 2014, 114, 5959–6039. [Google Scholar] [CrossRef] [PubMed]
- Maity, S. Development in SmI2 Catalyzed Reactions. Eur. J. Org. Chem. 2021, 37, 5312–5319. [Google Scholar] [CrossRef]
- Sahloul, K.; Sun, L.H.; Requet, A.; Chahine, Y.; Mellah, M.A. Samarium “Soluble” Anode: A New Source of SmI2 Reagent for Electrosynthetic Application. Chem. Eur. J. 2012, 18, 11205. [Google Scholar] [CrossRef]
- Zhang, Y.-F.; Mellah, M. Convenient Electrocatalytic Synthesis of Azobenzenes from Nitroaromatic Derivatives Using SmI2. ACS Catal. 2017, 7, 8480–8486. [Google Scholar] [CrossRef]
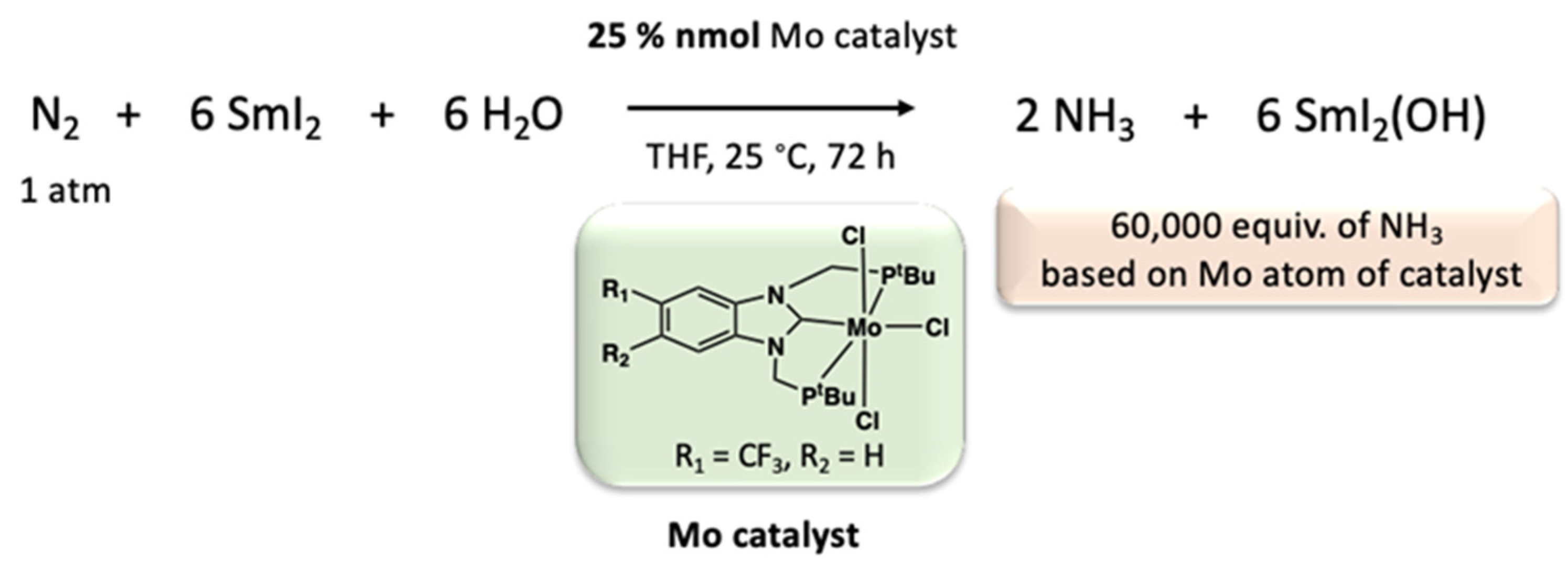
- Ashida, Y.; Mizushima, T.; Arashiba, K.; Egi, A.; Tanaka, H.; Yoshizawa, K.; Nishibayashi, Y. Catalytic Production of Ammonia from Dinitrogen Employing Molybdenum Complexes Bearing N-Heterocyclic Carbene-Based PCP-Type Pincer Ligands. Nat. Synth. 2023, 2, 635–644. [Google Scholar] [CrossRef]
- Evans, C.H. Biochemistry of the Lanthanides; Springer Science: New York, NY, USA, 1990; Volume 8. [Google Scholar]
- Pol, A.; Barends, T.R.M.; Dietl, A.; Khadem, A.F.; Eygensteyn, J.; Jetten, M.S.M.; Op den Camp, H.J.M. Rare earth metals are essential for methanotrophic life in volcanic mudpots. Environ. Microbiol. 2014, 16, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Cotruvo, J.A., Jr. The Chemistry of Lanthanides in Biology: Recent Discoveries, Emerging Principles, and Technological Applications. ACS Cent. Sci. 2019, 5, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Mattocks, J.A.; Cotruvo, J.A., Jr. Biological, biomolecular, and bio-inspired strategies for detection, extraction, and separations of lanthanides and actinides. Chem. Soc. Rev. 2020, 49, 8315. [Google Scholar] [CrossRef] [PubMed]
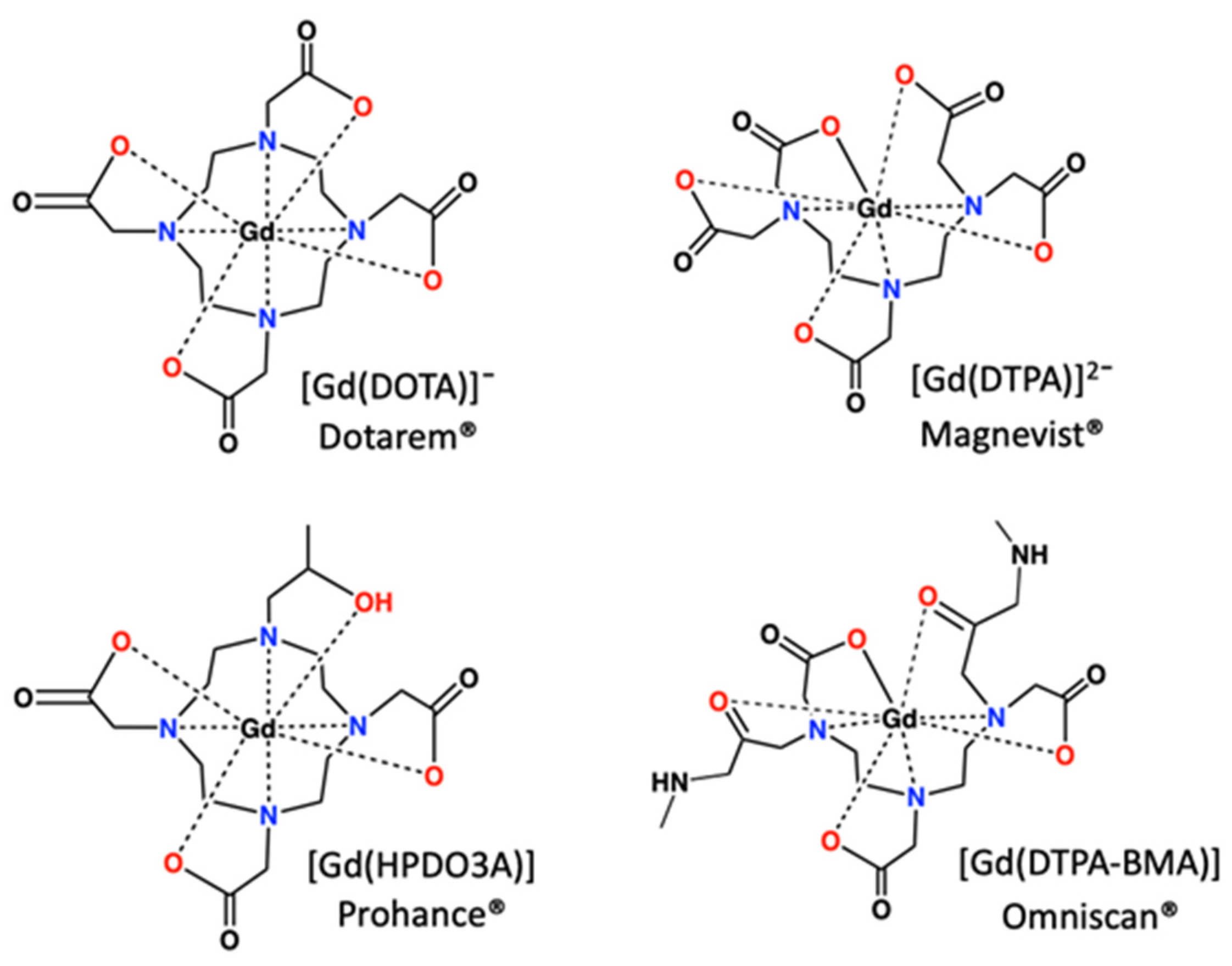
- Caravan, P. Strategies for increasing the sensitivity of gadolinium-based MRI contrast agents. Chem. Soc. Rev. 2006, 35, 512–523. [Google Scholar] [CrossRef]
- Ascenzi, P.; Bettinelli, M.; Bof, A.; Botta, M.; De Simone, G.; Luchinat, C.; Marengo, E.; Mei, H.; Aime, S. Rare earth elements (REE) in biology and medicine. Rend. Lincei Sci. Fis. Nat. 2020, 31, 821–833. [Google Scholar] [CrossRef]
- Teo, R.D.; Termini, J.; Gray, H.B. Lanthanides: Applications in Cancer Diagnosis and Therapy. J. Med. Chem. 2016, 59, 6012–6024. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef]
- Fricker, S.P. The therapeutic application of lanthanides. Chem. Soc. Rev. 2006, 35, 524–533. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Bottrill, M.; Kwok, L.; Long, N.J. Lanthanides in magnetic resonance imaging. Chem. Soc. Rev. 2006, 35, 557–571. [Google Scholar] [CrossRef]
- Fan, J.; He, M.; Wang, C.-J.; Zhang, M. Gadolinium Chloride Inhibits the Production of Liver Interleukin-27 and Mitigates Liver Injury in the CLP Mouse Model. Mediat. Inflamm. 2021, 2021, 2605973. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dong, C.; Tian, L.; Ji, X.; Yang, L.; Li, L. Gadolinium Chloride Restores the Function of the Gap Junctional Intercellular Communication between Hepatocytes in a Liver Injury. Int. J. Mol. Sci. 2019, 20, 3748. [Google Scholar] [CrossRef] [PubMed]
- Xian, T.; Meng, Q.; Gao, F.; Hu, M.; Wang, X. Functionalization of luminescent lanthanide complexes for biomedical applications. Coord. Chem. Rev. 2023, 474, 21486. [Google Scholar] [CrossRef]
- Park, J.Y.; Chang, Y.; Lee, G.H. Multi-Modal Imaging and Cancer Therapy Using Lanthanide Oxide Nanoparticles: Current Status and Perspectives. Curr. Med. Chem. 2015, 22, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Sadler, A.W.E.; Hogan, L.; Fraser, B.; Rendina, L.M. Cutting edge rare earth radiometals: Prospects for cancer theranostics. EJNMMI Radiopharm. Chem. 2022, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.F.G.; Nunes, R.F.; Coutinho, A.M.; Zaniboni, E.C.; Costa, L.B.; Barbosa, F.G.B.; Queiroz, M.A.; Cerri, G.G.; Buchpiguel, C.A. Theranostics in Nuclear Medicine: Emerging and Re-emerging Integrated Imaging and Therapies in the Era of Precision Oncology. Radiographics 2020, 40, 1715–1740. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.-Q.; Chau, C.V.; Arambula, J.F.; Gao, S. Lanthanide porphyrinoids as molecular theranostics. Chem. Soc. Rev. 2022, 51, 6177–6209. [Google Scholar] [CrossRef]
- Kapoor, S. Lanthanum and its rapidly emerging role as an anti-carcinogenic agent. J. Cell Biochem 2009, 106, 193. [Google Scholar] [CrossRef]
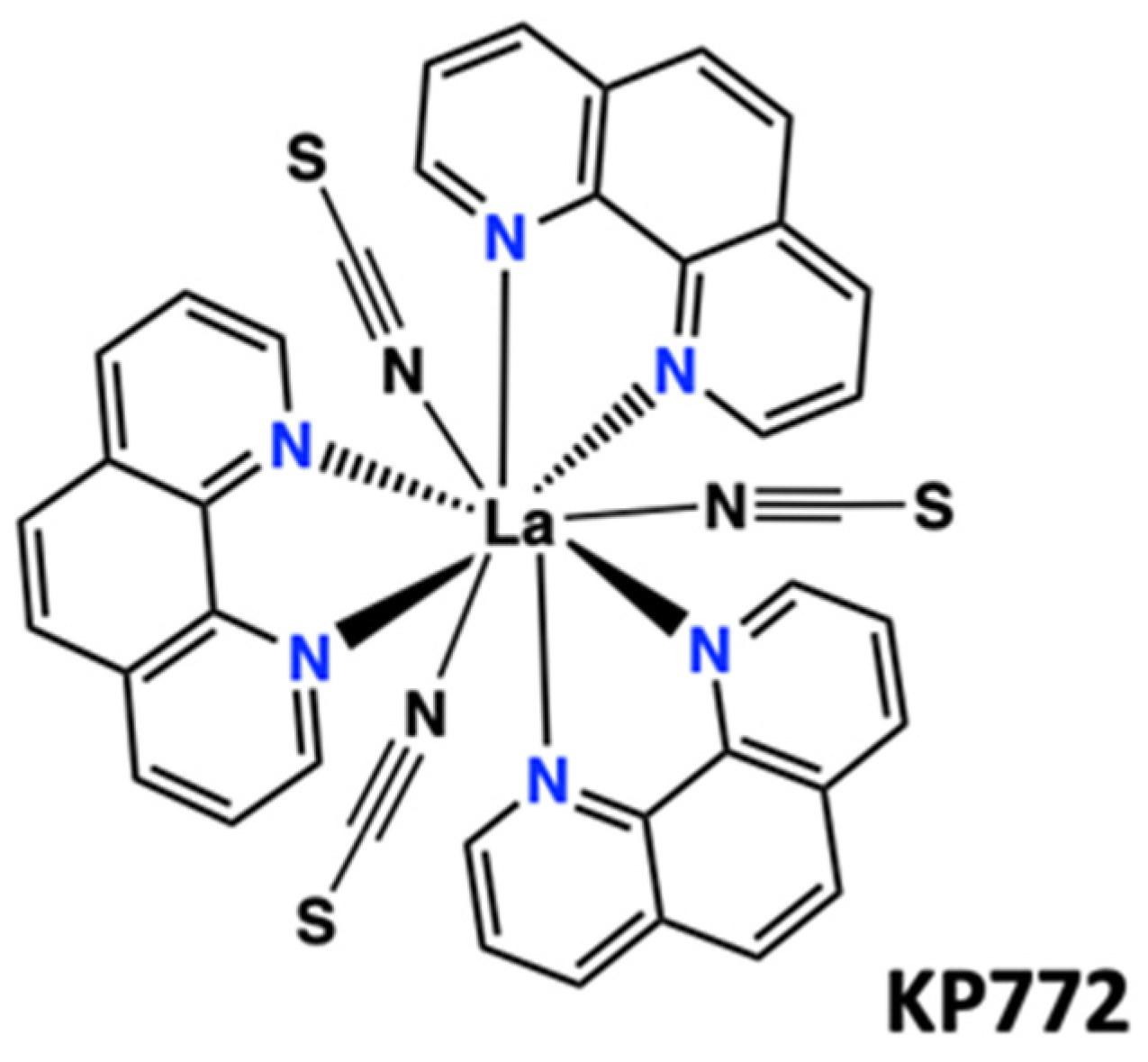
- Biba, F.; Groessl, M.; Egger, A.; Roller, A.; Hartinger, C.G.; Keppler, B.K. New Insights into the Chemistry of the Antineoplastic Lanthanum ComplexTris(1,10-phenanthroline)tris(thiocyanato-κN)lanthanum(III) (KP772) and Its Interaction with Biomolecules. Eur. J. Inorg. Chem. 2009, 2009, 4282–4287. [Google Scholar] [CrossRef]
- Hussain, A.; Gadadhar, S.; Goswami, T.K.; Karande, A.A.; Chakravarty, A.R. Photo-induced DNA cleavage activity and remarkable photocytotoxicity of lanthanide(III) complexes of a polypyridyl ligand. Dalton Trans. 2012, 41, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Lahiri, D.; Begum, M.S.A.; Saha, S.; Majumdar, R.; Dighe, R.R.; Chakravarty, A.R. Photocytotoxic Lanthanum(III) and Gadolinium(III) Complexes of Phenanthroline Bases Showing Light-Induced DNA Cleavage Activity. Inorg. Chem. 2010, 49, 4036–4045. [Google Scholar] [CrossRef] [PubMed]
- Kater, L.; Kater, B.; Jakupec, M.A.; Keppler, B.K.; Prokop, A. KP772 overcomes multiple drug resistance in malignant lymphoma and leukemia cells in vitro by inducing Bcl-2-independent apoptosis and upregulation of Harakiri. J. Biol. Inorg. Chem. 2021, 26, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Niroomand, S.; Jahanara, A.; Jahani, S.; Sargazi, G.; Patrick, B.O.; Noroozifar, M.; Khorasani-Motlagh, M. A novel binuclear Lanthanum complex containing 1,10-phenanthroline; from crystal structure to biological and antitumor Activity. J. Photochem. Photobiol. 2023, 441, 114711. [Google Scholar] [CrossRef]
- Campello, M.P.C.; Palma, E.; Correia, I.; Paulo, P.M.R.; Matos, A.; Rino, J.; Coimbra, J.; Pessoa, J.C.; Gambino, D.; Paulo, A.; et al. Lanthanide complexes with phenanthroline-based ligands: Insights into cell death mechanisms obtained by microscopy techniques. Dalton Trans. 2019, 48, 4611–4624. [Google Scholar] [CrossRef] [PubMed]
- Madanhire, T.; Pereira, M.C.; Davids, H.; Hosten, E.C.; Abrahams, A. Lanthanide(III) complexes with N-(2,6-dimethylphenyl)oxamate and 1,10-phenanthroline: Synthesis, characterisation and cytotoxicity against MCF-7, HEC-1A and THP-1 cell lines. Polyhedron 2020, 189, 114713. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Song, X.-Y.; Peng, Y.; Hong, X.; Liu, Y.-C.; Liang, H. High cytotoxicity of dihalo-substituted 8-qinolinolato-lanthanides. Dalton Trans. 2011, 40, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X. Insights of metal 8-hydroxyquinolinol complexes as potential anticancer drugs. J. Inorg. Biochem. 2023, 238, 112051. [Google Scholar] [CrossRef]
- Chen, L.J.; Yan, L.; Gu, Z.; Chen, Z.; Zhang, A.; Zhao, F. A Novel Drug Design Strategy: An Inspiration from Encaging Tumor by Metallofullerenol Gd@C82(OH)22. Molecules 2019, 24, 2387. [Google Scholar]
- Bünzli, J.-C.G. Lanthanide Luminescence for Biomedical Analyses and Imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Armelao, L.; Quici, S.; Barigelletti, F.; Accorsi, G.; Bottaro, G.; Cavazzini, M.; Tondello, E. Design of luminescent lanthanide complexes: From molecules to highly efficient photo-emitting materials. Coord. Chem. Rev. 2010, 254, 487–505. [Google Scholar] [CrossRef]
- Moore, E.G.; Samuel, A.P.S.; Raymond, K.N. From Antenna to Assay: Lessons Learned in Lanthanide Luminescence. Acc. Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, S.; Delalande, A.; Eliseeva, S.V.; Pallier, A.; Bonnet, C.S.; Szeremeta, F.; Même, S.; Pichon, C.; Petoud, S.; Tóth, E. Doxorubicin-Sensitized Luminescence of NIR-Emitting Ytterbium Liposomes: Towards Direct Monitoring of Drug Release. Angew. Chem. Int. Ed. 2021, 60, 23574–23577. [Google Scholar] [CrossRef]
- Thor, W.; Wu, Y.; Wang, L.; Zhang, Y.; Tanner, P.A.; Wong, K.-L. Charging and ultralong phosphorescence of lanthanide facilitated organic complex. Nat. Commun. 2021, 12, 6532. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Wu, Y.; Chau, H.-F.; Zhang, T.; Wong, C.T.; Yeung, Y.-H.; Lam, P.-L.; Bünzli, J.-C.G.; Charbonnière, L.J.; Law, G.-L.; et al. Multimodal Visualization of Bioorthogonal Systems by Off–On Luminescence and Enhanced Magnetic Resonance Imaging. Adv. Opt. Mater. 2023, 11, 2203057. [Google Scholar] [CrossRef]
- Ferreira da Rosa, P.P.; Kitagawa, Y.; Shoji, S.; Oyama, H.; Imaeda, K.; Nakayama, N.; Fushimi, K.; Uekusa, H.; Ueno, K.; Goto, H.; et al. Preparation of photonic molecular trains via softcrystal polymerization of lanthanide complexes. Nat. Commun. 2022, 13, 3660. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Xu, J.; Dos Santos, M.C.; Hildebrandt, N. Multiplexed Biosensing and Bioimaging Using Lanthanide-Based Time-Gated Förster Resonance Energy Transfer. Acc. Chem. Res. 2022, 55, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Fan, J.; Chen, B.; Qin, X.; Wang, J.; Wang, F.; Deng, R.; Liu, X. Rare-Earth Doping in Nanostructured Inorganic Materials. Chem. Rev. 2022, 122, 5519–5603. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Jiang, Y.; Hou, Z.; Cheng, Z.; Ma, P.; Li, C.; Lin, J. Highly luminescent lanthanide fluoride nanoparticles functionalized by aromatic carboxylate acids. RSC Adv. 2014, 4, 55100. [Google Scholar] [CrossRef]
- Cheignon, C.; Kassir, A.A.; Soro, L.K.; Charbonnière, L.J. Dye-sensitized lanthanide containing nanoparticles for luminescence based applications. Nanoscale 2022, 14, 13915–13949. [Google Scholar] [CrossRef]
- Knighton, R.C.; Soro, L.K.; Thor, W.; Strub, J.-M.; Cianférani, S.; Mély, Y.; Lenertz, M.; Wong, K.-L.; Platas-Iglesias, C.; Przybilla, F.; et al. Upconversion in a d–f [RuYb3] Supramolecular Assembly. J. Am. Chem. Soc. 2022, 144, 13356–13365. [Google Scholar] [CrossRef]
- Golesorkhi, B.; Naseri, S.; Guénée, L.; Taarit, I.; Alves, F.; Nozary, H.; Piguet, C. Ligand-Sensitized Near-Infrared to Visible Linear Light Upconversion in a Discrete Molecular Erbium Complex. J. Am. Chem. Soc. 2021, 143, 15326–15334. [Google Scholar] [CrossRef]
- Gee, W.J. Recent Trends Concerning Upconversion Nanoparticles and Near-IR Emissive Lanthanide Materials in the Context of Forensic Applications. Aust. J. Chem. 2019, 72, 164–173. [Google Scholar] [CrossRef]
- Liu, Y.; Aia, K.; Lu, L. Designing lanthanide-doped nanocrystals with both up- and down-conversion luminescence for anti-counterfeiting. Nanoscale 2011, 3, 4804–4810. [Google Scholar] [CrossRef]
- Shoji, S.; Saito, H.; Jitsuyama, Y.; Tomita, K.; Haoyang, Q.; Sakurai, Y.; Okazaki, Y.; Aikawa, K.; Konishi, Y.; Sasaki, K.; et al. Plant growth acceleration using a transparent Eu3+-painted UV-to-red conversion film. Sci. Rep. 2022, 12, 17155. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wang, Y.; Kuzmin, A.; Hua, Y.; Zhao, J.; Xu, S.; Prasad, P.N. Next generation lanthanide doped nanoscintillators and photon converters. eLight 2022, 2, 17. [Google Scholar] [CrossRef]
- Hou, B.; Yi, L.; Hu, D.; Luo, Z.; Gao, D.; Li, C.; Xing, B.; Wang, J.-W.; Lee, C.N.; Zhang, R.; et al. A swallowable X-ray dosimeter for the real-time monitoring of radiotherapy. Nat. Biomed. Eng. 2023, 7, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.D.S.; Balabhadra, S.; Carlos, L.D. Lanthanide-Based Thermometers: At the Cutting-Edge of Luminescence Thermometry. Adv. Opt. Mater. 2019, 7, 1801239. [Google Scholar] [CrossRef]
- Guan, H.; Qi, M.; Shi, L.; Liu, W.; Yang, L.; Dou, W. Ratiometric Luminescent Thermometer Based on the Lanthanide Metal–Organic Frameworks by Thermal Curing. ACS Appl. Mater. Interfaces 2023, 15, 18114–18124. [Google Scholar] [CrossRef]
- Zanella, S.; Aragon-Alberti, M.; Brite, C.D.S.; Salles, F.; Carlos, L.D.; Long, J. Luminescent Single-Molecule Magnets as Dual Magneto-Optical Molecular Thermometers. Angew. Chem. Int. Ed. 2023, 62, e2023069. [Google Scholar] [CrossRef]
- Carr, R.; Evans, N.H.; Parker, D. Lanthanide complexes as chiral probes exploiting circularly polarized luminescence. Chem. Soc. Rev. 2012, 41, 7673–7686. [Google Scholar] [CrossRef]
- Doistau, B.; Jiménez, J.-R.; Piguet, C. Beyond Chiral Organic (p-Block) Chromophores for Circularly Polarized Luminescence: The Success of d-Block and f-Block Chiral Complexes. Front. Chem. 2002, 8, 555. [Google Scholar] [CrossRef] [PubMed]
- Barry, D.E.; Kitchen, J.A.; Mercs, L.; Peacock, R.D.; Albrecht, M.; Gunnlaugsson, T. Chiral luminescent lanthanide complexes possessing strong (samarium, SmIII) circularly polarised luminescence (CPL), and their self-assembly into Langmuir–Blodgett films. Dalton Trans. 2019, 48, 11317–11325. [Google Scholar] [CrossRef] [PubMed]
- Dhbaibi, K.; Grasser, M.; Douib, H.; Dorcet, V.; Cador, O.; Vanthuyne, N.; Riobé, F.; Maury, O.; Guy, S.; Bensalah-Ledoux, A.; et al. Multifunctional Helicene-Based Ytterbium Coordination Polymer Displaying Circularly Polarized Luminescence, Slow Magnetic Relaxation and Room Temperature Magneto-Chiral Dichroism. Angew. Chem. Int. Ed. 2023, 62, e202215558. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.; Kuppusamy, S.K.; Heinrich, B.; Fuhr, O.; Hunger, D.; Ruben, M.; Goldner, P. Ultra-narrow optical linewidths in rare-earth molecular crystals. Nature 2022, 603, 241. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, G.; Mandl, G.A.; Maurizio, S.L.; Kaur, M.; Capobianco, J.A. The role of lanthanide luminescence in advancing technology. RSC Adv. 2023, 13, 17787–17811. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G. Lanthanide Photonics: Shaping the Nanoworld. Trends Chem. 2019, 1, 751–762. [Google Scholar] [CrossRef]
- Mor, O.E.; Ohana, T.; Borne, A.; Diskin-Posner, Y.; Asher, M.; Yaffe, O.; Shanzer, A.; Dayan, B. Tapered Optical Fibers Coated with Rare-Earth Complexes for Quantum Applications. ACS Photonics 2022, 9, 2676–2682. [Google Scholar] [CrossRef]
- Zhao, J.; Mei, D.; Wang, W.; Wu, Y.; Xue, D. Recent advances in nonlinear optical rare earth structures. J. Rare Earths 2021, 39, 1455–1466. [Google Scholar] [CrossRef]
- Li, P.; Xu, X.; Zhao, J.; Awasthi, P.; Qiao, X.; Du, J.; Fan, X.; Qian, G. Lanthanide doped fluorosilicate glass-ceramics: A review on experimental and theoretical progresses. J. Rare Earth 2022, 40, 169–192. [Google Scholar] [CrossRef]
- Chandrappa, V.; Basavapoornima, C.; Venkatramu, V.; Depuru, S.R.; Kaewkhao, J.; Pecharapa, W.; Jayasankar, C.K. A critical review and future prospects of Dy3+-doped glasses for white light emission applications. Optik 2022, 266, 169583. [Google Scholar] [CrossRef]
- Yin, X.; Deng, L.; Ruan, L.; Wu, Y.; Luo, F.; Qin, G.; Han, X.; Zhang, X. Progress for Single-Molecule Magnets Based on Rare Earth Elements. Mater. 2023, 16, 3568. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Finol, G.M.; Giménez-Santamarina, S.; Hu, Z.; Rosaleny, L.E.; Cardona-Serra, S.; Gaita-Ariño, A. Lanthanide molecular nanomagnets as probabilistic bits. Npj Comput. Mater. 2023, 9, 196. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400. [Google Scholar] [CrossRef] [PubMed]
- Gould, C.A.; McClain, K.R.; Reta, D.; Kragskow, J.G.C.; Marchiori, D.A.; Lachman, E.; Choi, E.-S.; Analytis, J.G.; Britt, R.D.; Chilton, N.F.; et al. Ultrahard magnetism from mixed-valence dilanthanide complexes with metal-metal bonding. Science 2022, 375, 198–202. [Google Scholar] [CrossRef]
- Evans, P.; Reta, D.; Goodwin, C.A.P.; Ortu, F.; Chilton, N.F.; Mills, D.P. A double-dysprosocenium single-molecule magnet bound together with neutral ligands. Chem. Commun. 2020, 56, 5677. [Google Scholar] [CrossRef]
- Bernot, K. Get under the Umbrella: A Comprehensive Gateway for Researchers on Lanthanide-Based Single-Molecule Magnets. Eur. J. Inorg. Chem. 2023, 26, e202300336. [Google Scholar] [CrossRef]
- Liu, F.; Spree, L.; Krylov, D.S.; Velkos, G.; Avdoshenko, S.M.; Popov, A.A. Single-Electron Lanthanide-Lanthanide Bonds Inside Fullerenes toward Robust Redox-Active Molecular Magnets. Acc. Chem. Res. 2019, 52, 2981–2993. [Google Scholar] [CrossRef]
- Shen, W.; Hu, S.; Lu, X. Endohedral Metallofullerenes: New Structures and Unseen Phenomena. Chem. Eur. J. 2020, 26, 5748–5757. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Ivanov, M.S.; Khomchenko, V.A.; Mamontova, E.; Thibaud, J.-M.; Rouquette, J.; Beaudhuin, M.; Granier, D.; Ferreira, R.A.S.; Carlos, L.D.; et al. Room temperature magnetoelectric coupling in a molecular ferroelectric ytterbium(III) complex. Science 2020, 367, 671–676. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.R.; Bates, J.E.; Ziller, J.W.; Furche, F.; Evans, W.J. Completing the Series of +2 Ions for the Lanthanide Elements: Synthesis of Molecular Complexes of Pr2+, Gd2+, Tb2+, and Lu2+. J. Am. Chem. Soc. 2013, 26, 9857–9868. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C.T.; Zivkovic, I.; Scopelliti, R.; Mazzanti, M. Molecular Complex of Tb in the +4 Oxidation State. J. Am. Chem. Soc. 2019, 141, 9827–9831. [Google Scholar] [CrossRef] [PubMed]
- Willauer, A.R.; Palumbo, C.T.; Fadaei-Tirani, F.; Zivkovic, I.; Douair, I.; Maron, L.; Mazzanti, M. Accessing the +IV Oxidation State in Molecular Complexes of Praseodymium. J. Am. Chem. Soc. 2020, 142, 5538–5542. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, F.-C.; Rajeshkumar, T.; Maron, L.; Scopelliti, R.; Sienkiewicz, A.; Mazzanti, M. Isolation and redox reactivity of cerium complexes in four redox states. Chem. Sci. 2023, 14, 6011. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yuan, S.; Li, J.; Wang, H.-Y.; Ge, J.-Y.; Drake, H.F.; Leong, C.F.; Yu, F.; D’Alessandro, D.M.; Kurmoo, M.; et al. Rare-Earth Metal Tetrathiafulvalene Carboxylate Frameworks as Redox-Switchable Single-Molecule Magnets. Chem. Eur. J. 2021, 27, 622–627. [Google Scholar] [CrossRef]
- Li, Q.; Yam, V.W.-W. Redox Luminescence Switch Based on Energy Transfer in CePO4:Tb3+ Nanowires. Angew. Chem. Int. Ed. 2007, 46, 3486–3489. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Maslova, O.V.; Morozov, A.G.; Dechert, S.; Demeshko, S.; Meyer, F. Genuine Redox Isomerism in a Rare-Earth-Metal Complex. Angew. Chem. Int. Ed. 2012, 51, 10584–10587. [Google Scholar] [CrossRef]
- Ariciu, A.-M.; Woen, D.H.; Huh, D.N.; Nodaraki, L.E.; Kostopoulos, A.K.; Goodwin, C.A.P.; Chilton, N.F.; McInnes, E.J.L.; Winpenny, R.E.P.; Evans, W.J.; et al. Engineering electronic structure to prolong relaxation times in molecular qubits by minimising orbital angular momentum. Nat. Commun. 2019, 10, 3330. [Google Scholar] [CrossRef]
- Richards, V. Molecular machines. Nat. Chem. 2016, 8, 1090. [Google Scholar] [CrossRef]
- Evans, N.H. Lanthanide-Containing Rotaxanes, Catenanes, and Knots. ChemPlusChem 2020, 85, 783–792. [Google Scholar] [CrossRef]
- Zhang, Y.; Kersell, H.; Stefak, R.; Echeverria, J.; Iancu, V.; Perera, U.G.E.; Li, Y.; Deshpande, A.; Braun, K.-F.; Joachim, C.; et al. Simultaneous and coordinated rotational switching of all molecular rotors in a network. Nat. Nanotechnol. 2016, 11, 706–713. [Google Scholar] [CrossRef]
- Frank, T.; Edelmann, F.T. Lanthanide amidinates and guanidinates: From laboratory curiosities to efficient homogeneous catalysts and precursors for rare-earth oxide thin films. Chem. Soc. Rev. 2009, 38, 2253–2268. [Google Scholar] [CrossRef]
- López-Cervantes, V.B.; Obeso, J.L.; Yañez-Aulestia, A.; Islas-Jácome, A.; Leyva, C.; González-Zamora, E.; Sánchez-González, E.; Ibarra, I.A. MFM-300(Sc): A chemically stable Sc(III)-based MOF material for multiple applications. Chem. Commun. 2023, 59, 10343–10359. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Gao, Y.; Du, Y.; Zhou, L.; Chen, X. Recent Advances in Developing Lanthanide Metal–Organic Frameworks for Ratiometric Fluorescent Sensing. Front. Chem. 2020, 8, 624592. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhao, Y.; Wang, J.; Pei, R. Lanthanide-based MOFs: Synthesis approaches and applications in cancer diagnosis and therapy. J. Mater. Chem. B 2022, 10, 9535–9564. [Google Scholar] [CrossRef] [PubMed]
- Gorai, T.; Schmitt, W.; Gunnlaugsson, T. Highlights of the development and application of luminescent lanthanide based coordination polymers, MOFs and functional nanomaterials. Dalton Trans. 2021, 50, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Echenique-Errandonea, E.; Mendes, R.F.; Figueira, F.; Choquesillo-Lazarte, D.; Beobide, G.; Cepeda, J.; Ananias, D.; Rodríguez-Diéguez, A.; Paz, F.A.A.; Seco, J.M. Multifunctional Lanthanide-Based Metal–Organic Frameworks Derived from 3-Amino-4-hydroxybenzoate: Single-Molecule Magnet Behavior, Luminescent Properties for Thermometry, and CO2 Adsorptive Capacity. Inorg. Chem. 2022, 61, 12977–12990. [Google Scholar] [CrossRef] [PubMed]
- Deneff, J.L.; Rohwer, L.E.S.; Butler, K.S.; Kaehr, B.; Vogel, D.J.; Luk, T.S.; Reyes, R.A.; Cruz-Cabrera, A.A.; Martin, J.E.; Gallis, D.F.S. Orthogonal luminescence lifetime encoding by intermetallic energy transfer in heterometallic rare-earth MOFs. Nat. Commun. 2023, 14, 981. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behrsing, T.; Blair, V.L.; Jaroschik, F.; Deacon, G.B.; Junk, P.C. Rare Earths—The Answer to Everything. Molecules 2024, 29, 688. https://doi.org/10.3390/molecules29030688
Behrsing T, Blair VL, Jaroschik F, Deacon GB, Junk PC. Rare Earths—The Answer to Everything. Molecules. 2024; 29(3):688. https://doi.org/10.3390/molecules29030688
Chicago/Turabian StyleBehrsing, Thomas, Victoria L. Blair, Florian Jaroschik, Glen B. Deacon, and Peter C. Junk. 2024. "Rare Earths—The Answer to Everything" Molecules 29, no. 3: 688. https://doi.org/10.3390/molecules29030688





