Discovery of a New Compound, Erinacerin W, from the Mycelia of Hericium erinaceus, with Immunomodulatory and Neuroprotective Effects
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Material Preparation
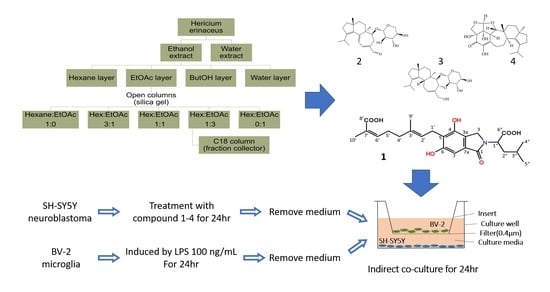
3.3. Extraction and Isolation
Erinacerin W (1)
3.4. Co-Culture of BV-2 Microglia and SH-SY5Y Neurons
3.5. Total RNA Isolation and Quantitative PCR (qPCR) Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.N.; Dantoine, T.; Dartigues, J.F.; et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): A randomised, placebo-controlled trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef]
- Ulziijargal, E.; Mau, J.L. Nutrient compositions of culinary-medicinal mushroom fruiting bodies and mycelia. Int. J. Med. Mushrooms 2011, 13, 343–349. [Google Scholar] [CrossRef]
- Phan, C.W.; David, P.; Naidu, M.; Wong, K.H.; Sabaratnam, V. Therapeutic potential of culinary-medicinal mushrooms for the management of neurodegenerative diseases: Diversity, metabolite, and mechanism. Crit. Rev. Biotechnol. 2015, 35, 355–368. [Google Scholar] [CrossRef]
- Li, G.; Yu, K.; Li, F.; Xu, K.; Li, J.; He, S.; Cao, S.; Tan, G. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J. Ethnopharmacol. 2014, 153, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.K.; Park, J.B.; Song, C.H. Hypolipidemic effect of an Exo-biopolymer produced from a submerged mycelial culture of Hericium erinaceus. Biosci. Biotechnol. Biochem. 2003, 67, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Tang, L.; Xie, Y.; Xie, L. Secondary Metabolites from Hericium erinaceus and Their Anti-Inflammatory Activities. Molecules 2022, 27, 2157. [Google Scholar] [CrossRef] [PubMed]
- Spiteller, P. Chemical ecology of fungi. Nat. Prod. Rep. 2015, 32, 971–993. [Google Scholar] [CrossRef] [PubMed]
- Snider, B.B.; Vo, N.H.; O’Nei, S.V.; Foxman, B.M. Synthesis of (±)-Allocyathin B2 and (+)-Erinacine, A.J. Am. Chem. Soc. 1996, 118, 7644–7645. [Google Scholar] [CrossRef]
- Ma, B.-J.; Shen, J.-W.; Yu, H.-Y.; Ruan, Y.; Wu, T.-T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology 2010, 1, 92–98. [Google Scholar] [CrossRef]
- Zhang, C.C.; Cao, C.Y.; Kubo, M.; Harada, K.; Yan, X.T.; Fukuyama, Y.; Gao, J.M. Chemical Constituents from Hericium erinaceus Promote Neuronal Survival and Potentiate Neurite Outgrowth via the TrkA/Erk1/2 Pathway. Int. J. Mol. Sci. 2017, 18, 1659. [Google Scholar] [CrossRef]
- Tan, Y.F.; Mo, J.S.; Wang, Y.K.; Zhang, W.; Jiang, Y.P.; Xu, K.P.; Tan, G.S.; Liu, S.; Li, J.; Wang, W.X. The ethnopharmacology, phytochemistry and pharmacology of the genus Hericium. J. Ethnopharmacol. 2024, 319, 117353. [Google Scholar] [CrossRef]
- Wang, K.; Bao, L.; Qi, Q.; Zhao, F.; Ma, K.; Pei, Y.; Liu, H. Erinacerins C–L, Isoindolin-1-ones with α-Glucosidase Inhibitory Activity from Cultures of the Medicinal Mushroom Hericium erinaceus. J. Nat. Prod. 2015, 78, 146–154. [Google Scholar] [CrossRef]
- Ashour, A.; Amen, Y.; Allam, A.E.; Kudo, T.; Nagata, M.; Ohnuki, K.; Shimizu, K. New isoindolinones from the fruiting bodies of the fungus Hericium erinaceus. Phytochem. Lett. 2019, 32, 10–14. [Google Scholar] [CrossRef]
- Lin, C.-F.; Shiao, Y.-J.; Chen, C.-C.; Tzeng, T.-T.; Chen, C.-C.; Lee, L.-Y.; Chen, W.-P.; Shen, C.-C. A xanthurenate and an isoindolinone from the mycelia of Hericium erinaceum. Phytochem. Lett. 2018, 26, 218–221. [Google Scholar] [CrossRef]
- Song, X.; Gaascht, F.; Schmidt-Dannert, C.; Salomon, C.E. Discovery of Antifungal and Biofilm Preventative Compounds from Mycelial Cultures of a Unique North American Hericium sp. Fungus. Molecules 2020, 25, 963. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.J.; Yoon, J.Y.; Kim, G.S.; Lee, S.E.; Lee, D.Y.; Choi, J.H.; Kim, S.Y.; Kang, K.S.; Cho, J.Y.; Kim, K.H. Benzyl alcohol derivatives from the mushroom Hericium erinaceum attenuate LPS-stimulated inflammatory response through the regulation of NF-κB and AP-1 activity. Immunopharmacol. Immunotoxicol. 2014, 36, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Lv, H.; Zhang, X. Erinacerins, Novel Glioma Inhibitors from Hericium erinaceus, Induce Apoptosis of U87 Cells through Bax/Capase-2 Pathway. Anticancer Agents Med. Chem. 2020, 20, 2082–2088. [Google Scholar] [CrossRef]
- Wang, K.; Bao, L.; Ma, K.; Liu, N.; Huang, Y.; Ren, J.; Wang, W.; Liu, H. Eight new alkaloids with PTP1B and α-glucosidase inhibitory activities from the medicinal mushroom Hericium erinaceus. Tetrahedron 2015, 71, 9557–9563. [Google Scholar] [CrossRef]
- Peng, Y.; Chu, S.; Yang, Y.; Zhang, Z.; Pang, Z.; Chen, N. Neuroinflammatory In Vitro Cell Culture Models and the Potential Applications for Neurological Disorders. Front. Pharmacol 2021, 12, 671734. [Google Scholar] [CrossRef] [PubMed]
- Pandur, E.; Varga, E.; Tamási, K.; Pap, R.; Nagy, J.; Sipos, K. Effect of Inflammatory Mediators Lipopolysaccharide and Lipoteichoic Acid on Iron Metabolism of Differentiated SH-SY5Y Cells Alters in the Presence of BV-2 Microglia. Int. J. Mol. Sci. 2018, 20, 17. [Google Scholar] [CrossRef]
- Clark, I.A.; Vissel, B. The meteorology of cytokine storms, and the clinical usefulness of this knowledge. Semin. Immunopathol. 2017, 39, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Isacson, O. The Consequences of Coronavirus-Induced Cytokine Storm Are Associated with Neurological Diseases, Which May Be Preventable. Front. Neurol. 2020, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Hsu, J.Y.; Chen, T.C.; Huang, C.C.; Wu, T.Y.; Chin, T.Y. Erinacine A Prevents Lipopolysaccharide-Mediated Glial Cell Activation to Protect Dopaminergic Neurons against Inflammatory Factor-Induced Cell Death In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 810. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Huang, C.S.; Chen, Y.H.; Chen, C.C.; Chen, C.C.; Chuang, C.H. Anti-Inflammatory Effect of Erinacine C on NO Production Through Down-Regulation of NF-κB and Activation of Nrf2-Mediated HO-1 in BV2 Microglial Cells Treated with LPS. Molecules 2019, 24, 3317. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Wang, J.; Wang, Q.; Ma, Q.; Chen, Y. Protocatechuic Acid Inhibits Inflammatory Responses in LPS-Stimulated BV2 Microglia via NF-κB and MAPKs Signaling Pathways. Neurochem. Res. 2015, 40, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Tzeng, T.T.; Chen, C.C.; Ni, C.L.; Lee, L.Y.; Chen, W.P.; Shiao, Y.J.; Shen, C.C. Erinacine S, a Rare Sesterterpene from the Mycelia of Hericium erinaceus. J. Nat. Prod. 2016, 79, 438–441. [Google Scholar] [CrossRef]
- Kawagishi, H.; Shimada, A.; Shirai, R.; Okamoto, K.; Ojima, F.; Sakamoto, H.; Ishiguro, Y.; Furukawa, S. Erinacines A, B and C, strong stimulators of nerve growth factor (NGF)-synthesis, from the mycelia of Hericium erinaceum. Tetrahedron Lett. 1994, 35, 1569–1572. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Y.; Zhang, Y.; Liu, F.; Rose, G.M.; He, X.; Yi, X.; Ren, R.; Li, Y.; Zhang, Y.; et al. Butein attenuates the cytotoxic effects of LPS-stimulated microglia on the SH-SY5Y neuronal cell line. Eur. J. Pharmacol. 2020, 868, 172858. [Google Scholar] [CrossRef]
- Jaisin, Y.; Thampithak, A.; Meesarapee, B.; Ratanachamnong, P.; Suksamrarn, A.; Phivthong-ngam, L.; Phumala-Morales, N.; Chongthammakun, S.; Govitrapong, P.; Sanvarinda, Y. Curcumin I protects the dopaminergic cell line SH-SY5Y from 6-hydroxydopamine-induced neurotoxicity through attenuation of p53-mediated apoptosis. Neurosci. Lett. 2011, 489, 192–196. [Google Scholar] [CrossRef]






| Position | δC, Type | δH (J in Hz) | HMBC | NOESY | COSY |
|---|---|---|---|---|---|
| 1 | 170.6, CH | ||||
| 2 | |||||
| 3 | 44.8, CH2 | 4.52 d (16.8); 4.27 d (16.8) | 3 a, 7 a, 4, 1 | 2″, 3″ | |
| 3 a | 119.7, C | ||||
| 4 | 150.4, C | ||||
| 5 | 120.4, C | ||||
| 6 | 156.6, C | ||||
| 7 | 100.7, CH | 6.78 s | 3 a, 5, 4, 6, 1 | 2′, 4′, 5′ | |
| 7 a | 130.0, C | ||||
| 1′ | 22.3, CH2 | 3.45 d (6.8) | 3 a, 5, 2′, 3′, 4, 6 | 2′ | |
| 2′ | 123.0, CH | 5.33 t (6.8) | 1′, 4′, 9′ | 1′, 4′, 5′, 6′ | 1′ |
| 3′ | 133.5, C | ||||
| 4′ | 38.0, CH2 | 2.11 t (7.6) | 2′, 3′, 5′, 6′ | 7, 2′, 5′, 6′, 10′ | 5′ |
| 5′ | 27.0, CH2 | 2.30 q (7.6) | 3′, 4′, 6′, 7′ | 2′, 4′, 6′, 7, 10′ | 4′, 6′ |
| 6′ | 142.3, CH | 6.75 t (7.6) | 4′, 5′, 8′, 10′ | 2′,4′, 5′, 9′ | 5′, 10′ |
| 7′ | 127.4, C | ||||
| 8′ | 170.3, C | ||||
| 9′ | 14.9, CH3 | 1.84 s | 2′, 4′ | 4′, 5′, 6′ | |
| 10′ | 11.0, CH3 | 1.78 s | 6′, 7′, 8′ | 4′, 5′ | 6′ |
| 1″ | 52.5, CH | 5.01 dd (4.4, 10.8) | 1, 3, 2″, 6″ | 2″, 4″, 5″, | 2″ |
| 2″ | 38.3, CH2 | 1.95 m | 1″, 3″, 4″, 5″ | 1″, 3, 7, 6′, | 1″, 3″ |
| 3″ | 24.9, CH | 1.5 m | 3, 2″, 4″, 5″ | 2″, 4″, 5″ | |
| 4″ | 20.1, CH3 | 1.00 d (7.6) | 2″, 3″, 5″ | 1″, 2″, 3″, 5″ | 3″, 5″ |
| 5″ | 22.1, CH3 | 1.00 d (7.6) | 2″, 3″, 4″ | 1″, 2″, 3″ 4″ | 3″, 4″ |
| 6″ | 170.6, C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-Y.; Chen, Y.-P.; Lin, T.-W.; Li, T.-J.; Chen, Y.-W.; Li, I.-C.; Chen, C.-C. Discovery of a New Compound, Erinacerin W, from the Mycelia of Hericium erinaceus, with Immunomodulatory and Neuroprotective Effects. Molecules 2024, 29, 812. https://doi.org/10.3390/molecules29040812
Lin J-Y, Chen Y-P, Lin T-W, Li T-J, Chen Y-W, Li I-C, Chen C-C. Discovery of a New Compound, Erinacerin W, from the Mycelia of Hericium erinaceus, with Immunomodulatory and Neuroprotective Effects. Molecules. 2024; 29(4):812. https://doi.org/10.3390/molecules29040812
Chicago/Turabian StyleLin, Jing-Yi, Yen-Po Chen, Ting-Wei Lin, Tsung-Ju Li, Yu-Wen Chen, I-Chen Li, and Chin-Chu Chen. 2024. "Discovery of a New Compound, Erinacerin W, from the Mycelia of Hericium erinaceus, with Immunomodulatory and Neuroprotective Effects" Molecules 29, no. 4: 812. https://doi.org/10.3390/molecules29040812