Design of Experimental Approach for Development of Rapid High Performance Liquid Chromatographic Process for Simultaneous Estimation of Metoprolol, Telmisartan, and Amlodipine from Formulation: Greenness and Whiteness Evaluation
Abstract
:1. Introduction
2. Materials and Methods
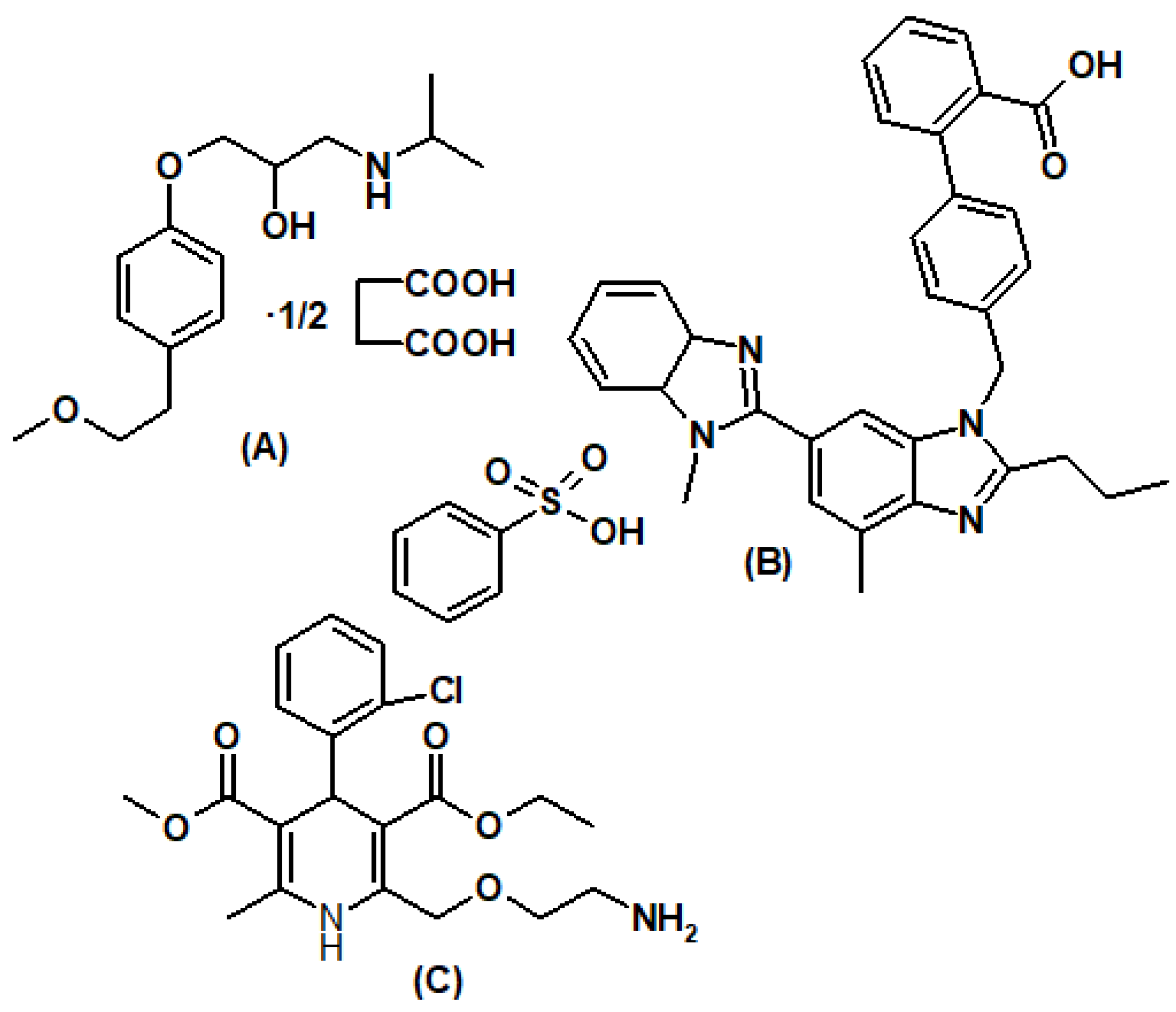
2.1. Materials
2.2. Instrument
2.3. Preparation of Mobile Phase
2.4. Preparation of Reference Standard Solutions
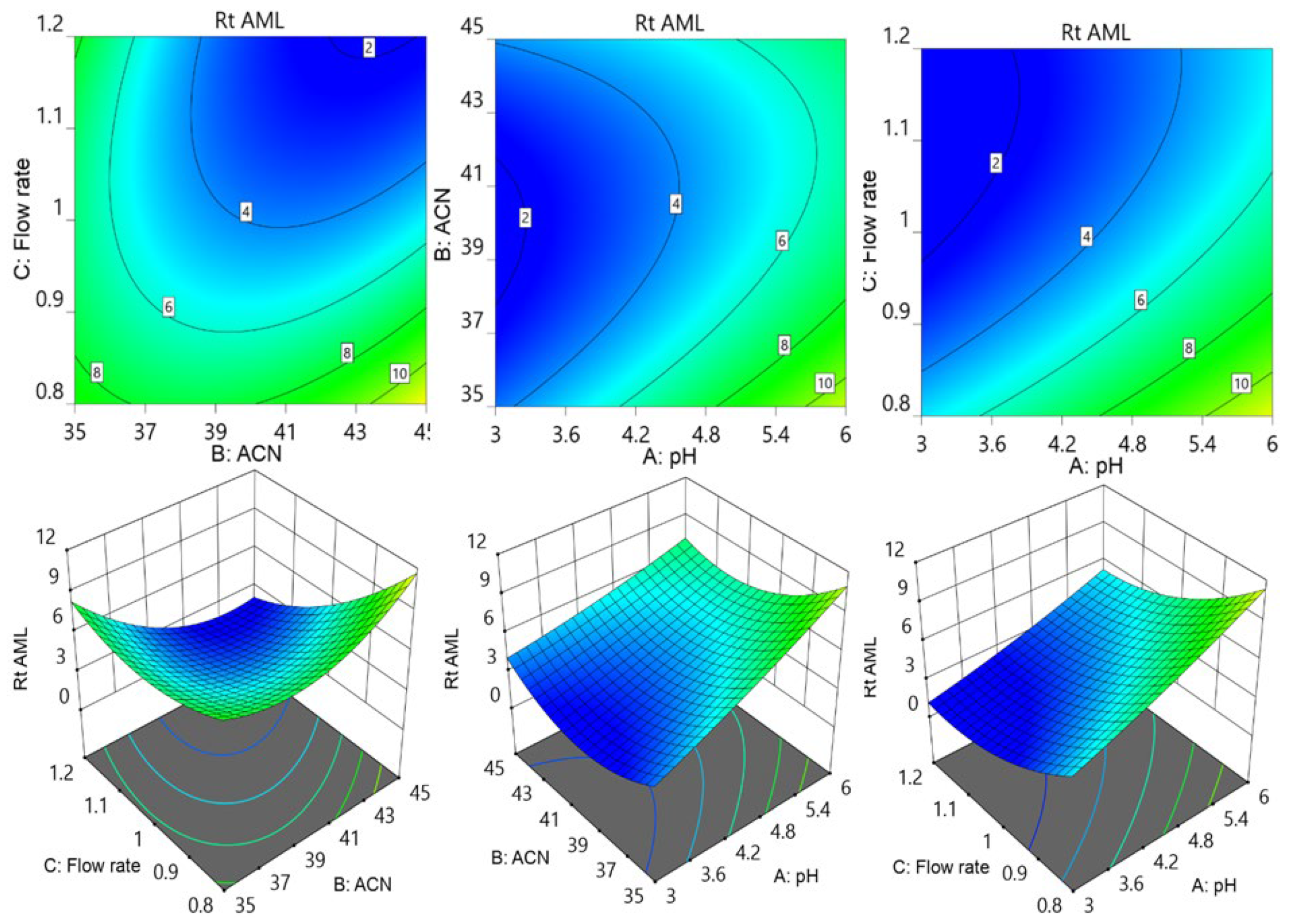
2.5. Box–Behnken Model to Optimize Chromatographic Conditions
2.6. Calibration Curve Construction
2.7. Sample Preparation for the Analysis of Formulation
2.8. Validation of the HPLC Method
2.8.1. System Suitability Study
2.8.2. Linearity and Sensitivity
2.8.3. Correctness and Precision
2.8.4. Robustness
2.9. Application of HPLC Method to Formulation
3. Results and Discussion
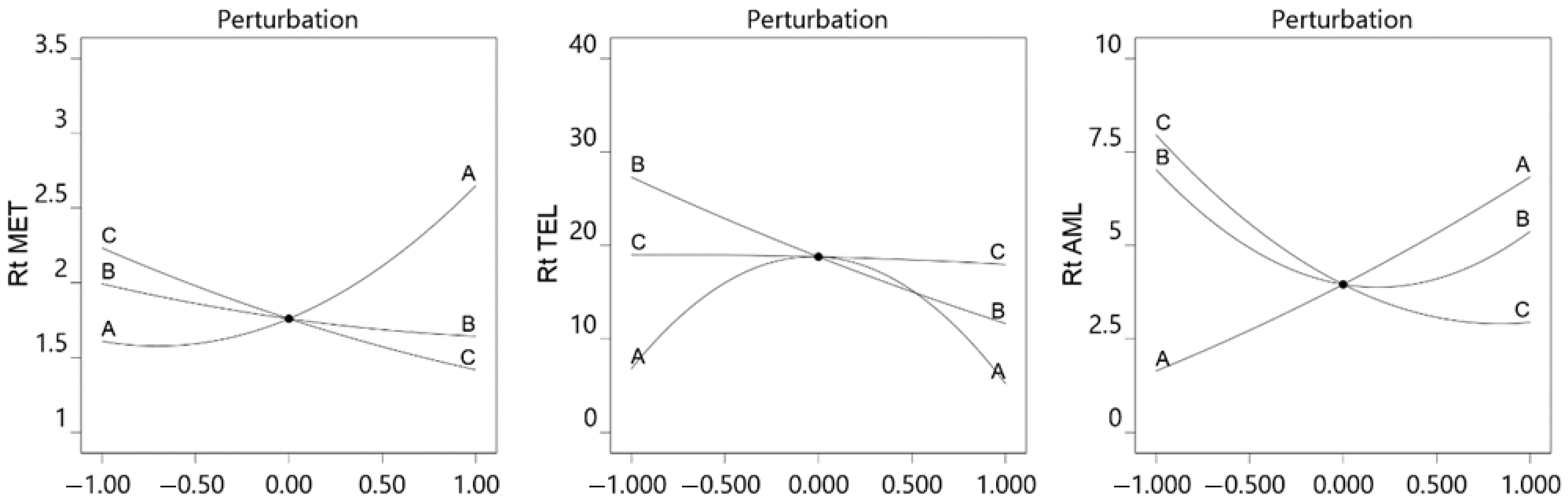
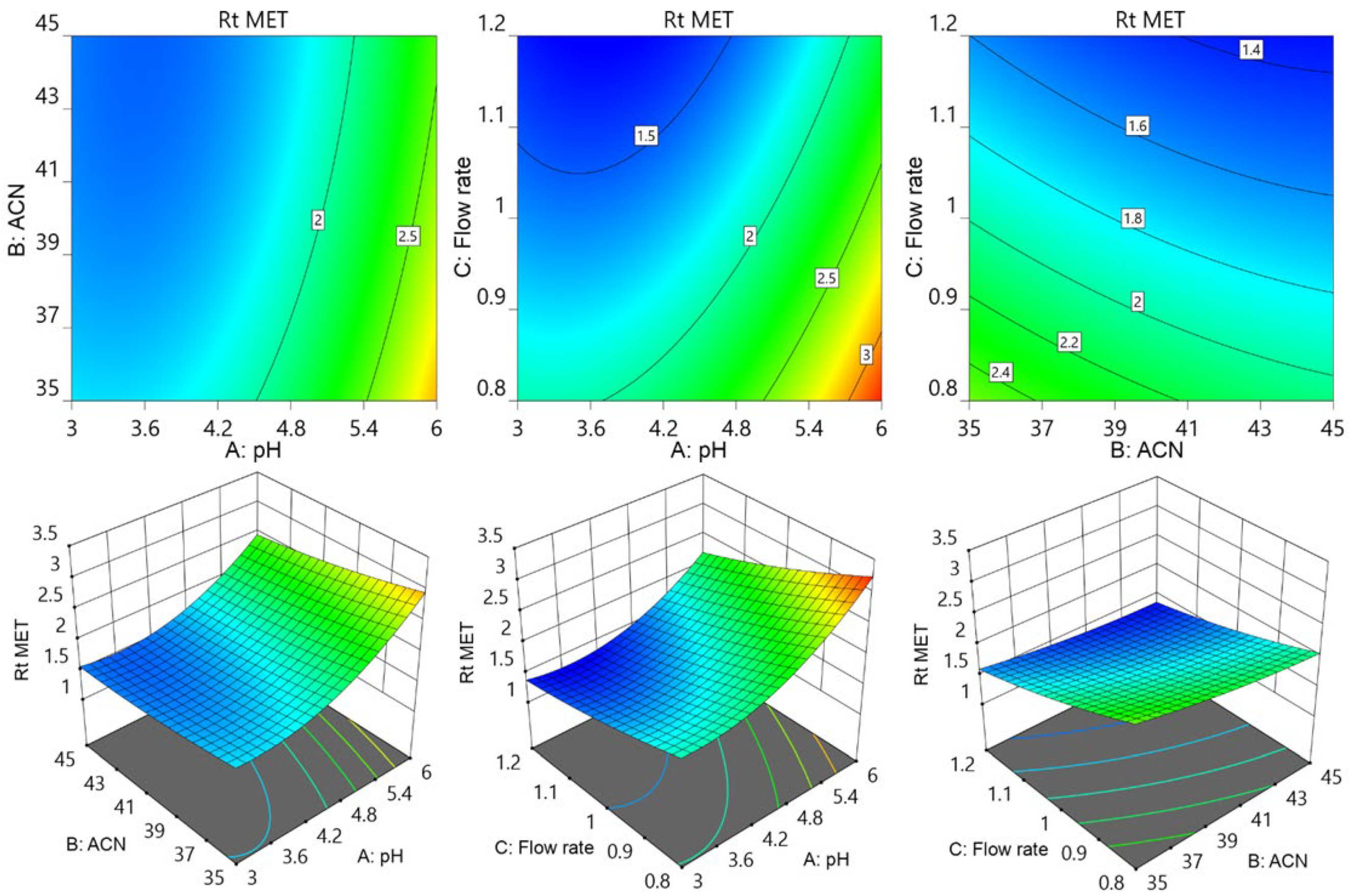
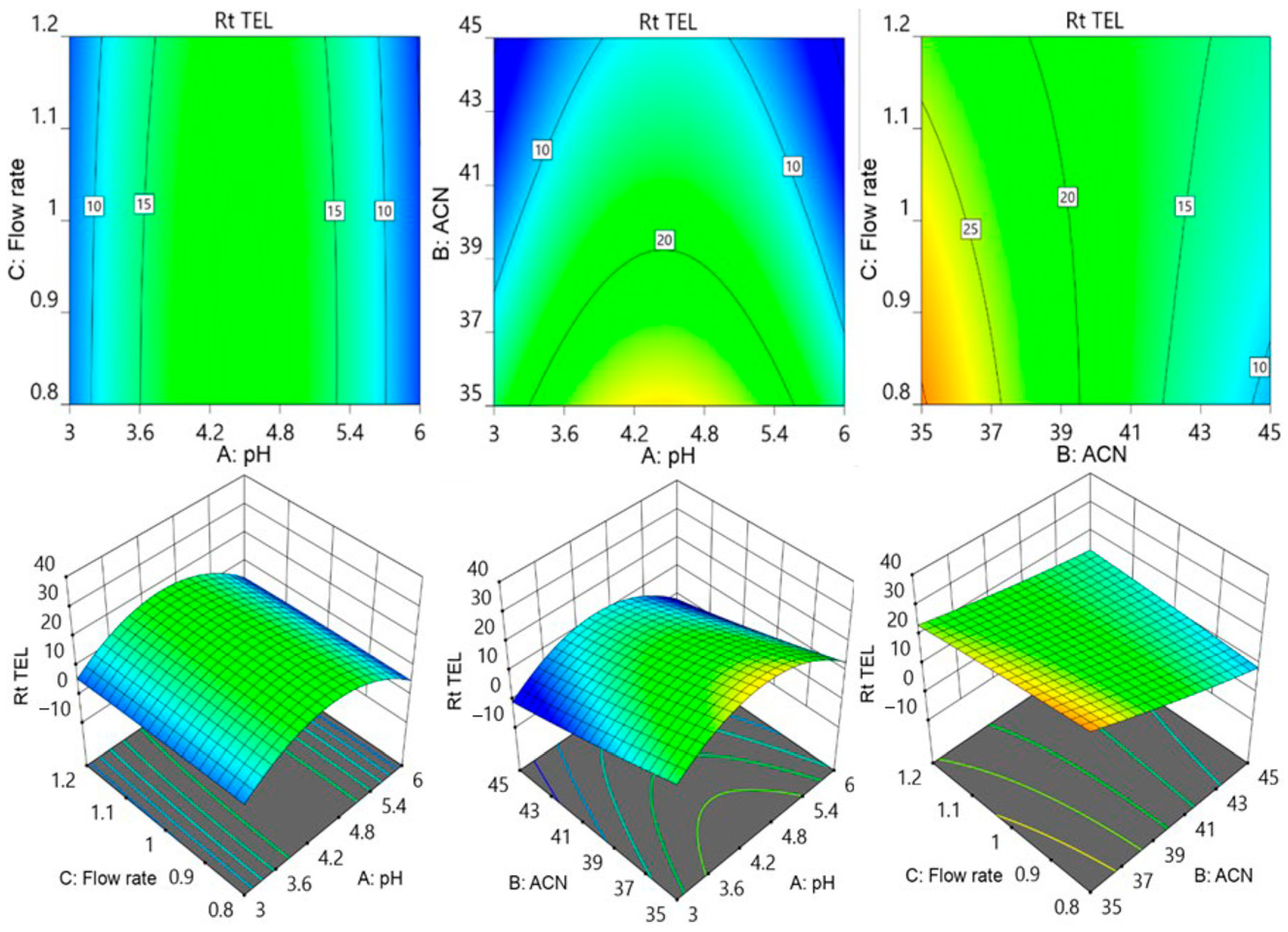
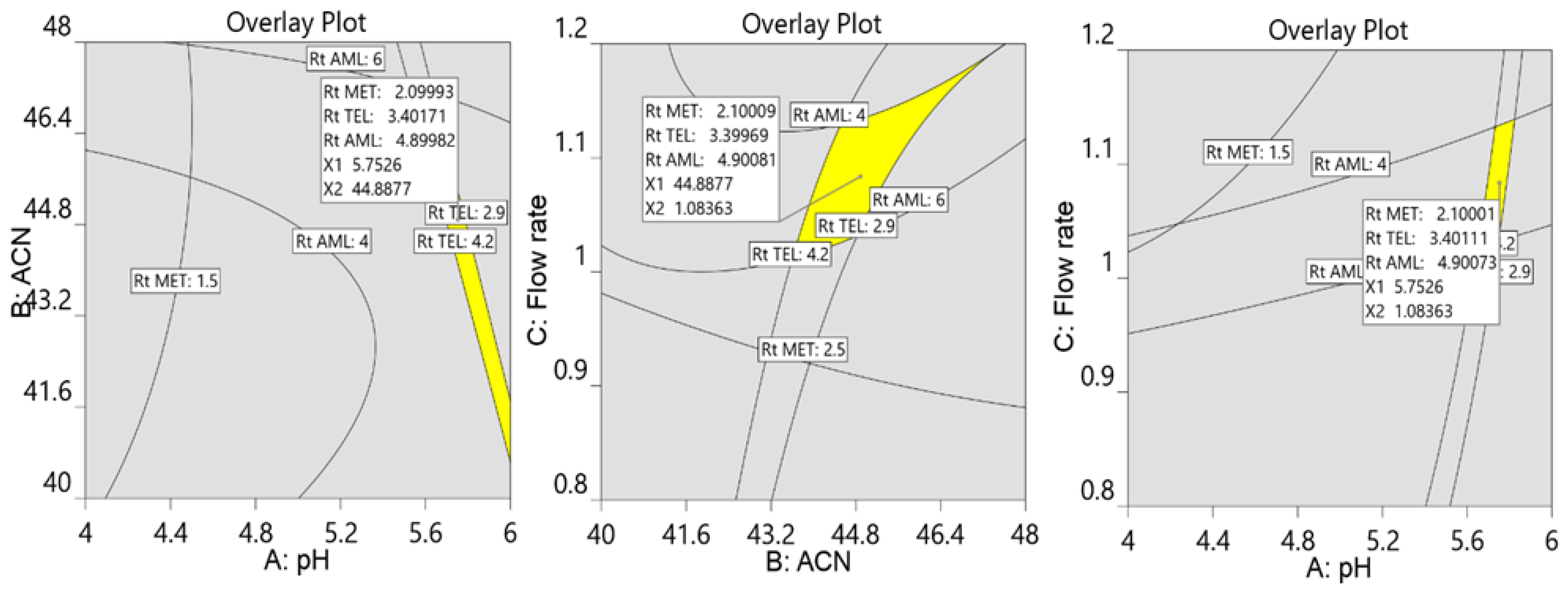
3.1. The Optimization of Chromatographic Parameters
3.2. HPLC Method Validation
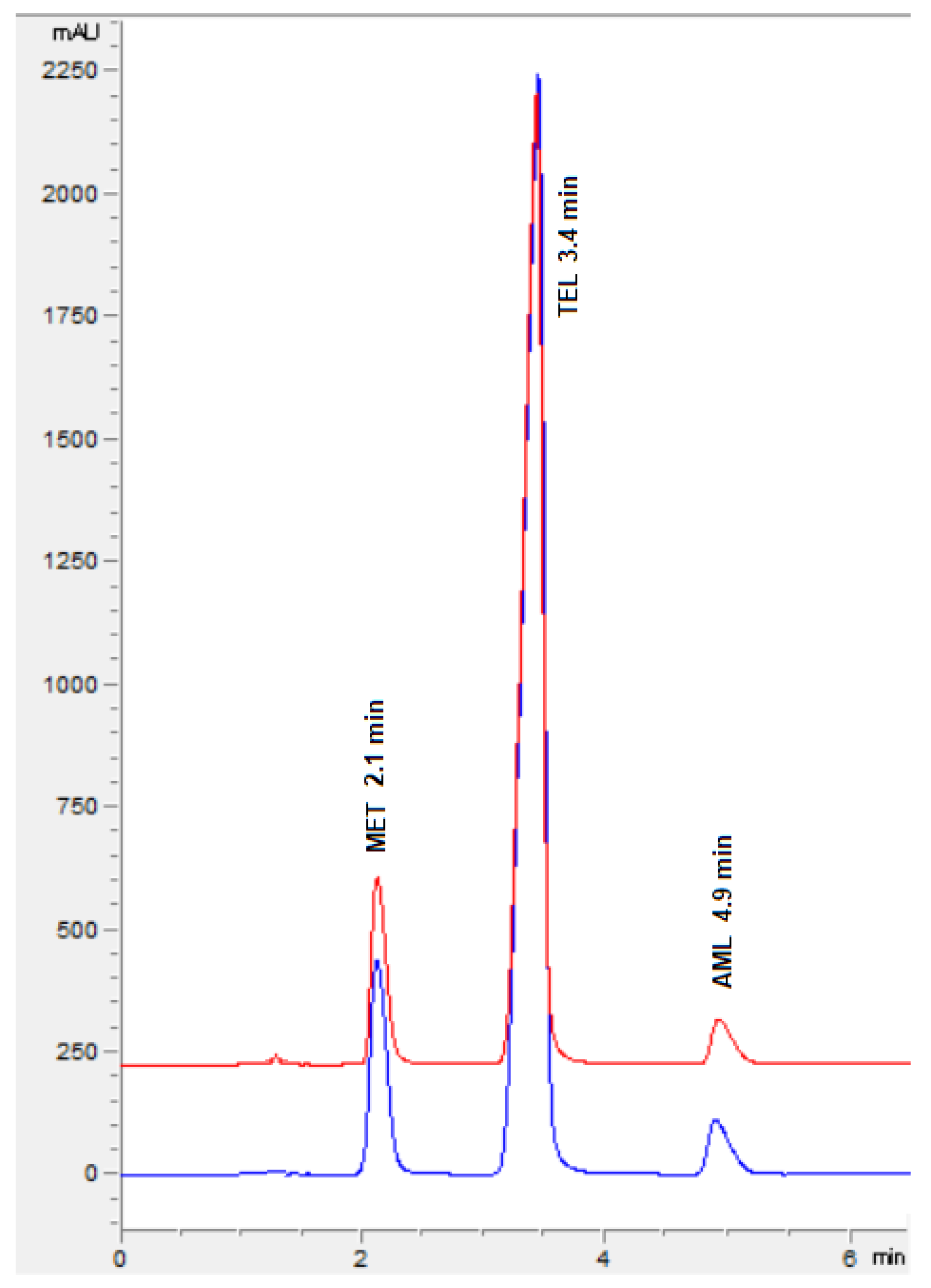
3.2.1. System Suitability Analysis
3.2.2. Linearity and Sensitivity
3.2.3. Correctness and Precision
3.2.4. Robustness
3.3. Analysis of Formulation
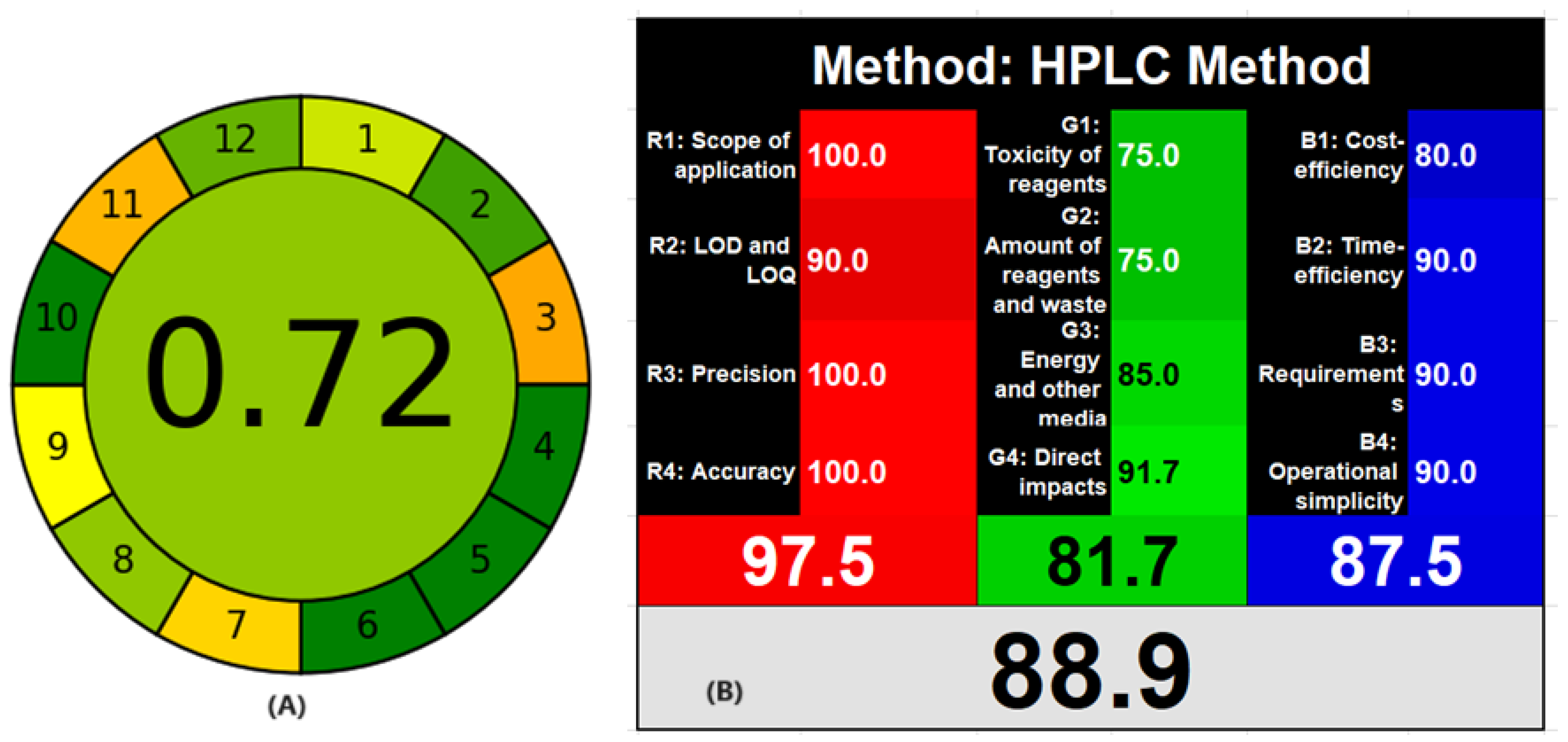
3.4. Assessment of Greenness and Whiteness of the HPLC Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-García, C.; Rubio-Guerra, A.F. Combination therapy in the treatment of hypertension. Drugs Context 2018, 6, 212531. [Google Scholar] [CrossRef]
- Hendriksen, L.C.; Omes-Smit, G.; Koch, B.C.P.; Ikram, M.A.; Stricker, B.H.; Visser, L.E. Sex-Based Difference in the Effect of Metoprolol on Heart Rate and Bradycardia in a Population-Based Setting. J. Pers. Med. 2022, 12, 870. [Google Scholar] [CrossRef]
- Kumari, K.; Toppo, M.S.; Majhi, L.; Kumar, A. Blood pressure-lowering effect of telmisartan compared to losartan among mild to moderate essential hypertensive adult subjects: A meta-analysis. J. Family Med. Prim. Care 2022, 11, 6227–6235. [Google Scholar] [CrossRef] [PubMed]
- Le, E.M. Calcium channel blockers for hypertension: Old, but still useful. Cardiovasc. Prev. Pharmacother. 2023, 5, 113–125. [Google Scholar] [CrossRef]
- Attimarad, M.; Venugopala, K.N.; Sreeharsha, N.; Chohan, M.S.; Shafi, S.; Nair, A.B.; Pottathil, S. A Rapid HPLC Method for the Concurrent Determination of Several Antihypertensive Drugs from Binary and Ternary Formulations. Separations 2021, 8, 86. [Google Scholar] [CrossRef]
- Elshanawane, A.A.; Abdelaziz, L.M.; Kamal, M.M.; Hafez, H.M. Quantitative Determination of Telmisartan, Ramipril, Amlodipine Besylate, and Atorvastatin Calcium by HPLC. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 195–206. [Google Scholar] [CrossRef]
- Attimarad, M.; Venugopala, K.N.; SreeHarsha, N.; Aldhubiab, B.E.; Nair, A.B. Validation of rapid RP-HPLC method for concurrent quantification of amlodipine and celecoxib in pure and formulation using an experimental design. Microchem. J. 2020, 152, 104365. [Google Scholar] [CrossRef]
- Panda, S.S.; Dutta, S.; Bera, R.K.V.V.; Jammula, S. Analytical eco-scale and quality by design-oriented liquid chromatography method for simultaneous quantification of metoprolol succinate, telmisartan, and cilnidipine in their fixed-dose combination. Sep. Sci. Plus 2021, 4, 128–136. [Google Scholar] [CrossRef]
- Thakker, N.; Shinde, G.; Dharamsi, A.; Choudhari, V. Development and Validation of SI/RS-UHPLC-PDA Method for Olmesartan Medoxomil and Metoprolol Succinate-Related Substance. Turk. J. Pharm. Sci. 2023, 20, 39–47. [Google Scholar] [CrossRef]
- Attimarad, M.; Chohan, M.S.; Elgorashe, R.E.E. Smart analysis of a ternary mixture of amlodipine, hydrochlorothiazide and telmisartan by manipulation of UV spectra: Development, validation and application to formulations. J. Mol. Struct. 2020, 1212, 128095. [Google Scholar] [CrossRef]
- Shahana, V.P.; Gayathri, R.M.; Rajesh, A.; Kathirvel, S. A Comprehensive Validation Method and Development of RP-HPLC for Simultaneous Estimation of Metoprolol, Telmisartan and Chlorthalidone in Bulk and its Formulation. Asian J. Res. Chem. 2018, 11, 827–834. [Google Scholar] [CrossRef]
- Kulkarni, P.; Gangrade, D. Simultaneous estimation of hydrochlorothiazide, amlodipine besylate and telmisartan in com-bined tablet dosage form by using RP- HPLC method. Int. J. Pharm. Sci. Res. 2017, 8, 268–276. [Google Scholar] [CrossRef]
- Jeelani, S.; Kouznetsova, N. A new stability-indicating HPLC-UV method for determination of amlodipine besylate and its impurities in drug substance. Heliyon 2023, 9, e19993. [Google Scholar] [CrossRef] [PubMed]
- Panchal, J.G.; Patel, R.V.; Mistry, B.R.; Menon, S.K. Development and Validation of Reversed-Phase LC Method for Simultaneous Determination Telmisartan, Amlodipine and Their Degradation Products in Fixed Dose Combination Tablets. Eurasian J. Anal. Chem. 2012, 7, 28–42. [Google Scholar]
- Patra, B.R.; Mohan, S.; Gowda, N. International conference on harmonization recommended forced degradation studies and development of a new validated isocratic reverse-phase ultra high-performance liquid chromatography method for the simultaneous estimation of telmisartan and amlodipine. Asian J. Pharm. Clin Res. 2019, 12, 250–258. [Google Scholar] [CrossRef]
- Lv, C.; Wei, C.; Bu, F.; Chen, R.; Wang, X.; Li, R.; Wang, B.; Guo, R. Determination of Amlodipine in Human Plasma by LC-MS/MS and Its Bioequivalence Study in Healthy Chinese Subjects. Pharmacol. Pharm. 2013, 4, 191–200. [Google Scholar] [CrossRef]
- Wang, B.; Sheng, L.; Li, Y. Simultaneous Determination of Telmisartan and Amlodipine in Dog Plasma by LC–MS-MS. J. Chromatogr. Sci. 2015, 53, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Ravi, V.B.; Inamadugu, J.K.; Pilli, N.R.; Sreenivasulu, V.; Ponneri, V. Simultaneous determination of telmisartan and amlodipine in human plasma by LC-MS/MS and its application in a human pharmacokinetic study. J Pharm Anal. 2012, 2, 319–326. [Google Scholar] [CrossRef]
- Adriana, M.; Gabriel, H.; Alepandru, V.R.; Ștefana, S.; Rupandra, S.; Hajnal, K. Simultaneous determination of Amlodipine and telmisartan from pharmaceutical products by way of capillary electrophoresis. Curr. Issues Pharm. Med. Sci. 2016, 29, 42–45. [Google Scholar] [CrossRef]
- Yenduri, S.; Sulthana, H.; Koppuravuri, N.P. Sustainability evaluation of existed HPLC based analytical methods for quantification of amlodipine besylate and telmisartan using RGB model: A whiteness approach. Green Anal. Chem. 2023, 6, 100074. [Google Scholar] [CrossRef]
- Rathod, S.D.; Patil, P.M.; Waghmare, S.S.A.; Chaudhari, P.D. UV-Spectrophotometric Method for Estimation of Telmisartan in Bulk and Tablet Dosage Form. Int. J. Pharm. Sci. Res. 2012, 3, 3936–3939. [Google Scholar] [CrossRef]
- Ashok, P.; Narenderan, S.T.; Meyyanathan, S.N.; Babu, B.; Vadivelan, R. Development and validation of a RP-HPLC method for estimation of telmisartan in human plasma. Int. J. App. Pharm. 2019, 11, 237–240. [Google Scholar] [CrossRef]
- Chabukswar, A.R.; Mohokar, M.N.; Choudhari, V.; Sharma, S.N.; Tambe, S.D.; Pagare, B.D. Absorption corrected method and isoabsorptive point method for simultaneous estimation of metoprolol and amlodipine in their combined dosage form. Int. J. Pharm. Sci. Res. 2012, 4, 240–244. [Google Scholar]
- Chabukswar, A.R.; Tambe, S.D.; Choudhari, V.P.; Sharma, S.N.; Mohokar, M.N.; Chate, S. Ratio derivative spectrophotometry method for simultaneous estimation of metoprolol and amlodipine in their combined dosage form. Res. J. Pharm. Technol. 2012, 5, 950–954. [Google Scholar]
- Jain, P.S.; Patel, M.K.; Bari, S.B.; Surana, S.J. Development and validation of HPTLC method for simultaneous determination of amlodipine besylate and metoprolol succinate in bulk and tablets. Indian J. Pharm. Sci. 2012, 74, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.M.; Hammad, S.F.; El-Malla, S.F.; Elshenawy, E.A. Simultaneous determination of amlodipine and metoprolol in their combined dosage form using a synchronous fluorescence spectrofluorimetric method. Luminescence 2018, 33, 364–369. [Google Scholar] [CrossRef]
- Soni, S.; Verma, R.; Verma, D.; Verma, A. Analytical method development and validation of metoprolol succinate by high performance liquid chromatograpy and ultraviolet spectroscopy technique. Res. J. Pharm. Technol. 2021, 14, 931–937. [Google Scholar] [CrossRef]
- Mahvash, I.; Shobha Rani, R.H. Estimation of metoprolol in human plasma by HPLC method. Int. J. Pharm. Pharm. Sci. 2014, 7, 442–446. [Google Scholar]
- Nawale, P.S.; Shirkhedkar, A.A.; Surana, S.J.; Patil, A.S. Normal and reversed-phase HPTLC methods for simultaneous estimation of Telmisartan and Metoprolol Succinate in pharmaceutical formulation. Int. Sch. Res. Not. 2012, 2012, 815353. [Google Scholar] [CrossRef]
- Nandi, U.; Dan, S.; Pal, T.K. Development and validation of a liquid chromatography–mass spectrometry method for simultaneous determination of metoprolol and telmisartan in rat plasma and its application to pharmacokinetic study. J. Pharm. Investig. 2015, 45, 329–340. [Google Scholar] [CrossRef]
- Jaiprakash, N.S.; Mrinmayee, D.; Zahid, Z.; Devanand, B.S.; Rohidas, A. Quality by design approach: Regulatory need. Arab. J. Chem. 2017, 10, S3412–S3425. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Imam, S.; Ahad, A.; Praveen, A.; Sultana, Y.; Ahmad, S.; Mujeeb, M. Analytical quality by design (AQbD) approach based HPTLC method for quantification of fisetin with superior recovery in formulations. Curr. Anal. Chem. 2020, 16, 149–157. [Google Scholar] [CrossRef]
- Fukuda, I.M.; Pinto, C.F.F.; Moreira, C.; Dos, S.; Saviano, A.M.; Lourenço, F.R. Design of Experiments (DoE) applied to Pharmaceutical and Analytical Quality by Design (QbD). Bra. J. Pharm. Sci. 2018, 54, e01006. [Google Scholar] [CrossRef]
- Rozet, E.; Pierre, L.; Jean-Francois, M.; Sondag, P.; Scherderac, T.; Bruno, B. Analytical procedure validation and the quality by design paradigm. J. Biopharm. Stat. 2015, 25, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.V.; Dash, R.N.; Reddy, B.J.; Venugopal, P.; Sandeep, P.; Madhavi, G. Quality by Design (QbD) approach to develop HPLC method for eberconazole nitrate: Application oxidative and photolytic degradation kinetics. J. Saudi Chem. Soc. 2016, 20, S313–S322. [Google Scholar] [CrossRef]
- Zafar, A.; El-Bagory, I.; Alruwaili, N.; Imam, S.S.; Alomar, F.A.; Elkomy, M.; Ahmad, N.; Aburaia, M. Quality by design (QbD) based development and validation of bioanalytical RP-HPLC method for dapagliflozin: Forced degradation and preclinical pharmacokinetic study. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 53–65. [Google Scholar] [CrossRef]
- Srinubabu, G.; Raju, C.A.I.; Sarath, N.; Kumar, P.K.; Rao, J.V.L.N.S. Development and validation of a HPLC method for the determination of voriconazole in pharmaceutical formulation using an experimental design. Talanta 2007, 71, 1424–1429. [Google Scholar] [CrossRef]
- Attimarad, M.; Elgorashe, R.E.E.; Subramaniam, R.; Islam, M.M.; Venugopala, K.N.; Nagaraja, S.; Balgoname, A.A. Development and Validation of Rapid RP-HPLC and Green Second-Derivative UV Spectroscopic Methods for Simultaneous Quantification of Metformin and Remogliflozin in Formulation Using Experimental Design. Separations 2020, 7, 59. [Google Scholar] [CrossRef]
- Jain, A.; Beg, S.; Saini, S.; Sharma, T.; Katare, O.P.; Singh, B. Application of chemometric approach for QbD-enabled development and validation of an RP-HPLC method for estimation of methotrexate. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 502–512. [Google Scholar] [CrossRef]
- Attimarad, M.; Venugopala, K.N.; Chohan, M.S.; Shinu, P.; David, M.; Molina, E.I.P.; Nair, A.B.; Sreeharsha, N.; Altaysan, A.I.; Balgoname, A.A. Multivariate Optimization of Chromatographic Conditions for Rapid Simultaneous Quantification of Antidiarrheal Drugs in Formulation Using Surface Response Methodology. Separations 2022, 9, 103. [Google Scholar] [CrossRef]
- Czyrski, A.; Sznura, J. The application of Box-Behnken-Design in the optimization of HPLC separation of fluoroquinolones. Sci. Rep. 2019, 9, 19458. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.L.; He, Y. Application of Box-Behnken designs in parameters optimization of differential pulse anodic stripping voltammetry for lead(II) determination in two electrolytes. Sci. Rep. 2017, 7, 2789. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, E.A.; El-Ashrey, M.K.; Mahmoud, S.T. An innovative combination of Box-Behnken design and ecofriendly approaches for the simultaneous determination of aspirin, clopidogrel, atorvastatin and rosuvastatin in their fixed-dose combination tablets. BMC Chem. 2023, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Nowak, P.M.; Kościelniak, P. What Color Is Your Method? Adaptation of the RGB Additive Color Model to Analytical Method714Evaluation. Anal. Chem. 2019, 91, 10343–10352. [Google Scholar] [CrossRef]
| X1 | X2 | X3 | Y1 | Y2 | Y3 | ||
|---|---|---|---|---|---|---|---|
| Standard | Run | A:pH of Buffer | B:Acetonitile Percent | C:Flow Rate | Rt MET | Rt TEL | Rt AML |
| 12 | 1 | 4.5 | 45 | 1.2 | 1.386 | 9.485 | 2.38 |
| 5 | 2 | 3 | 40 | 0.8 | 1.982 | 7.756 | 3.909 |
| 14 | 3 | 4.5 | 40 | 1 | 1.756 | 19.012 | 3.89 |
| 1 | 4 | 3 | 35 | 1 | 1.792 | 10.794 | 5.204 |
| 10 | 5 | 4.5 | 45 | 0.8 | 2.03 | 5.68 | 3.569 |
| 6 | 6 | 6 | 40 | 0.8 | 3.295 | 5.914 | 9.872 |
| 16 | 7 | 4.5 | 40 | 1 | 1.764 | 18.697 | 3.96 |
| 17 | 8 | 4.5 | 40 | 1 | 1.771 | 18.567 | 4.012 |
| 4 | 9 | 6 | 45 | 1 | 2.44 | 3.199 | 5.685 |
| 3 | 10 | 3 | 45 | 1 | 1.589 | 3.985 | 4.216 |
| 2 | 11 | 6 | 35 | 1 | 2.933 | 8.606 | 12.748 |
| 9 | 12 | 4.5 | 35 | 0.8 | 2.483 | 34.567 | 8.42 |
| 11 | 13 | 4.5 | 35 | 1.2 | 1.638 | 29.112 | 5.302 |
| 15 | 14 | 4.5 | 40 | 1 | 1.759 | 18.96 | 3.97 |
| 13 | 15 | 4.5 | 40 | 1 | 1.749 | 18.69 | 3.959 |
| 8 | 16 | 6 | 40 | 1.2 | 2.182 | 3.902 | 6.508 |
| 7 | 17 | 3 | 40 | 1.2 | 1.324 | 5.265 | 2.636 |
| HPLC Parameters | MET | TEL | AML | Acceptable Value |
|---|---|---|---|---|
| Retention time (min) ± SD | 2.15 ± 0.03 | 3.48 ± 0.02 | 4.98 ± 0.04 | - |
| Resolution ± SD | - | 6.52 ± 0.028 | 5.96 ± 0.019 | >2 |
| Capacity factor | 1.1 | 2.45 | 3.97 | >1 |
| Theoretical plate ± SD | 2452.45 ± 21.14 | 3551.70 ± 42.57 | 4672.01 ± 58.64 | >2000 |
| Linearity range (µg/mL) | 10–200 | 10–200 | 5–50 | - |
| Slope | 21.382 | 135.75 | 27.678 | - |
| Intercept | −12.91 | −179.67 | −11.18 | - |
| Correlation coefficient (R2) | 0.9999 | 0.9999 | 0.9998 | - |
| LOD (µg/mL) | 1.21 | 2.60 | 0.11 | - |
| LOQ (µg/mL) | 3.68 | 7.88 | 0.33 | - |
| Within-Day | Between-Day | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Amount of Drug [µg/mL] | Amount Found Mean [n = 3] ± SD | % RSD | % Recovery | % RE | Amount Found Mean [n = 9] ± SD | % RSD | % Recovery | % RE | |
| MET | 10.00 | 9.95 ± 0.19 | 1.91 | 99.50 | −0.50 | 9.89 ± 0.17 | 1.73 | 98.90 | −1.10 |
| 100.00 | 100.48 ± 1.45 | 1.44 | 100.48 | 0.48 | 98.45 ± 1.75 | 1.77 | 98.45 | −1.55 | |
| 200.00 | 197.85 ± 2.14 | 1.07 | 98.93 | −1.08 | 196.95 ± 3.14 | 1.58 | 98.48 | −1.53 | |
| TEL | 10.00 | 9.94 ± 0.16 | 1.61 | 99.40 | −0.60 | 10.13 ± 0.12 | 1.19 | 101.30 | 1.30 |
| 100.00 | 98.67 ± 1.79 | 1.81 | 98.67 | −1.33 | 99.07 ± 1.69 | 1.70 | 99.07 | −0.93 | |
| 200.00 | 198.03 ± 2.92 | 1.47 | 99.02 | −0.98 | 198.55 ± 3.46 | 1.74 | 99.28 | −0.72 | |
| AML | 5.00 | 5.04 ± 0.05 | 0.99 | 100.80 | 0.80 | 4.96 ± 0.08 | 1.63 | 99.20 | −0.80 |
| 25.00 | 24.63 ± 0.37 | 1.50 | 98.52 | −1.48 | 24.68 ± 0.43 | 1.69 | 98.72 | −1.28 | |
| 50.00 | 49.09 ± 0.86 | 1.75 | 98.18 | −1.82 | 49.19 ± 0.86 | 1.75 | 98.38 | −1.62 | |
| Average Peak Area ± SD | ||||
|---|---|---|---|---|
| Parameters | Conditions | MET | TEL | AML |
| mobile phase pH | 5.75 (−0.05) | 2135.78 ± 9.54 | 10,564.85 ± 88.38 | 268.59 ± 3.47 |
| 5.8 pH | 2126.45 ± 12.15 | 10,681.47 ± 91.07 | 266.11 ± 5.62 | |
| 5.85 (+0.05) | 2142.59 ± 8.37 | 10,576.43 ± 79.66 | 260.34 ± 4.05 | |
| %RSD | 0.38 | 0.61 | 1.60 | |
| Acetonitrile concentration | 43% (−2%) | 2118.97 ± 8.64 | 10,594.55 ± 65.37 | 257.96 ± 2.35 |
| 45% | 2126.45 ± 6.73 | 10,681.47 ± 95.67 | 266.11 ± 3.78 | |
| 47% (+2%) | 2131.34 ± 8.96 | 10,613.64 ± 83.55 | 261.47 ± 6.14 | |
| %RSD | 0.29 | 0.43 | 1.56 | |
| Wavelength (nm) | 229 (−2) | 2169.63 ± 11.47 | 10,712.38 ± 45.63 | 258.15 ± 3.18 |
| 230 nm | 2126.45 ± 8.68 | 10,681.47 ± 57.04 | 266.11 ± 4.09 | |
| 231 (+2) | 2095.86 ± 4.83 | 10,624.24 ± 37.58 | 271.75 ± 2.98 | |
| %RSD | 1.74 | 0.42 | 2.58 | |
| Flow rate mL/min | 1 (−1) | 2156.57 ± 12.75 | 10,908.36 ± 120.47 | 272.17 ± 3.55 |
| 1.1 mL/min | 2126.45 ± 10.68 | 10,681.47 ± 107.25 | 266.11 ± 5.92 | |
| 1.2 (+1) | 2097.46 ± 7.92 | 10,475.62 ± 104.67 | 258.38 ± 4.73 | |
| %RSD | 1.39 | 2.03 | 2.60 | |
| Formulation | Amount of Drug [mg/Tab] | Amount Found Mean [n = 3] ± SD | % RE | % Recovery |
|---|---|---|---|---|
| MET | 50 | 50.37 ± 1.49 | 0.74 | 100.74 |
| TEL | 40 | 39.56 ± 0.73 | −1.310 | 98.90 |
| AML | 5 | 4.96 ± 0.07 | −0.80 | 99.20 |
| Standard addition method | ||||
| MET | 25 | 24.53 ± 0.37 | −1.88 | 98.12 |
| 50 | 49.57 ± 0.63 | −0.86 | 99.14 | |
| 75 | 73.58 ± 1.07 | −1.89 | 98.11 | |
| Across Mean | 98.46 | |||
| % RSD | 0.89 | |||
| TEL | 20 | 19.66 ± 0.35 | −1.70 | 98.30 |
| 40 | 39.61 ± 0.51 | −0.98 | 99.03 | |
| 60 | 58.92 ± 0.79 | −1.80 | 98.20 | |
| Across Mean | 98.51 | |||
| % RSD | 1.09 | |||
| AML | 2.5 | 2.46 ± 0.05 | −1.6 | 98.40 |
| 5 | 5.06 ± 0.07 | 1.20 | 101.20 | |
| 7.5 | 7.40 ± 0.14 | −0.21 | 98.70 | |
| Across Mean | 99.43 | |||
| % RSD | 0.72 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attimarad, M.; Alali, M.J.; Alali, H.A.; Alabdulmuhsin, D.H.; Alnajdi, A.K.; Venugopala, K.N.; Nair, A.B. Design of Experimental Approach for Development of Rapid High Performance Liquid Chromatographic Process for Simultaneous Estimation of Metoprolol, Telmisartan, and Amlodipine from Formulation: Greenness and Whiteness Evaluation. Molecules 2024, 29, 1087. https://doi.org/10.3390/molecules29051087
Attimarad M, Alali MJ, Alali HA, Alabdulmuhsin DH, Alnajdi AK, Venugopala KN, Nair AB. Design of Experimental Approach for Development of Rapid High Performance Liquid Chromatographic Process for Simultaneous Estimation of Metoprolol, Telmisartan, and Amlodipine from Formulation: Greenness and Whiteness Evaluation. Molecules. 2024; 29(5):1087. https://doi.org/10.3390/molecules29051087
Chicago/Turabian StyleAttimarad, Mahesh, Mohammed Jassim Alali, Hussain Ali Alali, Dana Hisham Alabdulmuhsin, Aljohara Khalid Alnajdi, Katharigatta Narayanaswamy Venugopala, and Anroop B. Nair. 2024. "Design of Experimental Approach for Development of Rapid High Performance Liquid Chromatographic Process for Simultaneous Estimation of Metoprolol, Telmisartan, and Amlodipine from Formulation: Greenness and Whiteness Evaluation" Molecules 29, no. 5: 1087. https://doi.org/10.3390/molecules29051087







