Development of Pure Certified Reference Material of Cannabidiol
Abstract
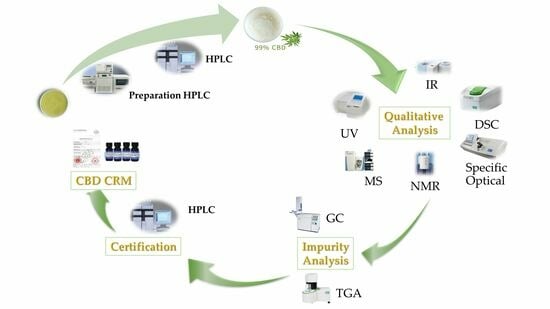
:1. Introduction
2. Results and Discussion
2.1. Purity Analysis
2.1.1. Analysis of the CBD Crude Extract
2.1.2. Preparation Process for the Purified CBD Product
2.1.3. Purity Analysis of the Purified CBD Product
2.1.4. Impurity Analysis of the Purified CBD Product
2.2. Qualitative Analysis
2.3. Homogeneity Test
2.4. Stability Test
2.4.1. Short-Term Stability
2.4.2. Long-Term Stability
2.4.3. Certification of the CBD CRM
- MSbetween—The between-group mean squares;
- MSwithin—The within-group mean squares;
- n—The number of the laboratory;
- p—The number of the determination in each laboratory;
- —The average value of the total.
2.5. Uncertainty Assessment
3. Materials and Methods
3.1. Apparatus and Reagents
3.2. Extraction and Purification of CBD
3.2.1. Purity Analysis of the CBD Crude Extract
3.2.2. Isolation and Purification of CBD
3.3. Analysis of the Purified CBD Product Purity
3.4. Qualitative Analysis
3.5. Homogeneity Test
3.6. Stability Test
3.6.1. Short-Term Stability Test
3.6.2. Long-Term Stability Test
3.7. Certification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krauke, Y.; Monks, K. Purification of Cannabidiol from CBD Oil by Preparative HPLC. Knauer Wissenschaftliche Geräte GmbH, VPH0074 Application Note 2020. Available online: https://api.semanticscholar.org/CorpusID:233305754 (accessed on 20 November 2023).
- Berman, P.; Futoran, K.; Lewitus, G.M.; Mukha, D.; Benami, M.; Shlomi, T.; Meiri, D. A new ESI-LC/MS approach for comprehensive metabolic profiling of phytocannabinoids in Cannabis. Sci. Rep. 2018, 8, 14280. [Google Scholar] [CrossRef] [PubMed]
- Britch, S.C.; Babalonis, S.; Walsh, S.L. Cannabidiol: Pharmacology and therapeutic targets. Psychopharmacology 2021, 238, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Lo Faro, A.F.; Pirani, F.; Berretta, P.; Pacifici, R.; Pichini, S.; Busardo, F.P. Pharmacology and legal status of cannabidiol. Ann. Dell Ist. Super. Di Sanita 2020, 56, 285–291. [Google Scholar] [CrossRef]
- Beal, K. Considerations in the addition of cannabis to chocolate. Curr. Opin. Food Sci. 2019, 28, 14–17. [Google Scholar] [CrossRef]
- Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. Commercial cannabis consumer products part 2: HPLC-DAD quantitative analysis of cannabis cannabinoids. Forensic Sci. Int. 2018, 289, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Wene, D.; Fan, Z.H. Qualitative and quantitative measurement of cannabinoids in cannabis using modified HPLC/DAD method. J. Pharm. Biomed. Anal. 2017, 146, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua-Olaizola, O.; Zarandona, I.; Ortiz, L.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Simultaneous quantification of major cannabinoids and metabolites in human urine and plasma by HPLC-MS/MS and enzyme-alkaline hydrolysis. Drug Test. Anal. 2017, 9, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, V.; Licata, M.; Pedrazzi, T.; Maran, D.; Bertelli, D.; Pellati, F.; Benvenuti, S. Development of a new method for the analysis of cannabinoids in honey by means of high-performance liquid chromatography coupled with electrospray ionisation-tandem mass spectrometry detection. J. Chromatogr. A 2019, 1597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hewavitharana, A.K.; Gloerfelt-Tarp, F.; Nolan, M.; Barkla, B.J.; Purdy, S.; Kretzschmar, T. Simultaneous Quantification of 17 Cannabinoids in Cannabis Inflorescence by Liquid Chromatography-Mass Spectrometry. Separations 2022, 9, 85. [Google Scholar] [CrossRef]
- Heinl, S.; Lerch, O.; Erdmann, F. Automated GC-MS Determination of Delta(9)-Tetrahydrocannabinol, Cannabinol and Cannabidiol in Hair(aEuro). J. Anal. Toxicol. 2016, 40, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, G.; Vento, F.; Alibrando, F.; Donnarumma, D.; Dugo, P.; Mondello, L. Cannabis Sativa L.: A comprehensive review on the analytical methodologies for cannabinoids and terpenes characterization. J. Chromatogr. A 2021, 1637, 461864. [Google Scholar] [CrossRef] [PubMed]
- De Backer, B.; Debrus, B.; Lebrun, P.; Theunis, L.; Dubois, N.; Decock, L.; Verstraete, A.; Hubert, P.; Charlier, C. Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, L.; Brighenti, V.; Rossi, M.C.; Sperlea, J.; Pellati, F.; Bertelli, D. Use of 13C-qNMR Spectroscopy for the Analysis of Non-Psychoactive Cannabinoids in Fibre-Type Cannabis sativa L. (Hemp). Molecules 2019, 24, 1138. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Simon, V.L.; Sahar, N.; Mirjana, M. Experimental investigation and thermodynamic modeling of cannabidiol solubility in plant oils and hydrophobic eutectic systems. J. Mol. Liq. 2023, 372, 121172. [Google Scholar] [CrossRef]
- Citti, C.; Linciano, P.; Forni, F.; Vandelli, M.A.; Gigli, G.; Lagana, A.; Cannazza, G. Analysis of impurities of cannabidiol from hemp. Isolation, characterization and synthesis of cannabidibutol, the novel cannabidiol butyl analog. J. Pharm. Biomed. Anal. 2019, 175, 112752. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fang, H.; Jin, W.; Chen, H.; Lin, Z.; Chen, X.; Zhang, Y.; Zhuan, H. Development of pure certified reference material of squalene. Microchem. J. 2023, 193, 108926. [Google Scholar] [CrossRef]
- He, C.; Gao, Q.; Ye, C.; Yang, G.; Zhang, P.; Yang, R.; Zhang, Q.; Ma, K. Development of a Purity Certified Reference Material for Vinyl Acetate. Molecules 2023, 28, 6245. [Google Scholar] [CrossRef] [PubMed]
- ISO Guide 35:2017. Reference Materials—Guidance for Characterization and Assessment of Homogeneity and Stability. International Standard Organization (ISO): Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/60281.html (accessed on 20 October 2023).
- Kumar, A.; Misra, D.K. A Review on the Statistical Methods and Implementation to Homogeneity Assessment of Certified Reference Materials in Relation to Uncertainty. Mapan J. Metrol. Soc. India 2020, 35, 457–470. [Google Scholar] [CrossRef]
- Sun, D.-M.; Song, L.; Wang, H.-Y.; Yu, C.-C.; Chu, Q.; Lan, T.; Zhang, W.-B. Establishment of Detection Methods for Five Cannabinoids in Hemp Cosmetics Based on HPLC. Anal. Sci. 2021, 37, 1821–1824. [Google Scholar] [CrossRef] [PubMed]











| Number | 1 | 2 | 3 | Mean (%) | RSD (%) |
|---|---|---|---|---|---|
| Moisture content (%) | 0.036 | 0.037 | 0.032 | 0.035 | 0.003 |
| Ash content (%) | 0.077 | 0.072 | 0.068 | 0.072 | 0.005 |
| Number | 1H-NMR δ/ppm | Value in the Literature [14] δ/ppm | Assignment | 13C-NMR δ/ppm | Value in the Literature [14] δ/ppm |
|---|---|---|---|---|---|
| 1 | 3.83 | 3.86 | 1H, br d, J = 8.4 Hz | 37.3 | 37.0 |
| 2 | 5.57 | 5.55 | 1H, s | 124.1 | 124.3 |
| 3 | - | - | - | 140.0 | 139.9 |
| 4 | 2.22, 2.11 | 2.20, 2.10 | 1H, m; 1H, m | 31.5 | 31.5 |
| 5 | 1.81 | 1.84 | 2H, m | 28.4 | 28.4 |
| 6 | 2.40 | 2.40 | 1H, m | 46.1 | 46.2 |
| 7 | - | 4.40 | 2H, m | 149.9 | 149.9 |
| 8 | 1.66 | 1.66 | 3H, s | 20.5 | 20.3 |
| 9 | 4.67, 4.66, 4.56 | 4.40 | 2H, m | 110.8 | 110.8 |
| 10 | 1.79 | 1.79 | 3H, s | 23.7 | 23.4 |
| 1′ | - | - | - | 113.7 | 113.8 |
| 2′ | 5.97 | - | 1H, br s, 2′-OH | 156.1 | 156.0 |
| 3′ | 6.28 | 6.26 | 1H, br s | 108.0 | 108.3 |
| 4′ | - | - | - | 143.0 | 142.9 |
| 5′ | 6.16 | 6.16 | 1H, br s | 109.8 | 108.3 |
| 6′ | 4.60 | - | 1H, br s, 6′-OH | 153.9 | 153.9 |
| 1′′ | 2.43 | 2.42 | 2H, t, J = 7.7 Hz | 35.5 | 35.5 |
| 2′′ | 1.56 | 1.57 | 2H, m | 30.4 | 30.4 |
| 3′′ | 1.30 | 1.30 | 2H, m | 30.6 | 30.7 |
| 4′′ | 1.31 | 1.31 | 2H, | 22.5 | 22.5 |
| 5′′ | 0.88 | 0.89 | 3H, t, J = 6.0 Hz | 14.0 | 14.1 |
| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (%) | 99.75 | 99.66 | 99.65 | 99.74 | 99.78 | 99.79 | 99.73 | 99.77 | 99.76 | 99.67 | 99.65 | 99.72 |
| 2 (%) | 99.77 | 99.71 | 99.73 | 99.67 | 99.65 | 99.76 | 99.75 | 99.83 | 99.78 | 99.58 | 99.67 | 99.65 |
| 3 (%) | 99.70 | 99.79 | 99.75 | 99.69 | 99.66 | 99.78 | 99.81 | 99.68 | 99.67 | 99.69 | 99.68 | 99.69 |
| Mean (%) | 99.74 | 99.72 | 99.71 | 99.70 | 99.70 | 99.78 | 99.76 | 99.76 | 99.74 | 99.65 | 99.67 | 99.69 |
| Overall Mean (%) | 99.72 | |||||||||||
| Variation Source | Degrees of Freedom | Values |
|---|---|---|
| Mean square between groups | 9 | S12 = 0.005875 |
| Mean square within groups | 20 | S22 = 0.003027 |
| F0.05 (11,24) | 2.22 | |
| F | F = S12/S22 = 1.9408 |
| Time (Day) | Values (%) | Means (%) | RSD (%) | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| 4 °C | 1 | 99.68 | 99.66 | 99.65 | 99.66 | 0.02 |
| 3 | 99.64 | 99.67 | 99.67 | 99.66 | 0.02 | |
| 7 | 99.65 | 99.68 | 99.67 | 99.67 | 0.02 | |
| 25 °C | 1 | 99.66 | 99.63 | 99.62 | 99.64 | 0.02 |
| 3 | 99.67 | 99.53 | 99.61 | 99.60 | 0.07 | |
| 7 | 99.56 | 99.67 | 99.65 | 99.63 | 0.06 | |
| 60 °C | 1 | 99.71 | 99.68 | 99.67 | 99.69 | 0.02 |
| 3 | 99.66 | 99.67 | 99.69 | 99.67 | 0.03 | |
| 7 | 99.66 | 99.69 | 99.67 | 99.67 | 0.02 | |
| Time (Month) | Values (%) | Means (%) | RSD (%) | Uncertainty (%) |
|---|---|---|---|---|
| 0 | 99.54 99.60 99.57 | 99.57 | 0.03 | 0.09 |
| 1 | 99.51 99.62 99.49 | 99.54 | 0.07 | |
| 2 | 99.47 99.44 99.49 | 99.46 | 0.03 | |
| 3 | 99.53 99.49 99.51 | 99.51 | 0.02 | |
| 6 | 99.45 99.55 99.50 | 99.50 | 0.05 | |
| 12 | 99.53 99.48 99.49 | 99.50 | 0.03 | |
| 18 | 99.62 99.65 99.66 | 99.64 | 0.02 |
| Number | Measured Value (%) | Mean (%) | SD (%) | RSD (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1–1 | 1–2 | 2–1 | 2–2 | 3–1 | 3–2 | ||||
| 1 | 99.51 | 99.49 | 99.48 | 99.48 | 99.50 | 99.50 | 99.49 | 0.01 | 0.01 |
| 2 | 99.72 | 99.72 | 99.72 | 99.72 | 99.72 | 99.72 | 99.72 | 0.00 | 0.00 |
| 3 | 99.55 | 99.52 | 99.52 | 99.52 | 99.52 | 99.53 | 99.53 | 0.02 | 0.02 |
| 4 | 99.62 | 99.62 | 99.62 | 99.60 | 99.59 | 99.60 | 99.61 | 0.01 | 0.01 |
| 5 | 99.79 | 99.90 | 99.81 | 99.82 | 99.82 | 99.83 | 99.83 | 0.04 | 0.04 |
| 6 | 99.72 | 99.72 | 99.72 | 99.72 | 99.72 | 99.72 | 99.72 | 0.00 | 0.00 |
| 7 | 99.80 | 99.81 | 99.81 | 99.78 | 99.78 | 99.78 | 99.79 | 0.01 | 0.01 |
| 8 | 99.71 | 99.71 | 99.73 | 99.74 | 99.72 | 99.72 | 99.72 | 0.01 | 0.01 |
| Number of the Laboratory | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Mean (%) | 99.49 | 99.72 | 99.53 | 99.61 | 99.83 | 99.72 | 99.79 | 99.72 |
| RSD (%) | 0.01 | 0.00 | 0.01 | 0.01 | 0.04 | 0.00 | 0.01 | 0.01 |
| (%) | 99.68 | |||||||
| S (%) | 0.12 | |||||||
| MSbetween | 0.088521 | |||||||
| MSwithin | 0.0063875 | |||||||
| Sr (%) | 0.08 | |||||||
| SL (%) | 0.12 | |||||||
| u (%) | 0.05 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Long, R.; Cao, F.; Zhao, X.; Lan, T.; Xu, D. Development of Pure Certified Reference Material of Cannabidiol. Molecules 2024, 29, 921. https://doi.org/10.3390/molecules29050921
Yu C, Long R, Cao F, Zhao X, Lan T, Xu D. Development of Pure Certified Reference Material of Cannabidiol. Molecules. 2024; 29(5):921. https://doi.org/10.3390/molecules29050921
Chicago/Turabian StyleYu, Congcong, Ruihan Long, Feng Cao, Xinying Zhao, Tao Lan, and Dunming Xu. 2024. "Development of Pure Certified Reference Material of Cannabidiol" Molecules 29, no. 5: 921. https://doi.org/10.3390/molecules29050921