1. Introduction
Rubbers have become essential materials in the industry with the advancement of plastic technology. The popularity of rubbers is due to good performance properties and the possibility of functionalization [
1]. The areas of particular interest in elastomer research include such features as self-healing, shape memory effect [
2,
3], superhydrophobicity [
4], reinforced mechanical properties, improved barrier properties [
5], and enhanced damping properties [
6,
7].
The opportunities provided by rubbers make it possible to create both elastomeric materials that perfectly dampen vibrations and those that can be repaired after breaking. For this reason, elastomers are widely used in such fields as automotive, aerospace, industrial equipment, and household appliances [
2,
3,
4,
7,
8,
9]. Noteworthy among rubbers is butyl rubber (IIR). It is a synthetic elastomer that is formed by the polymerization of isobutylene and isoprene. IIR vulcanizates exhibit very high anti-aging resistance and limited permeability to gases and liquids. In addition, they are resistant to weathering, ozone, hot air, acids, and bases. Butyl rubber is mainly used for products with reduced permeability to gases and liquids such as tire inner tubes, hoses, gaskets, and membranes [
10,
11,
12]. However, the latest research in the area of butyl rubber application shows that its use could be much wider.
El-Sabbagh S.H. et al. [
13] investigated the compatibility of natural rubber (NR) with butyl rubber to achieve elastomeric blends featuring both IIR and NR properties. The authors showed that macrophase separations form in the IIR/NR mix, which is disadvantageous for the properties of such blends. Moreover, a compatibilizer was used, which decreased the extent of phase separation and increased interfacial adhesion between phases, consequently reducing the size of dispersed phase domains, which led to better mechanical properties including tensile strength and a higher value of Young′s modulus. Sukharev N. et al. [
14] studied elastomeric blends containing butyl rubber and ethylene-propylene-diene rubber (EPDM). The study determined the influence of the proportion of elastomers in uncured blends and their effect on phase structure formation patterns in multicomponent polymer systems, changes in molecular mobility, and ozone resistance. The research has shown that there is such a ratio of EPDM to IIR to obtain the highest possible resistance to ozone. These materials can be successfully considered for use in applications where ozone concentrations are higher, i.e., at heights above 15 km or in the aerospace industry. A novel strategy to prepare interpenetrating polymer networks (IPNs) based on butyl rubber and poly(n-octadecyl acrylate) (PC18A) was developed by Tavsanli B. et al. [
15]. Solvent-free UV polymerization of the n-octadecyl acrylate monomer in the IIR melt at ambient temperature resulted in IPNs with self-healing and shape memory functions.
According to Guo X. et al. [
16], butyl rubber can be applied to materials with high damping electromagnetic interference or multi-absorbing materials, including absorbing electromagnetic waves. The study showed that the composites of butyl rubber with single-walled carbon nanotubes (IIR/SWCNT) achieved good mechanical performance (tensile strength reached 15 MPa), and the total electromagnetic shielding efficiency of the material increased to 23.8 dB. In addition, the authors showed that water-induced modification of the composite achieved good dispersion of SWCNTs to enhance electromagnetic shielding while maintaining a wide damping temperature range from −55 °C to 40 °C with a damping factor above 0.2. Chameswary J. et al. [
17] studied butyl rubber filled with barium titanate (BaTiO
3) with micro- or nanometric sizes. The research showed that such composites exhibited good mechanical properties, and they are flexible and absorb radio frequency vibrations. These results prove that these composites are proper candidates for the core of flexible dielectric waveguides and applications in flexible microwave substrates. Hao, S., et al. [
18] tested butyl rubber filled with graphene. Studies have shown that the proper formulation of such a composite makes it possible to achieve excellent mechanical properties, high conductivity, and high barrier to water vapor. The conductivity of the IIR/graphene nanocompound at a graphene content of 3.76% prepared by Liquid Phase Redispersion reached more than seven orders of magnitude higher than the conventional twin-roll mixing method. Due to the existence of homogeneously distributed networks of segregated graphene, the tensile strength and elongation at break for the IIR/graphene nanocomposites increased by 410 and 126%, respectively, at a graphene content of 3.76%. The IIR/graphene vulcanizate exhibited such electrical properties that it can be used as a wearable sensor and physiological signal detection.
The presented examples of filled IIR compositions show the advantages of using innovative substances such as elastomer fillers. However, for decades, the rubber industry, especially the tire industry, has mainly used carbon black as a reinforcing filler. Since its structure and surface can be changed over a wide range, carbon black can meet a wide variety of requirements. However, with the passing of time and the necessity to invent alternatives to carbon black, various types of silicas have been developed. In the 1940s and 1950s, the specific surface area and structure of silica were constantly adjusted to satisfy new requirements in the field of rubber materials [
19,
20,
21]. As the development proceeded, the advantages and disadvantages of silica compared to carbon black began to be perceived. Therefore, the research began for new and more easily available fillers for rubber, which will not cause as much carbon dioxide emissions as carbon black. The first and most obvious reason for using fillers is to improve mechanical properties, that is, to enhance the elastomer [
19,
22]. Fillers such as carbon black or silica can reinforce cured rubber, improving its tensile strength, durability, and wear resistance. This is particularly important in applications where the rubber products will be subjected to significant stress or abrasion. In addition to conventional fillers, various types of synthetic or natural substances are used, which also reinforce the composite by forming elastomer–filler and filler–filler interactions, thus creating a spatial network in the structure of the elastomer, which is responsible for carrying stresses [
19,
21,
22]. Another reason for using fillers is to reduce the cost of the rubber product. Fillers then act as diluents and reduce the amount of expensive rubber in the product. This is important to keep the right properties reducing production costs. Another reason is to control physical properties; fillers are used to adjust hardness, elasticity, and electrical and thermal conductivity. This allows manufacturers to customize rubber to suit specific requirements. In addition, the use of fillers allows the dimensional stability of rubber composites to be maintained. The incorporation of a filler into an elastomer matrix reduces shrinkage and increases the ability to retain shape over time. This is important in terms of providing the accuracy of manufactured parts, the efficiency of seals and gaskets, and the overall performance and reliability of rubber components in various applications [
20,
21,
22]. Additionally, some fillers improve the processability of rubber compounds by facilitating molding, extrusion, or other processing during production. The above reasons have contributed to the search for various fillers with unique properties, and the specific choice of filler will depend on the requirements of the product and the manufacturing process. Therefore, in recent years, more and more research has been conducted on alternatives to silica and carbon black [
19,
20,
21,
22,
23]. Substitution of standard reinforcing fillers with montmorillonite (MMT) has improved some of the desired properties of elastomeric compounds in addition to some reductions of the final weight and price of the final products [
24]. Montmorillonite organoclay has also been used as a filler in the IIR composites to improve their physical and mechanical properties [
25]. In this study, melt mixing in an internal mixer was selected as the method of dispersing organoclay in the elastomeric matrix, and the intercalation of rubber chains into the clay gallery was deduced from the increase in basal spacing of the silicate layers as was measured by XRD. The highest basal spacing was detected for the amount of MMT equal to 3 phr. Dispersion and distribution of the organoclay were observed by SEM. The authors proved that organoclay content and structure had a large impact on the mechanical and rheological properties of nanocomposites as well as the permeability of carbon dioxide gas through their films.
This study has focused on layered silicates and their effects on butyl rubber vulcanizates because it is a very promising group of compounds, that indicate suitable properties in the case of novel elastomeric materials. In this paper, the main focus was on the hydrophobic and barrier properties of butyl rubber vulcanizates filled with various phyllosilicates. A material whose surface exhibits hydrophobic or superhydrophobic properties is the target of much scientific research [
4]. The term “superhydrophobic” describes surfaces with a Young′s angle of more than 150° and indicates very low wettability. This angle is defined as the mechanical equilibrium of the drop under the action of three interfacial tensions: solid–vapor, γ
sv; solid–liquid, γ
sl; and liquid–vapor, γ
lv [
26,
27,
28]. This equilibrium relation is known as Young′s Equation (1):
where γ
lv, γ
sv, and γ
sl represent the liquid–vapor, solid–vapor, and solid–liquid interfacial tensions, respectively, and θ
Y is the Young′s contact angle [
28].
The popularity of such surfaces was started by the “lotus effect” described by Barthlott and Neinhus [
29,
30]. The lotus leaves owe their unusually high contact angle to a characteristic structure that scientists want to replicate for superhydrophobic materials. Examples of applications for such products could include self-cleaning materials [
31], anti-corrosion [
32], anti-icing [
32,
33], protective coatings, and impermeability to liquids.
Hydrophobicity microscopically is the tendency of chemical molecules to repel water molecules from each other, while hydrophobicity macroscopically is the property of a material’s surface to be non-wettable by water. Associated with this phenomenon in polymeric materials are such terms as surface tension, surface free energy, and contact angle [
34]. The wettability of polymer surfaces, including elastomers, depends on the contact angle, e.g., the angle formed by the tangent to the surface of a droplet on the surface of a solid, and it is highly dependent on the surface tension [
35,
36,
37].
The various values of Young′s angle lead to different phenomena at the solid–liquid interface. Therefore, several values of ϴ are distinguished as follows:
- −
ϴ = 0, when the liquid wets the surface of the solid and tends to spontaneously spread on it; this state is defined as the critical value of the surface tension of the liquid;
- −
0 < ϴ < π/2, when the liquid spills on the surface and tends to shrink on the surface within a limited range;
- −
ϴ > π/2, when the liquid does not spread on the surface and tends to shrink on the surface of the solid, forming droplets [
35,
36,
37].
In the context of the water–polymer interface, it must be considered that the contact angle is a measure of the hydrophilicity of the surface of a polymer material. Because water exhibits the highest surface tension compared to other liquids, the character of the polymer surface can be determined by the value of the contact angle. A hydrophilic surface is distinguished by the fact that the applied droplet spreads, thereby wetting the surface (the angle ϴ is small), for example, it is worth mentioning the surfaces of non-oxidized metal or glass (ϴ < 20°). On the opposite side, according to the literature, a hydrophobic surface is characterized by the fact that the applied water droplet does not spread on the polymer surface (the angle ϴ is large), such as the surface of polypropylene (ϴ = 110°) or polystyrene (ϴ > 97°) [
34].
Other important parameters describing elastomeric materials are their barrier properties. These are the features that determine whether a type of polymer can be used as, for example, a film or protective coating against a toxic substance. Three coefficients are used to describe them: permeability coefficient, diffusion coefficient, and solubility coefficient [
5,
38,
39]. To describe the permeation of gases through an elastomer, a diffusion mechanism is used, which occurs across the material due to a pressure gradient. In this case, the constant volume and variable pressure method is used to measure this coefficient. A vacuum was applied to both sides of the polymeric material, and the permeability coefficient P is described by the following Formula (2):
where V is the total amount of gas permeation through the sample into a cell, t
f is the sample thickness, A is the sample area, R is the gas constant, T is the absolute temperature, Δp is the pressure gradient across the sample, dp/dt is the transmission rate [
5,
38,
39].
In addition, the permeability coefficient (P) combines the effects of both the diffusion coefficient (D) and the solubility coefficient (S) and can be explained as Formula (3):
where D is the diffusion coefficient, which describes the kinetic aspect of transport, S is the solubility coefficient, which is related to the affinity of the penetrating substance (gas or liquid).
The above relation holds true when the value of D is independent of concentration and the value of S follows Henry’s law. It is used to describe gas transport in polymeric composites reinforced with impermeable nanofillers. In the above diffusion–solubility model, penetrant molecules initially dissolve into the high-pressure face of a film then diffuse across it through thickness and finally desorb at the low-pressure face. Thus, the permeability of a penetrant depends on both its diffusivity and solubility. These properties can be changed by the molecular structure of the polymer and environmental factors [
38,
40].
To increase the barrier properties of elastomers, layered fillers such as montmorillonite, graphene, vermiculite, or halloysite are introduced [
39,
40,
41,
42,
43,
44,
45]. The layers of this filler must “split” and form a difficult path for the substance to penetrate the material under investigation. This process is called exfoliation and is not simple to achieve when the filler is incorporated in situ into the polymer. Studies show that the most effective way to stop gas diffusion is to position the filler layers in an orientation perpendicular to the movement of the penetrating gas and increase the interactions at the polymer–filler interface [
41,
42,
43,
46].
It is common knowledge that the morphology and dispersion of layered aluminosilicates are key factors affecting the barrier properties of the composite. Therefore, proper dispersion and achieving a high degree of exfoliation of the layered nanofiller in the polymer matrix are the most important challenges in producing nanocomposites with improved barrier properties. According to the literature, there are three possible morphologies in nanocomposites (
Figure 1) [
38,
47,
48]:
- −
Tactoid (conventional composite), when there is no separation of layered filler packages, micrometer-sized structures are present in the polymer medium;
- −
Intercalated nanocomposite, when the polymer is located between parallel filler galleries, separation of filler layers occurs;
- −
Exfoliated nanocomposite, when the structure with the highest degree of dispersion of the filler in the polymer matrix, the polymer chains cause separation of the filler layers [
49,
50,
51].
The study aimed to produce new elastomeric materials containing butyl rubber and characterized them by enhanced hydrophobicity and barrier properties and reduced chemical degradation. To test the research, six various rubber compositions were prepared, which differed in the kind of filler. Phyllosilicates were chosen as fillers in the tested IIR compositions because they have the ability to interact with organic matter [
49,
50,
51,
52,
53,
54,
55], as well as the tendency to separate the packages that constitute them into nanometer-thick layers, which theoretically allows the obtaining of materials classified as nanocomposites [
56,
57,
58,
59,
60,
61].
3. Materials and Methods
3.1. Materials
In this work, butyl rubber, IIR (type: Butyl 206) with 2.3% mol. unsaturated bonds, a density of 0.91 g/mL, and Mooney viscosity of ML 1 + 8 (125 °C): 51 (delivered by ExxonMobil Chemicals&Specialties, Irving, TX, USA).
The curing system consisted of the following substances:
- -
Sulfur (S) as a cross-linking agent, with a density of 1.8–2.36 g/mL (delivered by Chempur, Piekary Śląskie, Poland);
- -
Zinc oxide (ZnO) as a cross-linking activator, with a density of 5.6 g/mL (delivered by Chempur, Piekary Śląskie, Poland);
- -
Tetramethylthiuram disulfide (TMTD) as a cross-linking accelerator, with a density of 1.29 g/mL (delivered by Brenntag Polska Sp. z o. o., Kędzierzyn-Koźle, Polska);
- -
Stearic acid (SA) as a cross-linking activator and dispersing agent, with a density of 0.94 g/mL (delivery by Chempur, Piekary Śląskie, Poland).
The following fillers were used:
- -
Silica (ARS), type: Arsil, with a density of 2.20 g/mL (delivered by Zakłady Chemiczne “Rudniki” S.A., Rudniki, Poland);
- -
Vermiculite (VER), FlameHunter VE MIC, with a density of 0.85–1.00 g/mL, and an average particle size of 250–710 μm (>80%) (delivered by NYSA Chem® Sp. z o. o., Wrocław, Poland);
- -
Montmorillonite (MMT, NanoBent ZR), modified with a quaternary ammonium salt with two short- and two long-chain alkyl substituents, with an average particle size of 20–60 μm (56%), ≤20 μm (44%) and layer separation of 2.0–2.4 nm (delivered by ZGM “Zębiec”, Zębiec, Poland);
- -
Perlite (PER), type: EP100F, with a density of 0.06–0.14 g/mL (delivered by Perlipol, Bełchatów, Poland);
- -
Halloysite tubes (HNT), with a density of 2.53 g/mL (delivered by Sigma-Aldrich Chemie, Steinheim am Albuch, Germany).
3.2. Compounding and Vulcanization
The IIR composites were prepared using a two-roll mill (type: Laborwalzwerk, Krupp-Gruson, Magdeburg-Buckau, Germany) with a roll diameter of 200 mm and a roll length of 450 mm, at a roll temperature of 30–35 °C. The total time to create the composition was 5–8 min. First, the rubber was plasticized and the ingredients were incorporated in the following order: stearic acid, ZnO, filler, accelerator, and sulfur. The obtained rubber composites were stored separately in tightly closed foils at room temperature.
The produced mixes were vulcanized in hydraulic presses in appropriate metal molds. The vulcanization parameters were a temperature of 160 °C, a pressure of 150–180 bar, and a cure time of 30 min.
3.3. Characteristics of the Cross-Linking Process
The vulcanization process is characterized by determining the cure kinetics, and the equilibrium swelling. The cure kinetics of the IIR composites were determined using the Alpha Technologies (MDR 2000) oscillating disk rheometer (Alpha Technologies, Hudson, OH, USA) at 160 °C (ASTM D5289-17 standard [
86]), which was employed to determine the following parameters: scorch time (t
02); vulcanization time (t
90); and minimal torque (T
min); maximum torque increment (ΔT
max), which is the difference between the torque after heating and minimal torque values. The degree of vulcanization (conversion) in the vulcametric studies was defined according to Formula (4) [
87].
where T
t is the torque value at a given time during the vulcanization (in this case: T
15), T
0 is the torque value at time zero, T
h is the torque value at the end of vulcanization (in this case: T
30).
The cure rate index (CRI) was designated according to Formula (5):
Swelling behavior was assessed using toluene (according to ASTM D471 [
88]). From each vulcanizate, four test pieces of 25–60 mg of different shapes were cut out, weighed using an electrical balance, and swollen in toluene until equilibrium was reached (for 72 h). After this time, the swollen samples were removed from toluene and washed with diethyl ether, and their weights were determined again. The samples were dried to a constant weight at a temperature of 50 °C and then reweighed.
Equilibrium volume swelling (Q
v) was calculated using Formula (6):
where Q
w is the value of the equilibrium mass swelling (mg/mg), d
v is the vulcanizate density (g/mL), and d
s is the solvent density (g/mL).
Equilibrium weight swelling was calculated from Formula (7):
where m
s is the swollen sample weight (mg), m
d is the dry sample weight (mg), and m
d* is the reduced sample weight (mg). The reduced sample weight was calculated from Formula (8):
where m
0 is the initial sample weight (mg), m
m is the mineral content in the blend (mg), and m
t is the total weight of the blend (mg).
Negative equilibrium weight swelling (
−Q
w), interpreted as the amount of leaching substances, was calculated from Formula (9):
The rubber volume fraction (V
R) was calculated from Formula (10):
The degree of cross-linking (α
c) was determined using Formula (11):
3.4. Determination of Surface Morphology
The morphology of the vulcanizates was assessed using a scanning electron microscope (SEM). This was a Hitachi Tabletop Microscope TM-1000 (Tokyo, Japan) product. The preparation of the samples for measurement consisted of placing a double-sided self-adhesive foil onto a special table and gluing the testing sample to it. Then, a gold layer was applied to the prepared sample using the Cressington Sputter Coater 108 auto vacuum sputtering machine (Redding, CA, USA) at a pressure greater than 40 mbar for 60 s. The samples prepared in this way were placed into the scanning electron microscope chamber, and the measurement was performed.
3.5. Determination of Dynamic and Mechanical Properties
For the vulcanizates, the following properties were determined: strength properties, hysteresis losses and Mullins effect, tear resistance, hardness, elasticity, loss modules, and Payne effect.
Measurements of the tensile properties were carried out using a testing machine (Zwick1435/Roell GmbH & Co. KG, Ulm, Germany). The parameters determined from this test were stress at elongation of 100, 200, and 300% (Se100, Se200, Se300); tensile strength (TSb); and relative elongation at break (Eb). Each property was determined for five samples. The test was conducted at a constant speed of 500 mm/min.
The hysteresis losses were determined using a testing machine (Zwick1435/Roell GmbH & Co. KG, Ulm, Germany). Each test was conducted for three samples, which were stretched five times to 200% elongation at a stretching speed of 500 mm/min, and the initial force was 0.1 N. The Mullins effect was determined according to Formula (12):
where W
1 is the hysteresis loss at the first extension of the sample (N∙mm) and W
5 is the hysteresis loss at the fifth extension of the sample (N∙mm).
The tear strength (T
s) was tested in accordance with method A of the standard ISO 34-1:2022 [
89] using a testing machine (Zwick1435/Roell GmbH & Co. KG, Ulm, Germany). Rectangular specimens with dimensions of 100 mm × 15 mm and a cut of 40 mm were used for the tests.
Hardness (HA) was tested on the Shore A scale using a Zwick/Roell hardness tester according to ISO-48-4:2018 [
90]. Each test was performed ten times. The samples were in the shapes of cylinders, with diameters of 80 mm and heights of 6 mm.
The dynamic properties of the vulcanizates were determined by the minimum and maximum storage modulus (G′
min, G′
max), the maximum loss modulus (G′
max), and the Payne effect (ΔG′, Formula (13)) at room temperature. The test was performed using the Ares G2 rotational rheometer (New Castle, UK) according to ISO 4664-1:2022 [
91]. The tested samples, in the form of discs with dimensions of 25 mm × 2 mm, were placed between special measuring plates of the apparatus. The parameters that were used were as follows: a soak time of 10 s, an angular frequency of 10 rad/s, a logarithmic sweep with strain from 0.005 to 70% s, 20 points per decade, and an initial force of 5 N.
where G′
max is the maximum storage modulus (MPa) and G′
min is the minimum storage modulus (MPa).
3.6. Resistance to Thermo-Oxidative Aging
The thermal aging of the IIR vulcanizates was carried out in a forced circulating aging oven at 70 °C for 7 days. After conditioning at room temperature for 24 h, the changes of mechanical properties (stress at 100%, 200%, or 300% strain, tensile strength, elongation at break) were evaluated based on the aging factor (AF) according to Formula (14):
where
is the tensile strength after thermo-oxidative aging (MPa), TS
b is the tensile strength before thermo-oxidative aging (MPa),
is the elongation at break after thermo-oxidative aging (%), and E
b is the elongation at break before thermo-oxidative aging (%).
3.7. Determination of Hydrophobicity
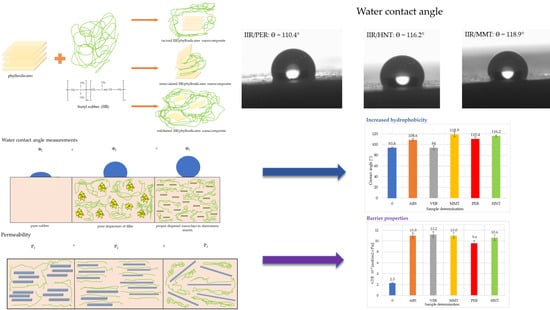
The contact angle of the vulcanizates surface was determined using a goniometer from DataPhysics Instruments GmbH OCA 15EC (Filderstadt, Germany). The embedded drop method was used. At the beginning of the measurement, a drop of water with a volume of ~5 µL was placed on the surface of the vulcanizate using a Hamilton microsyringe. Then, using a special program, a photo of the drop was taken within 10 s so that the boundary between the surface and the drop was visible. The contact angle was measured by analysis in a computer program adapted for this study. A minimum of 5 drops were deposited into each sample and the average value of the contact angle was calculated.
3.8. Determination of Barrier Properties
The barrier properties were measured using a device that tests gas permeability using the manometric method. The measurement of barrier properties was based on a method that used pressure differences in measurement chambers on both sides of the tested sample. The apparatus consisted of a measuring cell in which the test sample was placed. The measuring cell was divided into two parts, i.e., an atmospheric pressure chamber and a low-pressure chamber. The test gas (air) was supplied to the chamber at atmospheric pressure. The low-pressure chamber contained a high-sensitivity sensor that measured pressure changes occurring in the measuring chamber as a result of gas permeating through the partition between both chambers containing the tested sample. A vacuum pump was connected to the low-pressure chamber, generating low pressure in the chamber (<10 Pa). From the results obtained, the gas transmission rate (GTR) was calculated according to Formula (15):
where V
c is the volume of the low-pressure chamber (l), R is the gas constant (8.31 × 10
3) [(l·Pa)/(K·mol)], T is the measurement temperature (K), P
u is the gas pressure in the high-pressure chamber (Pa), A is the area of gas permeation through the sample (m
2), dp/dt is the pressure changes per unit of time (Pa/s).
The coefficient gas permeability (P) was determined according to Formula (16):
where d is the sample thickness (m).
3.9. Chemical Degradation
Chemical degradation studies for the obtained vulcanizates were carried out in accordance with the standard EN ISO 374-4:2019 [
92]. Samples with a diameter of 20 mm were acclimatized at (23 ± 2 °C) for 24 h as per EN ISO 2231:1995 (PN-EN ISO 2231:1999) [
93]. Then they were secured in glass vials containing 2 mL of heptane or methanol sealed with septa having a center hole 12 mm in diameter. The samples were placed under the septa. Vials prepared in this way were inverted to make sure that the test chemical was in direct contact with the vulcanizate surface for one hour.
Subsequently, the mechanical parameter of puncture force was determined in a comparative system, namely, for vulcanizates exposed and not exposed to the test chemicals (
Figure 9); the latter served as reference values. Puncture force was measured at a probe advance rate of 100 mm/min (the initial distance between the puncture probe and the sample surface was 100 mm). The mean difference (
n = 3) of the puncture force for samples exposed and not exposed to the test chemicals was expressed as a percentage.
3.10. Abrasion Resistance
Abrasion resistance was tested according to the standard PN-EN 388:2017-02 [
94] with a modified maximum number of rubs. Samples with a diameter of 38 mm were acclimatized at a temperature of (23 ± 2) °C and a relative humidity of (50 ± 5)% for 24 h according to EN ISO 2231:1995 (PN-EN ISO 2231:1999). The mechanical parameter of resistance to cyclical abrasion was determined after 500 rubs executed at a force of (9 ± 0.2) kPa using a Martindale apparatus (James Heal, Sterling, VA, USA).