Broadening the Voltage Window of 3D-Printed MXene Micro-Supercapacitors with a Hybridized Electrolyte
Abstract
:1. Introduction
2. Results and Discussion
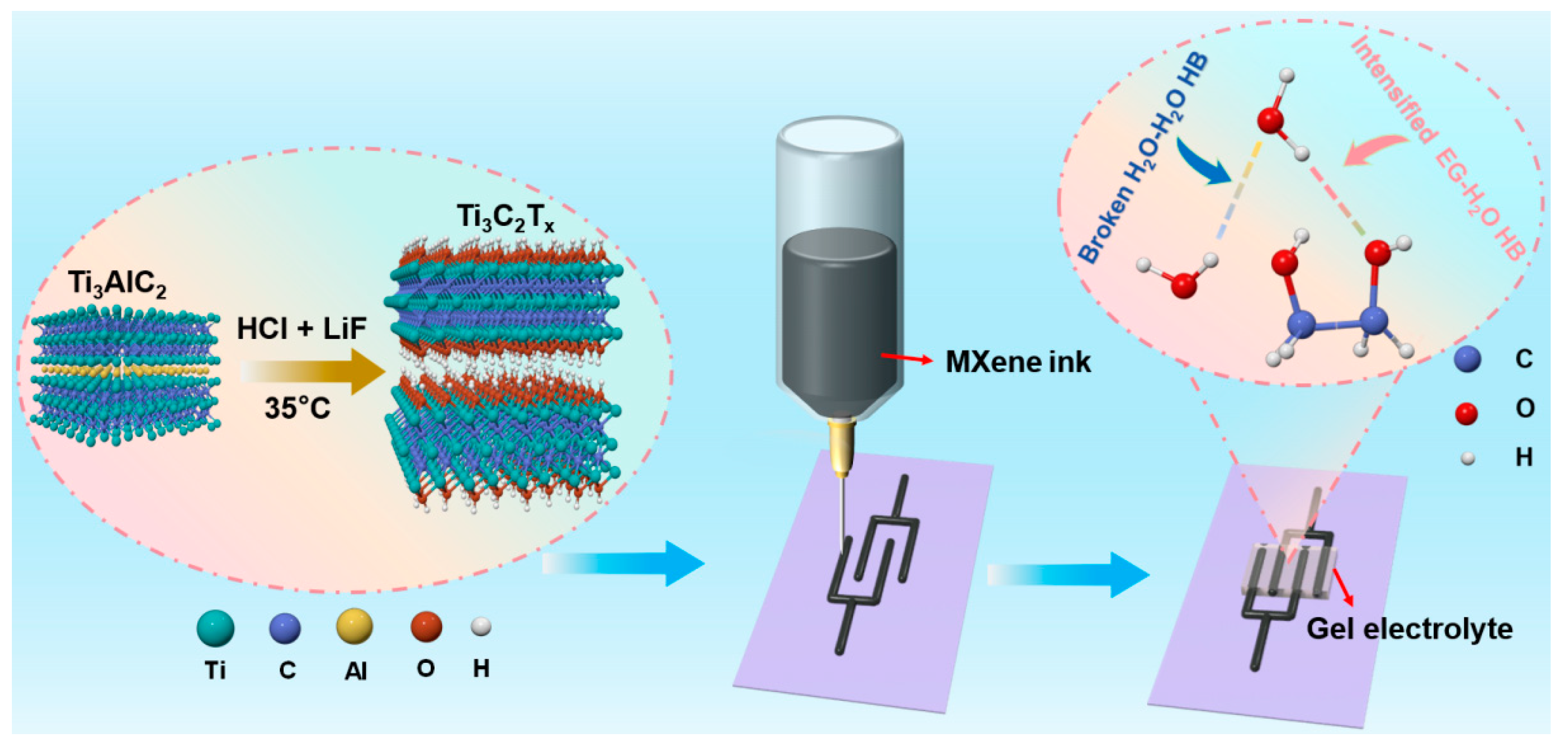
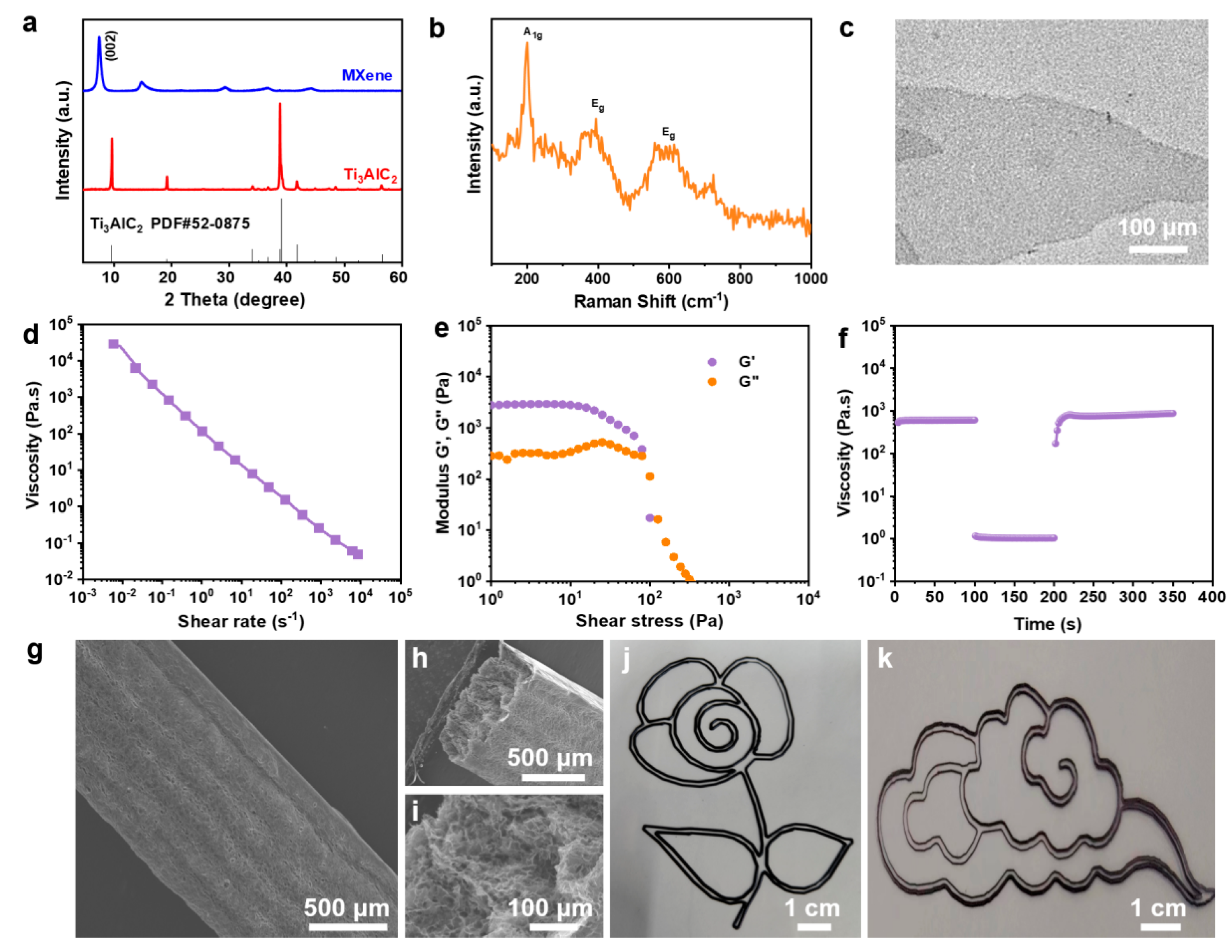
2.1. Material Fabrication and Characterization
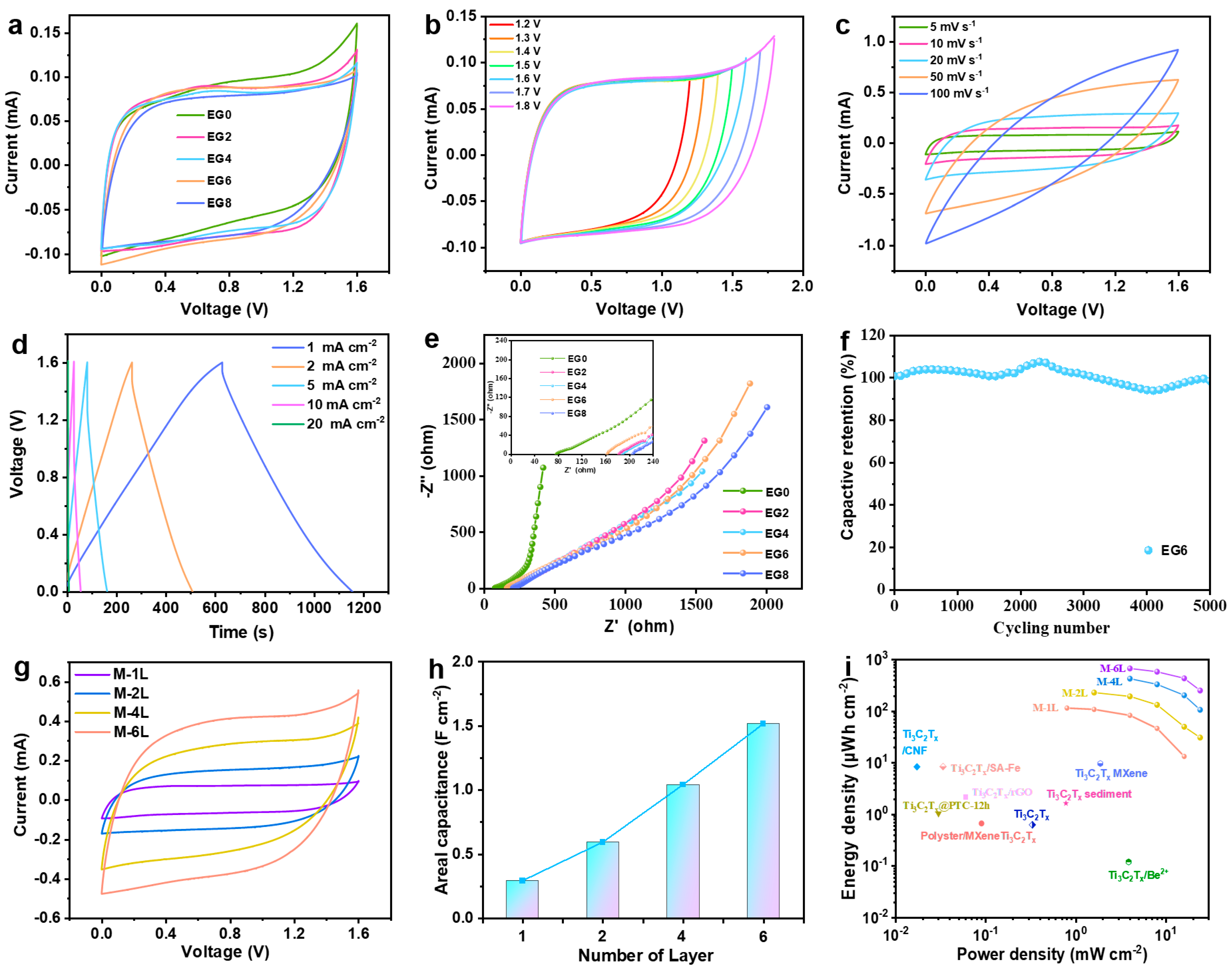
2.2. Characterization of NaCl/H2O/EG Hybrid Electrolytes
2.3. Electrochemical Performance of MXene MSCs
2.4. Integrative Electrochemical Performance of MSCs
3. Experimental Section
3.1. Fabrication of Ti3C2Tx MXene Ink
3.2. Preparation of Gel Electrolyte
3.3. Fabrication of MSCs via 3D Printing
3.4. Material Characterization
3.5. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, B.; Liu, F.; Sun, G.; Gao, J.; Xu, T.; Xiao, Y.; Shao, C.; Jin, X.; Yang, H.; Zhao, Y.; et al. Compact assembly and programmable integration of supercapacitors. Adv. Mater. 2020, 32, 1907005. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Zhu, F.; Schmidt, O.G. Recent progress in micro-supercapacitor design, integration, and functionalization. Small Methods 2018, 3, 1800367. [Google Scholar] [CrossRef]
- Jia, J.; Zhu, Y.; Das, P.; Ma, J.; Wang, S.; Zhu, G.; Wu, Z.-S. Advancing MXene-based integrated microsystems with micro-supercapacitors and/or sensors: Rational design, key progress, and challenging perspectives. J. Mater. 2023, 9, 1242–1262. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Cheng, L.; Mo, F.; Chen, L.; Yu, S.; Wei, J. 3D printed supercapacitor: Techniques, materials, designs, and applications. Adv. Funct. Mater. 2022, 33, 2208034. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z.; Qian, Q.; Wang, J.; Ren, J.; Chen, H.; Xing, W.; Zhou, N. Customizable supercapacitors via 3D printed gel electrolyte. Adv. Funct. Mater. 2023, 33, 2214301. [Google Scholar] [CrossRef]
- Jiang, T. Fabrication technology, research and development status, development trend of the ternary laminated structure MAX phase ceramics materials. Adv. Ceram. 2023, 44, 1–23. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, X.; Jin, S.; Xia, Q.; Chang, Y.; Wang, L.; Zhou, A. Synthesis of Mo2C MXene with high electrochemical performance by alkali hydrothermal etching. J. Adv. Ceram. 2023, 12, 1889–1901. [Google Scholar] [CrossRef]
- Qin, T.; Chen, H.; Zhang, Y.; Chen, X.; Liu, L.; Yan, D.; Ma, S.; Hou, J.; Yu, F.; Peng, S. Modulating surface chemistry of heteroatom-rich micropore carbon cloth electrode for aqueous 2.1 V high-voltage window all-carbon supercapacitor. J. Power Sources 2019, 431, 232–238. [Google Scholar] [CrossRef]
- Yu, L.; Fan, Z.; Shao, Y.; Tian, Z.; Sun, J.; Liu, Z. Versatile N-doped MXene ink for printed electrochemical energy storage application. Adv. Energy Mater. 2019, 9, 1901839. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Ma, J.; Das, P.; Zheng, S.; Wu, Z.-S. Recent status and future perspectives of 2D MXene for micro-supercapacitors and micro-batteries. Energy Storage Mater. 2022, 51, 500–526. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, D.; Cheng, M.; Zhou, J.; Owusu, K.A.; Mai, L.; Yu, Y. A new view of supercapacitors: Integrated supercapacitors. Adv. Energy Mater. 2019, 9, 1901081. [Google Scholar] [CrossRef]
- Li, L.; Meng, J.; Bao, X.; Huang, Y.; Yan, X.P.; Qian, H.L.; Zhang, C.; Liu, T. Direct-ink-write 3D printing of programmable micro-supercapacitors from MXene-regulating conducting polymer inks. Adv. Energy Mater. 2023, 13, 2203683. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Fan, X.; Shi, X.; Liang, J. 3D-printed stretchable micro-supercapacitor with remarkable areal performance. Adv. Energy Mater. 2020, 10, 1903794. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Zhang, X.; Li, X.; Zhang, G.; Cao, P. 3D printed micro-electrochemical energy storage devices: From design to integration. Adv. Funct. Mater. 2021, 31, 2104909. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Q.; Ma, J.; Das, P.; Zhang, L.; Liu, H.; Wang, S.; Li, H.; Wu, Z.-S. Three-dimensional (3D)-printed MXene high-voltage aqueous micro-supercapacitors with ultrahigh areal energy density and low-temperature tolerance. Carbon Energy 2024, e481. [Google Scholar] [CrossRef]
- Guo, T.; Zhou, D.; Pang, L.; Sun, S.; Zhou, T.; Su, J. Perspectives on working voltage of aqueous supercapacitors. Small 2022, 18, 2106360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Wang, Y.; Cheng, T.; Yao, L.Q.; Li, X.; Lai, W.Y.; Huang, W. Printed supercapacitors: Materials, printing and applications. Chem. Soc. Rev. 2019, 48, 3229–3264. [Google Scholar] [CrossRef]
- Orangi, J.; Hamade, F.; Davis, V.A.; Beidaghi, M. 3D printing of additive-free 2D Ti3C2Tx(MXene) ink for fabrication of micro-supercapacitors with ultra-high energy densities. ACS Nano 2020, 14, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, M.C.; Liu, C.; Wu, Q.; Mei, C. 3D printed Ti3C2TxMXene/cellulose nanofiber architectures for solid-state supercapacitors: Ink rheology, 3D printability, and electrochemical performance. Adv. Funct. Mater. 2021, 32, 2109593. [Google Scholar] [CrossRef]
- Chen, M.-T.; Zhang, T.-Y.; Qiu, D.-Q.; Fang, Z.-K.; Zhu, W.-H.; Chen, B.-Y.; Dai, Y.-M. Study on the supercapacitor properties of Fe/Ni composite oxides. Adv. Ceram. 2022, 43, 300–303. [Google Scholar]
- Jiang, Q.; Lei, Y.; Liang, H.; Xi, K.; Xia, C.; Alshareef, H.N. Review of MXene electrochemical microsupercapacitors. Energy Storage Mater. 2020, 27, 78–95. [Google Scholar] [CrossRef]
- Li, K.; Li, J.; Zhu, Q.; Xu, B. Three-dimensional MXenes for supercapacitors: A review. Small Methods 2022, 6, 2101537. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Wang, X.; Bai, W.; Sun, H.; Pan, F.; Chi, Y.; Wang, Z. High-temperature supercapacitors based on MXene with ultrahigh volumetric capacitance. ACS Mater. Lett. 2023, 5, 2084–2095. [Google Scholar] [CrossRef]
- Jin, X.; Song, L.; Dai, C.; Xiao, Y.; Han, Y.; Zhang, X.; Li, X.; Bai, C.; Zhang, J.; Zhao, Y.; et al. An aqueous anti-freezing and heat-tolerant symmetric microsupercapacitor with 2.3 V output voltage. Adv. Energy Mater. 2021, 11, 2101523. [Google Scholar] [CrossRef]
- Leong, K.W.; Pan, W.; Wang, Y.; Luo, S.; Zhao, X.; Leung, D.Y.C. Reversibility of a high-voltage, Cl–-regulated, aqueous mg metal battery enabled by a water-in-salt electrolyte. ACS Energy Lett. 2022, 7, 2657–2666. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Huang, J.; Liu, M. Low temperature tolerant organohydrogel electrolytes for flexible solid-state supercapacitors. Adv. Energy Mater. 2018, 8, 1801967. [Google Scholar] [CrossRef]
- Tang, X.; Xiao, D.; Xu, Z.; Liu, Q.; Ding, B.; Dou, H.; Zhang, X. A novel ionic liquid-based electrolyte assisting the high performance of low-temperature supercapacitors. J. Mater. Chem. A 2022, 10, 18374–18382. [Google Scholar] [CrossRef]
- Zhou, Y.; Ghaffari, M.; Lin, M.; Xu, H.; Xie, H.; Koo, C.M.; Zhang, Q.M. High performance supercapacitor under extremely low environmental temperature. RSC Adv. 2015, 5, 71699–71703. [Google Scholar] [CrossRef]
- Hou, X.; Wang, R.; He, X.; Pollard, T.P.; Ju, X.; Du, L.; Paillard, E.; Frielinghaus, H.; Barnsley, L.C.; Borodin, O.; et al. Stabilizing the solid-electrolyte interphase with polyacrylamide for high-voltage aqueous lithium-ion batteries. Angew. Chem. Int. Ed. 2021, 60, 22812–22817. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, S.; Chen, N.; Li, Y.; Lai, J.; Ma, Y.; Chen, J.; Wu, F.; Chen, R. A universal strategy for high-voltage aqueous batteries via lone pair electrons as the hydrogen bond-breaker. Energy Environ. Sci. 2022, 15, 2653–2663. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, P.; Liu, Y.; Zhu, M.; Dai, T.; Tie, Z.; Jin, Z. Wide-voltage-window amphiphilic supramolecule excluded-volume electrolytes for ultra-durable full-cell aqueous potassium-ion batteries. Chem. Eng. J. 2023, 459, 141623. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, K.; Gao, C.; Wang, J.; Wu, W.; Zhao, F.; Qu, L.; Zhao, Y. High water-retaining, antifreeze micro-supercapacitor without encapsulation for all-weather. J. Power Sources 2022, 545, 231909. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, W.; Wu, Y.; Wu, X.; Zhang, X.; Jiang, S.; Zhao, B.; Chen, Y.; Yang, C.; Ding, L.; et al. High-performance all-printed flexible micro-supercapacitors with hierarchical encapsulation. Energy Environ. Mater. 2024, e12657. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Z.; Ma, Y.; Bin, D.; Wang, Y.; Xia, Y. Recent progress of rechargeable batteries using mild aqueous electrolytes. Small Methods 2019, 3, 1800272. [Google Scholar] [CrossRef]
- Xu, J.; Ji, X.; Zhang, J.; Yang, C.; Wang, P.; Liu, S.; Ludwig, K.; Chen, F.; Kofinas, P.; Wang, C. Aqueous electrolyte design for super-stable 2.5 V LiMn2O4 || Li4Ti5O12 pouch cells. Nat. Energy 2022, 7, 186–193. [Google Scholar] [CrossRef]
- Yuan, G.; Wan, T.; BaQais, A.; Mu, Y.; Cui, D.; Amin, M.A.; Li, X.; Xu, B.B.; Zhu, X.; Algadi, H.; et al. Boron and fluorine Co-doped laser-induced graphene towards high-performance micro-supercapacitors. Carbon 2023, 212, 118101. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, J.; Das, P.; Wang, S.; Wu, Z.-S. High-voltage MXene-based supercapacitors: Present status and future perspectives. Small Methods 2023, 7, 2201609. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Zhang, L.; Li, Z.; Dou, H.; Zhang, X. Design strategies and research progress for water-in-salt electrolytes. Energy Storage Mater. 2022, 44, 10–28. [Google Scholar] [CrossRef]
- Liang, T.; Hou, R.; Dou, Q.; Zhang, H.; Yan, X. The applications of water-in-salt electrolytes in electrochemical energy storage devices. Adv. Funct. Mater. 2021, 31, 2006749. [Google Scholar] [CrossRef]
- Abbas, Q.; Béguin, F. High voltage AC/AC electrochemical capacitor operating at low temperature in salt aqueous electrolyte. J. Power Sources 2016, 318, 235–241. [Google Scholar] [CrossRef]
- Kamboj, N.; Dey, R.S. Exploring the chemistry of “organic/water-in-salt” electrolyte in graphene-polypyrrole based high-voltage (2.4 V) microsupercapacitor. Electrochim. Acta 2022, 421, 140499. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, S.; Lu, P.; Ma, J.; Das, P.; Su, F.; Cheng, H.M.; Wu, Z.S. Kinetic regulation of MXene with water-in-LiCl electrolyte for high-voltage micro-supercapacitors. Nat. Sci. Rev. 2022, 9, nwac024. [Google Scholar] [CrossRef]
- Hu, Z.; Song, Z.; Huang, Z.; Tao, S.; Song, B.; Cao, Z.; Hu, X.; Wu, J.; Li, F.; Deng, W.; et al. Reconstructing hydrogen bond network enables high voltage aqueous zinc-ion supercapacitors. Angew. Chem. Int. Ed. 2023, 62, e202309601. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Li, P.; Du, X.; Ma, M.; Liang, Z.; Su, Y.; Xiong, L. Ultrahigh-voltage aqueous electrolyte for wide-temperature supercapacitors. J. Mater. Chem. A 2023, 11, 15532–15539. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, G.; Zhu, Y.; Lin, M.C.; Chen, H.; Li, Y.Y.; Hung, W.H.; Zhou, B.; Wang, X.; Bai, Y.; et al. High-safety and high-energy-density lithium metal batteries in a novel ionic-liquid electrolyte. Adv. Mater. 2020, 32, 2001741. [Google Scholar] [CrossRef]
- Chang, N.; Li, T.; Li, R.; Wang, S.; Yin, Y.; Zhang, H.; Li, X. An aqueous hybrid electrolyte for low-temperature zinc-based energy storage devices. Energy Environ. Sci. 2020, 13, 3527–3535. [Google Scholar] [CrossRef]
- Wang, W.; Yang, C.; Chi, X.; Liu, J.; Wen, B.; Liu, Y. Small-molecular crowding electrolyte enables high-voltage and high-rate supercapacitors. Energy Technol. 2021, 9, 2100684. [Google Scholar] [CrossRef]
- Li, S.; Shi, Q.; Li, Y.; Yang, J.; Chang, T.H.; Jiang, J.; Chen, P.Y. Intercalation of metal ions into Ti3C2TxMXene electrodes for high-areal-capacitance microsupercapacitors with neutral multivalent electrolytes. Adv. Funct. Mater. 2020, 30, 2003721. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2TxMXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, N.; Ma, Y.; Wang, S.; Liu, W.; Luo, C.; Zhang, H.; Cheng, F.; Rao, J.; Hu, X.; et al. Highly self-healable 3D microsupercapacitor with MXene-graphene composite aerogel. ACS Nano 2018, 12, 4224–4232. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, R.; Zhang, H.; Cheng, K. Aqueous MXene inks for inkjet-printing microsupercapacitors with ultrahigh energy densities. J. Colloid Interface Sci. 2023, 645, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Abdolhosseinzadeh, S.; Schneider, R.; Verma, A.; Heier, J.; Nuesch, F.; Zhang, C.J. Turning trash into treasure: Additive free MXene sediment inks for screen-printed micro-supercapacitors. Adv. Mater. 2020, 32, 2000716. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wei, L.; Wu, X.; Jiang, C.; Yao, Y.; Peng, B.; Chen, H.; Huangfu, J.; Ying, Y.; Zhang, C.J.; et al. Room-temperature high-precision printing of flexible wireless electronics based on MXene inks. Nat. Commun. 2022, 13, 3223. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Tebyetekerwa, M.; Marriam, I.; Li, W.; Wu, Y.; Peng, S.; Ramakrishna, S.; Yang, S.; Zhu, M. Polyester@MXene nanofibers-based yarn electrodes. J. Power Sources 2018, 396, 683–690. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Huang, Y.; Zhao, X.; Shi, Y.; Qu, J.; Yang, C.; Xie, J.; Wang, J.; Li, L.; et al. Duplex printing of all-in-one integrated electronic devices for temperature monitoring. J. Mater. Chem. A 2019, 7, 972–978. [Google Scholar] [CrossRef]
- Zhang, C.; Kremer, M.P.; Seral-Ascaso, A.; Park, S.H.; McEvoy, N.; Anasori, B.; Gogotsi, Y.; Nicolosi, V. Stamping of flexible, coplanar micro-supercapacitors using MXene inks. Adv. Funct. Mater. 2018, 28, 1705506. [Google Scholar] [CrossRef]
- Zhu, G.; Hou, Y.; Lu, J.; Zhang, H.; Zhuang, Z.; Baig, M.M.; Khan, M.Z.; Akram, M.A.; Dong, S.; Liu, P.; et al. MXene decorated 3D-printed carbon black-based electrodes for solid-state micro-supercapacitors. J. Mater. Chem. A 2023, 11, 25422–25428. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Jia, H.; Chen, X.; Li, J.; Chen, Y.; Jia, J.; Zhao, G.; Yu, L.; Zhu, G.; Zhu, Y. Broadening the Voltage Window of 3D-Printed MXene Micro-Supercapacitors with a Hybridized Electrolyte. Molecules 2024, 29, 1393. https://doi.org/10.3390/molecules29061393
Jiang X, Jia H, Chen X, Li J, Chen Y, Jia J, Zhao G, Yu L, Zhu G, Zhu Y. Broadening the Voltage Window of 3D-Printed MXene Micro-Supercapacitors with a Hybridized Electrolyte. Molecules. 2024; 29(6):1393. https://doi.org/10.3390/molecules29061393
Chicago/Turabian StyleJiang, Xin, Haowen Jia, Xuan Chen, Jiajia Li, Yanling Chen, Jin Jia, Guangzhen Zhao, Lianghao Yu, Guang Zhu, and Yuanyuan Zhu. 2024. "Broadening the Voltage Window of 3D-Printed MXene Micro-Supercapacitors with a Hybridized Electrolyte" Molecules 29, no. 6: 1393. https://doi.org/10.3390/molecules29061393





