Improvement in Electrochemical Performance of Waste Sugarcane Bagasse-Derived Carbon via Hybridization with SiO2 Nanospheres
Abstract
:1. Introduction
2. Results and Discussions
3. Experimental
3.1. Materials
3.2. Activation and Chemical Modification of Sugarcane Bagasse Fibers
3.3. Carbonization of Activated SB and TEOS-Modified SB Fibers
3.4. Structural Characterizations
3.5. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, S.; Chand, P. Supercapacitor and electrochemical techniques: A brief review. Results Chem. 2023, 5, 100885. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Gao, S.; Zhang, H.; Wang, H.; Li, Q. Photo-assisted charging of carbon fiber paper-supported CeO2/MnO2 heterojunction and its long-lasting capacitance enhancement in dark. J. Adv. Ceram. 2022, 11, 1735–1750. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Shankar, A.B.; Rohita, D.S.; Rao, T.N.; Karthik, M. Conversion of Biomass Waste into High Performance Supercapacitor Electrodes for Real-Time Supercapacitor Applications. ACS Sustain. Chem. Eng. 2019, 7, 17175–17185. [Google Scholar] [CrossRef]
- Castro-Gutierrez, J.; Celzard, A.; Fierro, V. Energy storage in supercapacitors: Focus on tannin-derived carbon electrodes. Front. Mater. 2020, 7, 217. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy storage devices. Renew. Sustain. Ener. Rev. 2020, 137, 110464. [Google Scholar] [CrossRef]
- Hatfield-Dodds, S.; Schandl, H.; Newth, D.; Obersteiner, M.; Cai, Y.; Baynes, T.; West, J.; Havlik, P. Assessing global resource use and greenhouse emissions to 2050, with ambitious resource efficiency and climate mitigation policies. J. Clean. Prod. 2017, 144, 403–414. [Google Scholar] [CrossRef]
- Ostergaard, P.A.; Duic, N.; Noorollahi, Y.; Mikulcic, H.; Kalogirou, S. Sustainable Development using renewable energy technology. Renew. Energy 2019, 146, 2430–2437. [Google Scholar] [CrossRef]
- Sattayarut, V.; Wanchaem, T.; Ukkakimapan, P.; Yordsri, V.; Dulyaseree, P.; Phonyiem, M.; Endo, M. Nitrogen self-doped activated carbons via the direct activation of Samanea saman leaves for high energy density supercapacitors. RSC Advan. 2019, 9, 21724–21732. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Luo, L.; Luo, L.; Deng, J.; Wu, X.; Fan, M.; Weigang, Z. High energy density supercapacitors with hierarchical nitrogen-doped porous carbon as active material obtained from bio-waste. Renew. Energy 2021, 175, 760–769. [Google Scholar] [CrossRef]
- Kalak, T. Potential Use of Industrial Biomass Waste as a Sustainable Energy Source in the Future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- Iqbal, S.; Khatoon, H.; Pandit, A.H.; Ahmad, S. Recent development of carbon-based materials for energy storage devices. Mater. Sci. Energy Technol. 2019, 2, 417–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, H.; Zhou, Q.; Liu, K.; Ma, W.; Fan, S. Biomass-derived carbon for supercapacitors electrodes—A review of recent advances. Inorg. Chem. Comm. 2023, 153, 110768. [Google Scholar] [CrossRef]
- Yang, H.; Ye, S.; Zhou, J.; Liang, T. Biomass-derived porous carbon materials for supercapacitor. Front. Chem. 2019, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, G.P.; Bhattarai, D.P.; Maharjan, B.; Kim, K.S.; Park, C.H.; Kim, C.S. Synthesis and characterizations of activated carbon from Wisteria sinensis seeds biomass for energy storage applications. J. Ind. Eng. Chem. 2019, 72, 265–272. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sour. 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Wang, H.; Wen, J. Biomass porous carbon-based composite for high performance supercapacitor. Mater. Res. Expr. 2020, 7, 115601. [Google Scholar] [CrossRef]
- Sahu, R.K.; Gangil, S.; Bhargav, V.K.; Sahu, P.; Ghritalahre, B. Synthesizing biomass into nano carbon for use in high-performance supercapacitors—A brief critical review. J. Energy Storage 2023, 72, 108348. [Google Scholar] [CrossRef]
- Devi, N.S.; Hariram, M.; Vivekanandan, S. Modification techniques to improve the capacitive performance of biocarbon materials. J. Energy Storage 2021, 33, 101870. [Google Scholar] [CrossRef]
- Iqbal, S.; Rasheed, T.; Bilal, M.; Shah, K.H.; Bilal, M.; Sheraz, T.A. Biomass-derived nitrogen-rich porous carbon composite for supercapacitor application. J. Mater. Sci. Mater. Electron. 2022, 33, 14793–14804. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Zhang, J.; Hou, S.; Hao, C.; Zhang, J. Recent advances in flexible supercapacitors. J. Solid State Electrochem. 2022, 26, 2627–2658. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Tailoring porosity in carbon materials for supercapacitor applications. Mater. Horiz. 2014, 1, 157–168. [Google Scholar] [CrossRef]
- Saraf, M.; Natarajan, K.; Mobin, S.M. Robust nanocomposite of nitrogen-doped reduced graphene oxide and MnO2 nanorods for high-performance supercapacitors and nonenzymatic peroxide sensors. ACS Sustain. Chem. Eng. 2018, 6, 10489–10504. [Google Scholar] [CrossRef]
- Li, L.; Ji, P.; Geng, C.; Li, Y.; Meng, L.; Zhou, B.; Liang, J.; Peng, J.; Su, X. Facile synthesis of high-entropy (Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4 nanopowders and their electrochemical properties as supercapacitor electrode. J. Energy Storage 2023, 73, 109182. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Anand, S.; Dey, B.; Yang, D.J.; Choudhury, A. Asymmetric supercapacitors based on porous MnMoS4 nanosheets-anchored carbon nanofiber and N, S-doped carbon nanofiber electrodes. J. Alloys Compd. 2022, 906, 164271. [Google Scholar] [CrossRef]
- Guo, M.; Liu, Y.; Zhang, F.; Cheng, F.; Cheng, C.; Miao, Y.; Gao, F.; Yu, J. Inactive Al3+-doped La(CoCrFeMnNiAlx)1/(5+x)O3 high-entropy perovskite oxides as high performance supercapacitor electrodes. J. Adv. Ceram. 2022, 11, 742–753. [Google Scholar] [CrossRef]
- Anand, S.; Ahmad, M.W.; Saidi, A.K.A.A.; Yang, D.J.; Choudhury, A. Polyaniline nanofiber decorated carbon nanofiber hybrid mat for flexible electrochemical supercapacitor. Mater. Chem. Phys. 2020, 254, 123480. [Google Scholar] [CrossRef]
- Choudhury, A.; Dey, B.; Mohapatra, S.S.; Kim, D.W.; Yang, K.S.; Yang, D.J. Flexible and freestanding supercapacitor based on nanostructured poly (m-aminophenol)/carbon nanofiber hybrid mats with high energy and power densities. Nanotechnology 2018, 29, 165401. [Google Scholar] [CrossRef] [PubMed]
- Saraf, M.; Chacon, B.; Ippolito, S.; Lord, R.W.; Anayee, M.; Wang, R.; Inman, A.; Shuck, C.E.; Gogotsi, Y. Enhancing charge storage of Mo2Ti2C3 MXene by partial oxidation. Adv. Funct. Mater. 2024, 34, 2306815. [Google Scholar] [CrossRef]
- Saraf, M.; Zhang, T.; Averianov, T.; Shuck, C.E.; Lord, R.W.; Pomerantseva, E.; Gogotsi, Y. Vanadium and niobium MXenes—Bilayered V2O5 asymmetric supercapacitors. Small Methods 2023, 7, 2201551. [Google Scholar] [CrossRef]
- Saraf, M.; Shuck, C.E.; Norouzi, N.; Matthews, K.; Inman, A.; Zhang, T.; Pomerantseva, E.; Gogotsi, Y. Free-standing α-MoO3/Ti3C2 MXene hybrid electrode in water-in-salt electrolytes. Energy Environ. Mater. 2023, 6, e12516. [Google Scholar] [CrossRef]
- Hu, W.; Wang, B.; Yu, Y.; Wang, N.; Wu, X. Biomass derived carbon containing in-situ constructed nickel-based hydroxide nanostructures based on MnO2 template for high performance asymmetric supercapacitors. J. Alloys Compd. 2021, 884, 161149. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, X.; Zhou, D.; Liu, C. Biomass-derived hierarchical porous carbon/silicon carbide composite for electrochemical supercapacitor. Colloids Surf. A. 2021, 620, 126567. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.J. MnO2 and biomass-derived 3D porous carbon composites electrodes for high performance supercapacitor applications. J. Alloys Compd. 2018, 741, 360–367. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Manaf, S.A.A.; Divyashree, D.; Feng, C.K.; Hegde, G. Superior supercapacitive performance in porous nanocarbons. J. Energy Chem. 2016, 25, 734–739. [Google Scholar] [CrossRef]
- Ali, G.A.M.; Manaf, S.A.B.A.; Kumar, A.; Chong, K.F.; Hegde, G. High performance supercapacitor using catalysis free porous carbon nanoparticles. J. Phys. D Appl. Phys. 2014, 47, 495307. [Google Scholar] [CrossRef]
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 2006, 313, 1760–1763. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hegde, G.; Manaf, S.A.B.A.; Ngaini, Z.; Sharma, K.V. Catalyst free silica templated porous carbon nanoparticles from bio-waste materials. Chem. Commun. 2014, 50, 12702–12705. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, S.; Mi, R.; Mei, J.; Liu, L.M.; Cao, L.; Lau, W.M.; Liu, H. Porous structure design of carbon xerogels for advanced supercapacitor. Appl. Energy 2015, 153, 32–40. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; da Silva, E.L.; Oliveira, A.P.S.; Matsushima, J.T.; Cuña, A.; Marcuzzo, J.S.; Gonçalves, E.S.; Baldan, M.R. High-performance supercapacitor electrode based on activated carbon fiber felt/iron oxides. Mater. Today Commun. 2019, 21, 100553. [Google Scholar] [CrossRef]
- Li, R.; Huang, J.; Li, J.; Cao, L.; Zhong, X.; Yu, A.; Lu, G. Nitrogen-doped porous hard carbons derived from shaddock peel for high-capacity lithium-ion battery anodes. J. Electroanal. Chem. 2020, 862, 114044. [Google Scholar] [CrossRef]
- Gong, Y.; Li, D.; Fu, Q.; Zhang, Y.; Pan, C. Nitrogen self-doped porous carbon for high-performance supercapacitors. ACS Appl. Ener. Mater. 2020, 3, 1585–1592. [Google Scholar] [CrossRef]
- Rodriguez Correa, C.; Otto, T.; Kruse, A. Influence of the biomass components on the pore formation of activated carbon. Biomass Bioenergy 2017, 97, 53–64. [Google Scholar] [CrossRef]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F.; Chambat, G. Contributions of hemicellulose, cellulose and lignin to the mass and the porous properties of chars and steam activated carbons from various lignocellulosic precursors. Bioresour. Technol. 2009, 100, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Liu, K.; He, S.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Byrne, J.A.; McLaughlin, J.A.D.; Elhissi, A.; Ahmed, W. Comparison between FTIR and XPS characterization of amino acid glycine adsorption onto diamond-like carbon (DLC) and silicon doped DLC. Appl. Surf. Sci. 2013, 273, 507–514. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, J.; Chen, Y.; Hao, X.; Jin, X. Characterization, preparation, and reaction mechanism of hemp stem based activated carbon. Results Phys. 2017, 7, 1628–1633. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Lan, W.; Li, D.; Zhang, A.; Liu, C. Construction of sugarcane bagasse-derived porous and flexible carbon nanofibers by electrospinning for supercapacitors. Indus. Crops Prod. 2021, 170, 113700. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Peng, Y.; Wang, X.; Wang, J.; Zhao, J. 3-dimensional interconnected framework of N-doped porous carbon based on sugarcane bagasse for application in supercapacitors and lithium-ion batteries. J. Power Sourc. 2018, 390, 186–196. [Google Scholar] [CrossRef]
- Wahid, M.; Puthusseri, D.; Phase, D.; Ogale, S. Enhanced capacitance retention in a supercapacitor made of carbon from sugarcane bagasse by hydrothermal pretreatment. Energy Fuel 2014, 28, 4233–4240. [Google Scholar] [CrossRef]
- Okonkwo, C.A.; Menkiti, M.C.; Obiora-Okafo, I.A.; Ezenwa, O.N. Controlled pyrolysis of sugarcane bagasse enhanced mesoporous carbon for improving capacitance of supercapacitor electrode. Biomass Bioenergy 2021, 146, 105996. [Google Scholar] [CrossRef]
- Pongpanyanatea, K.; Roddecha, S.; Piyanirund, C.; Phraewphiphat, T.; Hasin, P. Dispersed MnO2 nanoparticles/sugarcane bagasse-derived carbon composite as an anode material for lithium-ion batteries. RSC Adv. 2024, 14, 2354–2368. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, J.; Wang, B.; Feng, H.; Yu, Y.; Sakai, E. Polyaniline/sugarcane bagasse derived biocarbon composites with superior performance in supercapacitors. J. Electroanal. Chem. 2017, 801, 360–367. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, W.; Luo, J.; Fan, J.; Wu, Z.; Zhu, H.; Huang, J.; Zhang, X. High-efficient and pH-sensitive orange luminescence from silicon-doped carbon dots for information encryption and bio-imaging. J. Colloid Interf. Sci. 2022, 607, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ouyang, J.; Wang, H.; Wang, W.; Chui, P.; Sun, K. Synthesis and characterization of core–shell structured SiO2@YVO4:Yb3+, Er3+ microspheres. Appl. Surf. Sci. 2012, 258, 3689–3694. [Google Scholar] [CrossRef]
- Ishak, S.; Mandal, S.; Lee, H.; Singh, J.K. pH-controlled synthesis of sustainable lauric acid/SiO2 phase change material for scalable thermal energy storage. Sci. Rep. 2021, 11, 15012. [Google Scholar] [CrossRef] [PubMed]
- Nassifa, N.; Livage, J. From diatoms to silica-based biohybrids. Chem. Soc. Rev. 2011, 40, 849–859. [Google Scholar] [CrossRef]
- Kim, C.; Park, S.H.; Cho, J.K.; Lee, D.Y.; Park, T.J.; Lee, W.J.; Yang, K.S. Raman spectroscopic evaluation of polyacrylonitrile-based carbon nanofibers prepared by electrospinning. J. Raman Spectrosc. 2004, 35, 928–933. [Google Scholar] [CrossRef]
- Gong, X.; Zheng, S.; Zhao, X.; Vomiero, A. Engineering high-emissive silicon-doped carbon nanodots towards efficient large-area luminescent solar concentrators. Nano Energy 2022, 10, 107617. [Google Scholar] [CrossRef]
- Hariyanto, B.; Wardani, D.A.P.; Kurniawati, N.; Har, P.; Darmawan, N.; Irzaman. X-ray peak profile analysis of silica by Williamson–Hall and size-strain plot methods. J. Phys. Conf. Ser. 2021, 012106. [Google Scholar] [CrossRef]
- Yin, L.; Wu, M.; Li, Y.; Wu, G.; Wang, Y. Synthesis of SiO2@carbon-graphene hybrids as anode materials of lithium-ion batteries. New Carbon Mater. 2017, 32, 311–318. [Google Scholar] [CrossRef]
- Huang, S.; Yang, D.; Zhang, W.; Qiu, X.; Li, Q.; Li, C. Dual-templated synthesis of mesoporous lignin-derived honeycomb-like porous carbon/SiO2 composites for high-performance Li-ion battery. Microporous Mesoporous Mater. 2021, 317, 111004. [Google Scholar] [CrossRef]
- Yulianti, R.T.; Destyorini, F.; Irmawati, Y.; Priyono, S.; Fauzi, M.H.; Umar, A.A.; Uyama, H.; Fauzia, V.; Yudianti, R. High capacitance performance of hierarchically SiO2 self-doped porous activated carbon derived from palm empty fruit bunches. J. Energy Stor. 2023, 71, 108153. [Google Scholar] [CrossRef]
- Supriya1, S.; Bhat, V.S.; Jayeoye, T.J.; Rujiralai, T.; Chong, K.F.; Hegde, G. An investigation on temperature-dependant surface properties of porous carbon nanoparticles derived from biomass. J. Nanostruct. Chem. 2022, 12, 495–511. [Google Scholar] [CrossRef]
- Chen, L.; Ji, T.; Mu, L.; Shi, Y.; Brisbin, L.; Guo, Z.; Khan, M.A.; Young, D.P.; Zhu, J. Facile synthesis of mesoporous carbon nanocomposites from natural biomass for efficient dye adsorption and selective heavy metal removal. RSC Adv. 2016, 6, 2259–2269. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, S.P.; Shao, Z. Intercalation pseudocapacitance in electrochemical energy storage: Recent advances in fundamental understanding and materials development. Mater. Adv. 2020, 7, 100072. [Google Scholar] [CrossRef]
- Joshi, A.; Lalwani, S.; Singh, G.; Sharma, R.K. Highly oxygen deficient, bimodal mesoporous silica-based supercapacitor with enhanced charge storage characteristics. Electrochim. Acta. 2019, 297, 705–714. [Google Scholar] [CrossRef]
- Sankar, K.V.; Surendran, S.; Pandi, K.; Allin, A.M.; Nithya, V.D.; Lee, Y.S.; Selvan, R.K. Studies on the electrochemical intercalation/de-intercalation mechanism of NiMn2O4 for high stable pseudocapacitor electrodes. RSC Adv. 2015, 5, 27649–27656. [Google Scholar] [CrossRef]
- Sathiya, M.; Prakash, A.S.; Ramesh, K.; Tarascon, J.M.; Shukla, A.K. V2O5-Anchored carbon nanotubes for enhanced electrochemical energy storage. Mesoporous J. Am. Chem. Soc. 2011, 133, 16291–16299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Xiao, J.; Jiang, H.; Hu, T.; Meng, C. Copper oxide/cuprous oxide/hierarchical porous biomass-derived carbon hybrid composites for high-performance supercapacitor electrode. J. Alloys Compd. 2019, 782, 1103–1113. [Google Scholar] [CrossRef]
- Zhao, N.; Deng, L.; Luo, D.; Zhang, P. One-step fabrication of biomass-derived hierarchically porous carbon/MnO nanosheets composites for symmetric hybrid supercapacitor. Appl. Surf. Sci. 2020, 526, 146696. [Google Scholar] [CrossRef]
- Yin, Y.; Yan, S.; Ni, Z.; Jin, C.; Zhao, L. Economical synthesized Mn3O4/biomass-derived carbon from vegetable sponge composites and its excellent supercapacitive behavior. Biomass Convers. Bioref. 2023, 13, 12115–12124. [Google Scholar] [CrossRef]
- Ren, Y.; Xu, Q.; Zhang, J.; Yang, H.; Wang, B.; Yang, D.; Hu, J.; Liu, Z. Functionalization of biomass carbonaceous aerogels: Selective preparation of MnO2@CA composites for supercapacitors. ACS Appl. Mater. Interf. 2014, 6, 9689–9697. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, Q.; Peng, C.; Shu, N.; Pan, F.; Wang, J.; Zhu, Y. Increasing S dopant and specific surface area of N/S-codoped porous carbon by in-situ polymerization of PEDOT into biomass precursor for high performance supercapacitor. Appl. Surf. Sci. 2020, 502, 144191. [Google Scholar] [CrossRef]
- Jiang, L.; Ren, Z.; Chen, S.; Zhang, Q.; Lu, X.; Zhang, H.; Wan, G. Bio-derived three-dimensional hierarchical carbon-graphene-TiO2 as electrode for supercapacitors. Sci. Rep. 2018, 8, 4412. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Gao, X.; Wang, J.; Yu, J.; Dong, H.; Zhang, Q.; Yu, L.; Dong, L. Synthesis and supercapacitance of Co3O4 supported on porous carbon derived from wheat flour. ECS J. Solid State Sci. Technol. 2018, 7, M161–M165. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Anand, S.; Dey, B.; Fatima, A.; Yang, D.J.; Choudhury, A. N/P/O/S heteroatom-doped porous carbon nanofiber mats derived from a polyacrylonitrile/l-cysteine/P2O5 precursor for flexible electrochemical supercapacitors. ACS Appl. Energy Mater. 2021, 4, 12177–12190. [Google Scholar] [CrossRef]
- Sun, J.; Niu, J.; Liu, M.; Ji, J.; Dou, M.; Wang, F. Biomass-derived nitrogen-doped porous carbons with tailored hierarchical porosity and high specific surface area for high energy and power density supercapacitors. Appl. Surf. Sci. 2018, 427, 807–813. [Google Scholar] [CrossRef]
- Wang, D.; Min, Y.; Yu, Y. Facile synthesis of wheat bran-derived honeycomb-like hierarchical carbon for advanced symmetric supercapacitor applications. J. Solid State Electrochem. 2014, 19, 577–584. [Google Scholar] [CrossRef]
- Zhao, G.; Li, Y.; Zhu, G.; Shi, J.; Lu, T.; Pan, L. Biomass-based N, P, and S self-doped porous carbon for high performance supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 12052–12060. [Google Scholar] [CrossRef]
- Sudhan, N.; Subramani, K.; Karnan, M.; Ilayaraja, N.; Satish, M. Biomass-derived activated porous carbon from rice straw for a high-energy symmetric supercapacitor in aqueous and non-aqueous electrolytes. Energy Fuels 2017, 31, 977–985. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Deng, M. Eco-friendly preparation of biomass-derived porous carbon and its electrochemical properties. ACS Omega 2022, 7, 22689–22697. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Peng, Y.; Wang, X.; Wang, N.; Wang, J.; Zhao, J. Nitrogen-doped biomass-based hierarchical porous carbon with large mesoporous volume for application in energy storage. Chem. Eng. J. 2018, 348, 850–859. [Google Scholar] [CrossRef]
- Yue, X.; Yang, H.; Cao, Y.; Jiang, L.; Li, H.; Shi, F.; Liu, J. Nitrogen-doped cornstalk-based biomass porous carbon with uniform hierarchical pores for high-performance symmetric supercapacitors. J. Mater. Sci. 2022, 57, 3645–3661. [Google Scholar] [CrossRef]
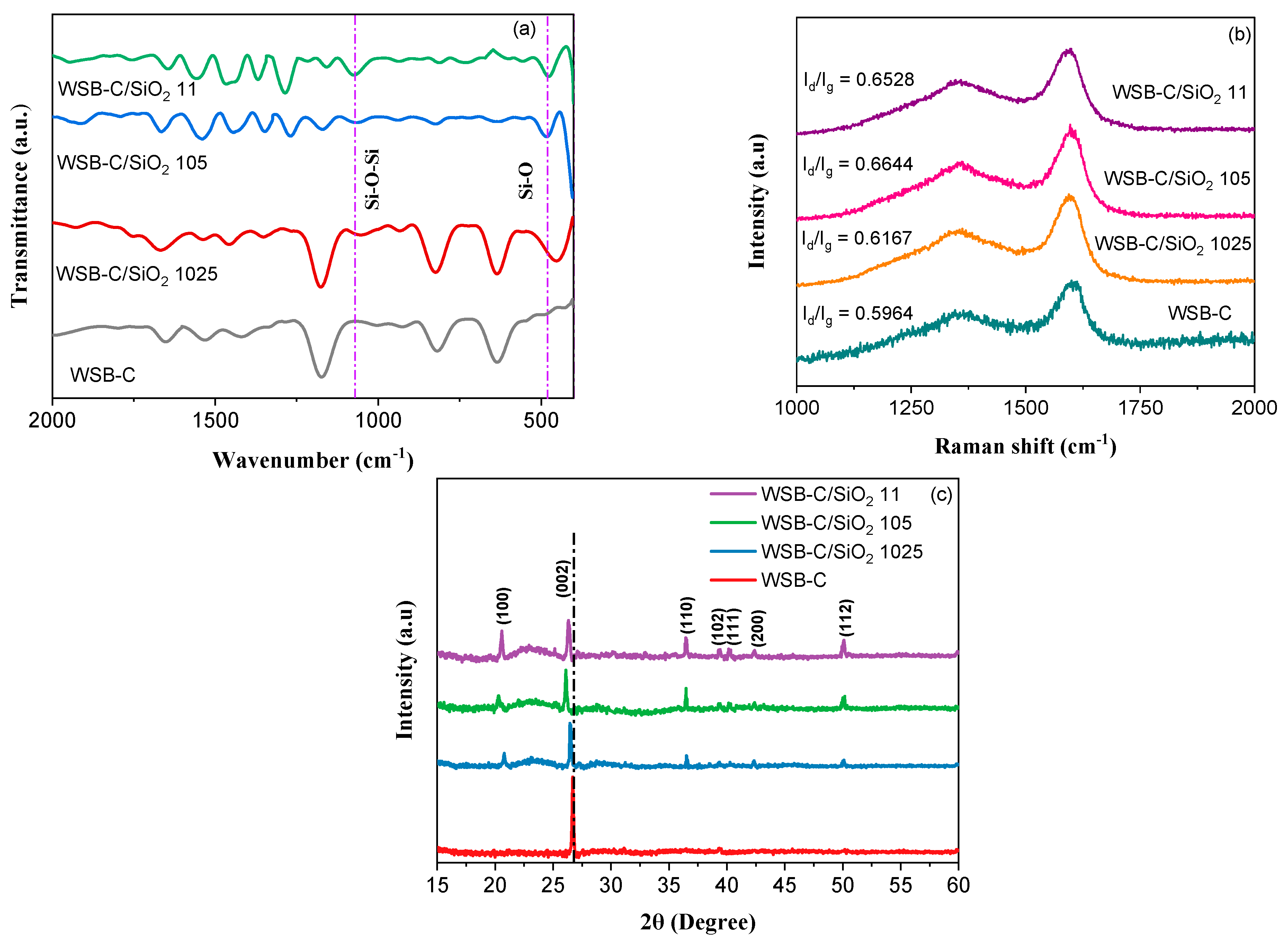
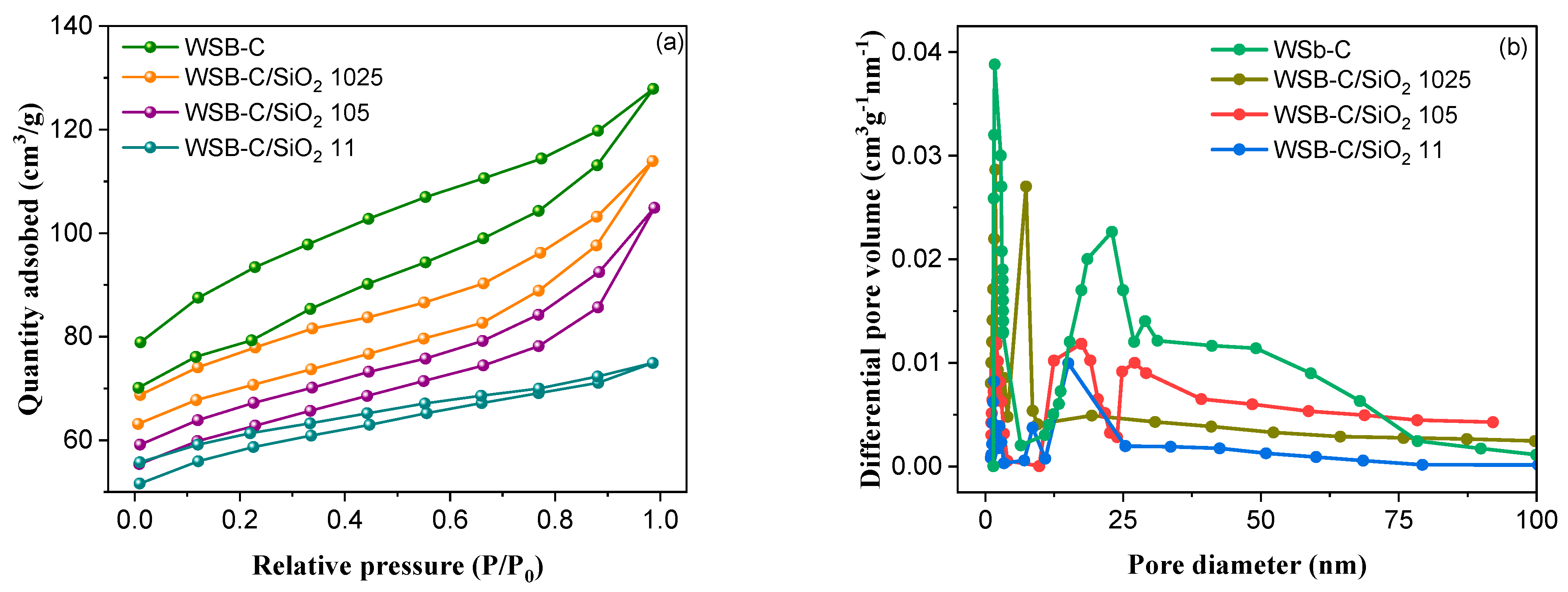




| Sample | WSB-C | WSB-C/SiO2 1024 | WSB-C/SiO2 105 | WSB-C/SiO2 11 |
|---|---|---|---|---|
| SBET (m2/g) | 342.8 | 279.9 | 207.7 | 115.7 |
| Vtotal (cm3/g) | 0.0426 | 0.389 | 0.0288 | 0.0219 |
| Mesopore (%) | 44.7 | 57.4 | 74.2 | 68.8 |
| Dav (nm) | 3.46 | 3.63 | 3.92 | 4.47 |
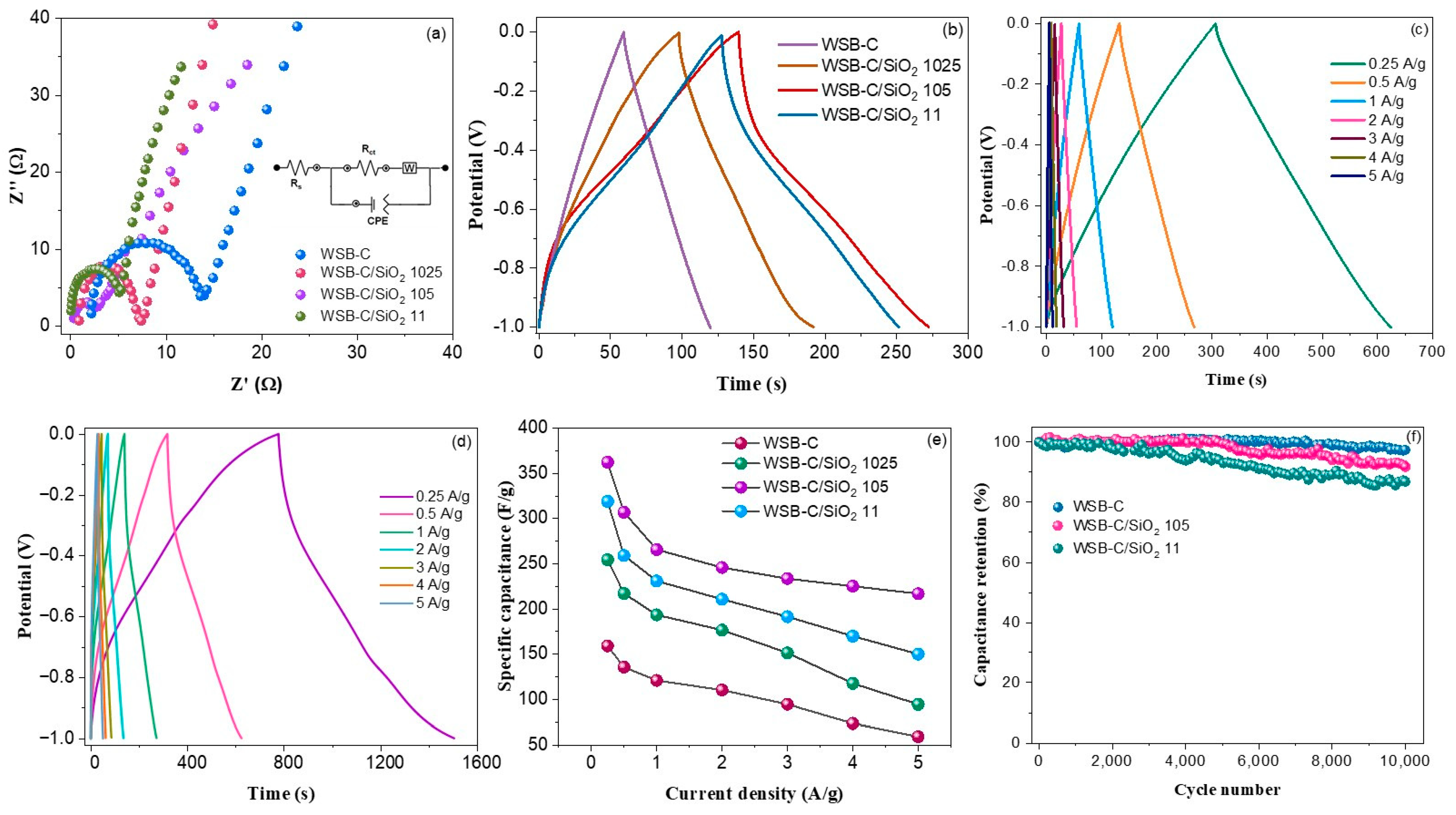
| Sample | WSB-C | WSB-C/SiO2 1024 | WSB-C/SiO2 105 | WSB-C/SiO2 11 |
|---|---|---|---|---|
| Rs (Ω) | 2.17 | 0.876 | 0.114 | 0.064 |
| RCT (Ω) | 11.59 | 6.39 | 2.57 | 4.38 |
| Zw (Ω/s0.5) | 48.7 | 12.67 | 3.23 | 8.18 |
| CPE (µF) | 11.46 | 15.63 | 23.92 | 19.47 |
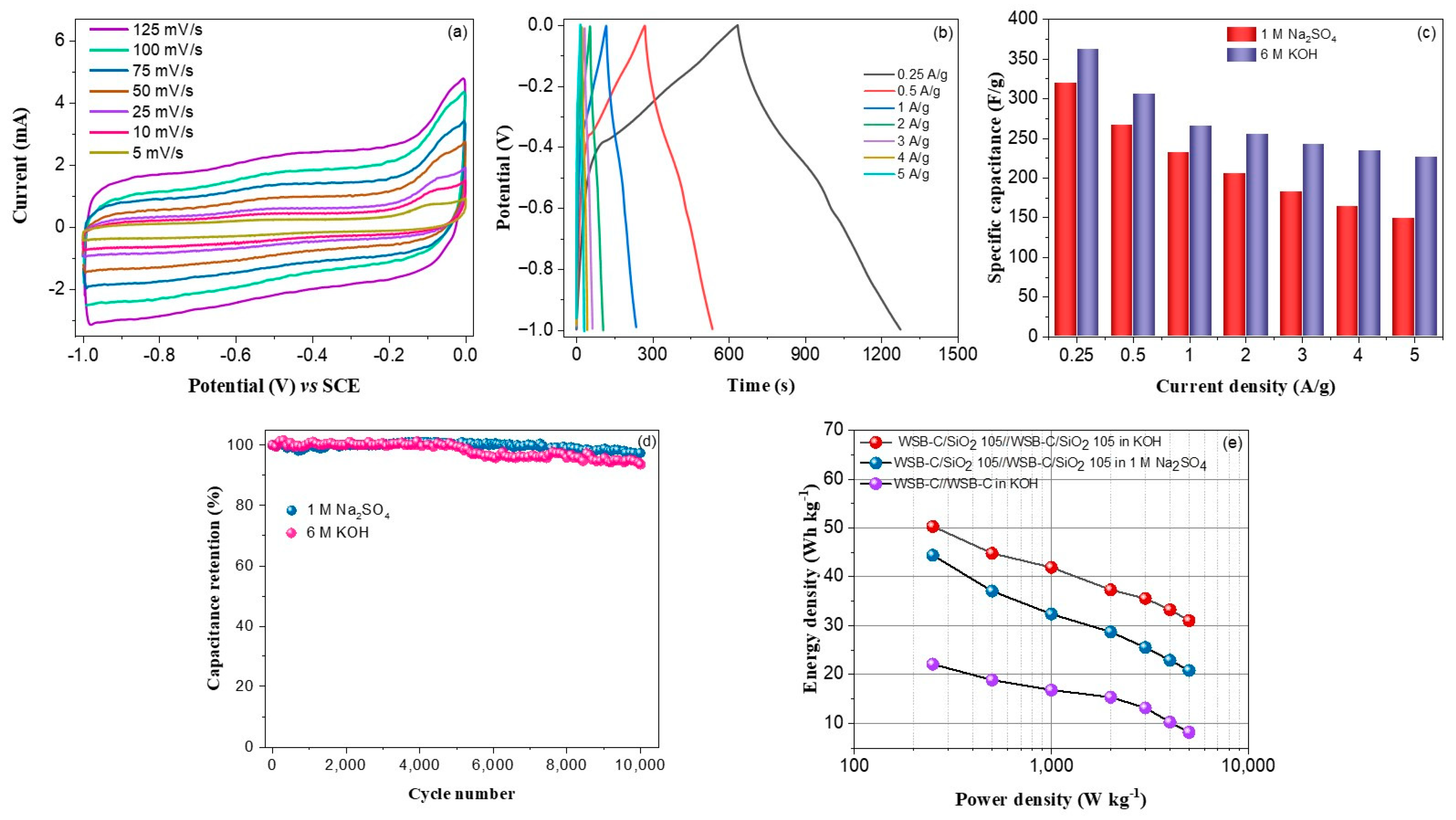
| Biomass | Biomass-Derived Carbon (Cbiomass) Composites | Electrolyte | Potential Range (V) | Sp. Capacitance (F/g) | Cycling Stability | Ref. |
|---|---|---|---|---|---|---|
| Bamboo leaves | Cbiomass/CuO/Cu2O | 1 M HCl | −1.0 to +0.3 | 147@1 A/g | 93% after 5000 cycles | [69] |
| Wasted litchi shell | Cbiomass/MnO | 6 M KOH | −1.0 to +0.2 | [email protected] A/g | 93.5% after 5000 cycles | [70] |
| Vegetable sponge | Cbiomass/Mn3O4 Composite | 1 M Na2SO4 | 0 to 1.0 | [email protected] A/g | 89.5% after 4500 cycles | [71] |
| Watermelon | Cbiomass/MnO2 | 6 M KOH | −1.0 to 0 | [email protected] A/g | 60% after 1000 cycles | [72] |
| Waste bamboo shoot shells | Cbiomass/PEDOT | 1 M H2SO4 | 0 to 1.0 | [email protected] A/g | 87% after 10,000 cycles | [73] |
| Loofah | Cbiomass/TiO2 | 1 M H2SO4 | 0 to 1.0 | 250.8@1 A/g | 84% after 100 cycles | [74] |
| Wheat flour | Cbiomass/Co3O4 | 2 M KOH | −0.8 to +0.4 | [email protected] A/g | 80% after 1000 cycles | [75] |
| Waste sugarcane bagasse | Cbiomass/SiO2 | 6 M KOH | −1.0–0.0 | [email protected] A/g | 91.7 after 10,000 cycles | Present |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwi, M.M.A.; Singh, J.; Choudhury, A.; Hossain, S.S.; Butt, A.N. Improvement in Electrochemical Performance of Waste Sugarcane Bagasse-Derived Carbon via Hybridization with SiO2 Nanospheres. Molecules 2024, 29, 1569. https://doi.org/10.3390/molecules29071569
Alwi MMA, Singh J, Choudhury A, Hossain SS, Butt AN. Improvement in Electrochemical Performance of Waste Sugarcane Bagasse-Derived Carbon via Hybridization with SiO2 Nanospheres. Molecules. 2024; 29(7):1569. https://doi.org/10.3390/molecules29071569
Chicago/Turabian StyleAlwi, Muhammad Mudassir Ahmad, Jyoti Singh, Arup Choudhury, SK Safdar Hossain, and Akbar Niaz Butt. 2024. "Improvement in Electrochemical Performance of Waste Sugarcane Bagasse-Derived Carbon via Hybridization with SiO2 Nanospheres" Molecules 29, no. 7: 1569. https://doi.org/10.3390/molecules29071569





