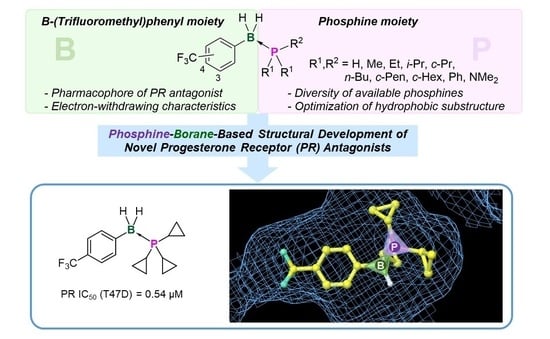
Design, Synthesis, and Evaluation of B-(Trifluoromethyl)phenyl Phosphine–Borane Derivatives as Novel Progesterone Receptor Antagonists
Abstract
:1. Introduction
2. Results and Discussion
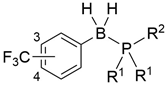
2.1. Molecular Design
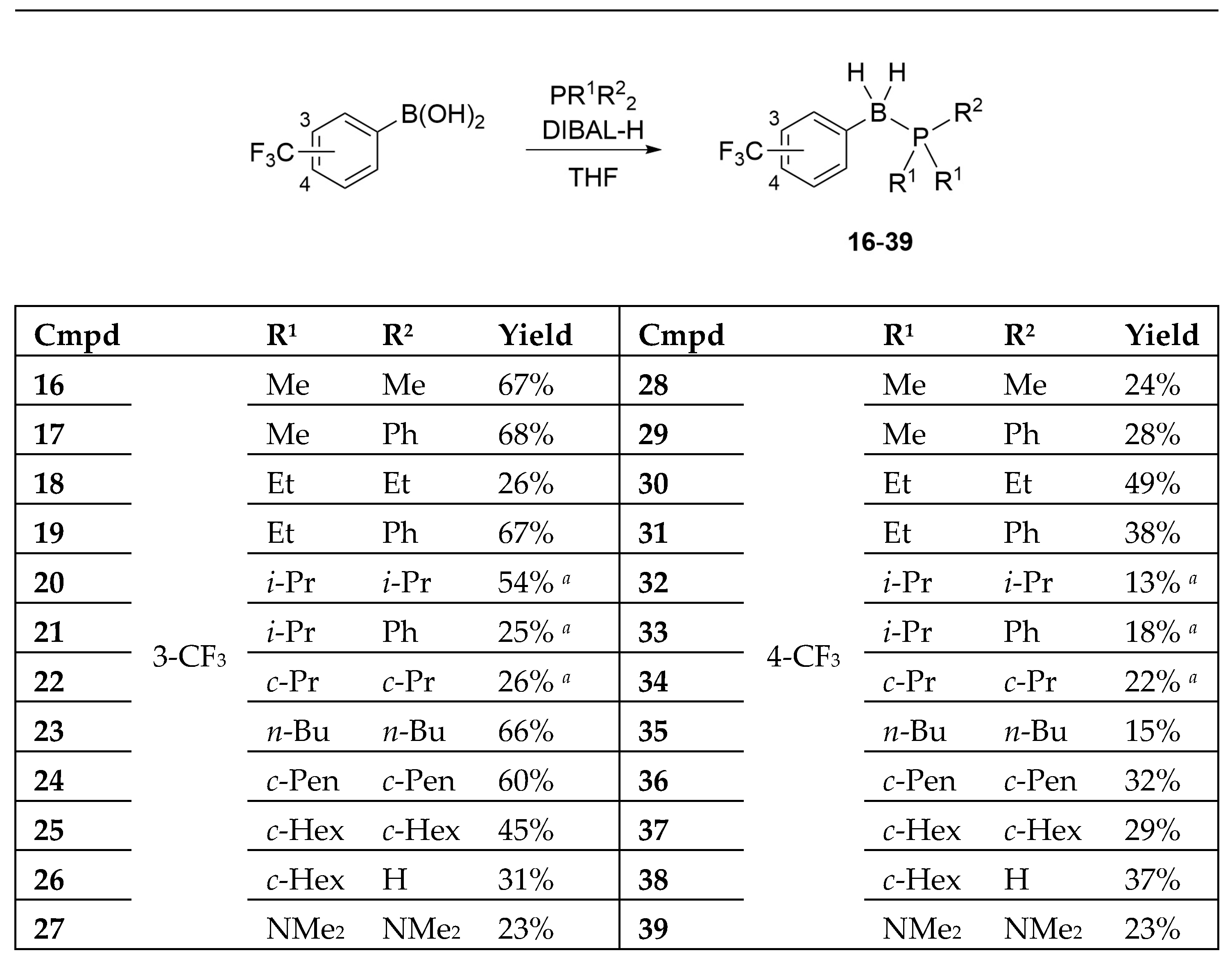
2.2. Synthesis
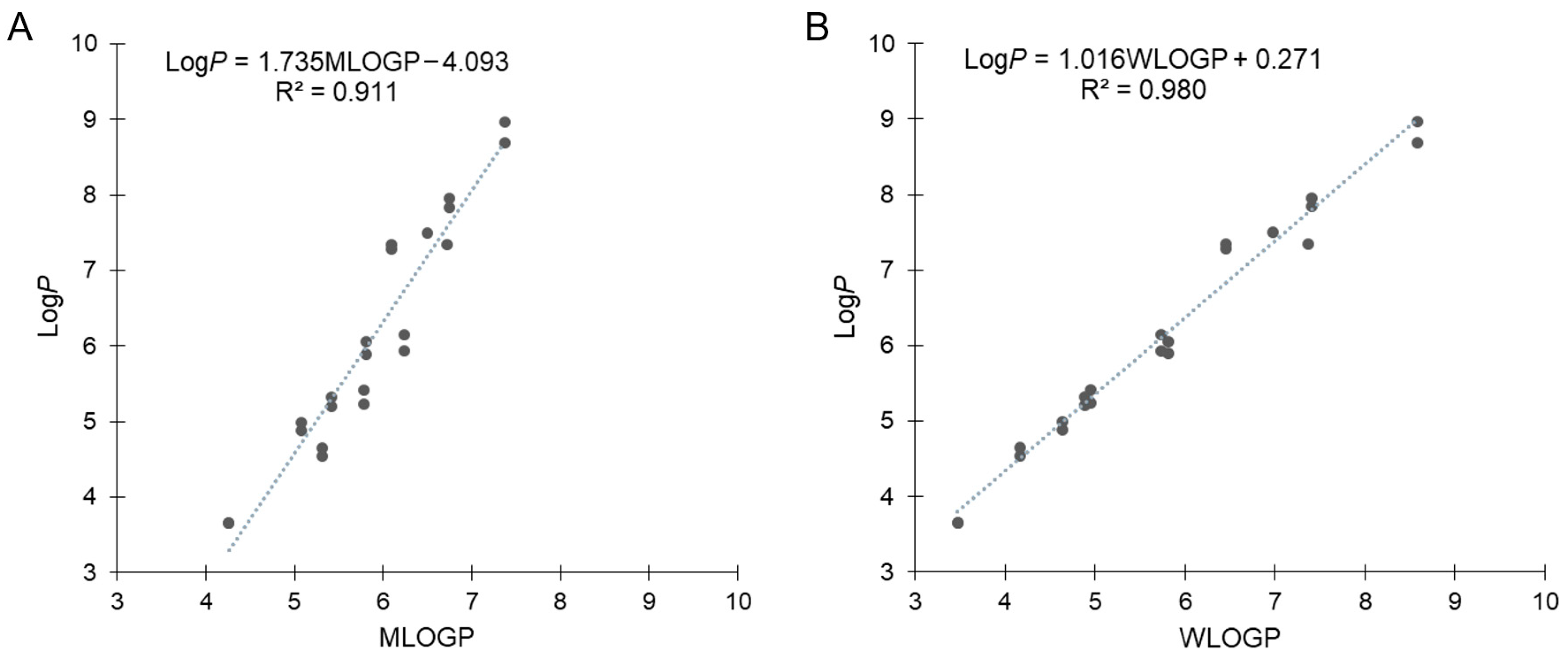
2.3. Hydrophobicity
2.4. PR Antagonistic Activity
2.5. Docking Simulations
3. Materials and Methods
3.1. Chemistry
3.2. General Procedure for the Synthesis of Phosphine–Borane Derivatives 16–39
3.2.1. B-3-(Trifluoromethyl)phenyl Trimethylphosphine–Borane (16)
3.2.2. B-(3-Trifluoromethyl)phenyl Dimethyl(phenyl)phosphine–Borane (17)
3.2.3. B-3-(Trifluoromethyl)phenyl Triethylphosphine–Borane (18)
3.2.4. B-3-(Trifluoromethyl)phenyl Diethyl(phenyl)phosphine–Borane (19)
3.2.5. B-3-(Trifluoromethyl)phenyl Triisopropylphosphine–Borane (20)
3.2.6. B-3-(Trifluoromethyl)phenyl Diisopropyl(phenyl)phosphine–Borane (21)
3.2.7. B-3-(Trifluoromethyl)phenyl Tricyclopropylphosphine–Borane (22)
3.2.8. B-3-(Trifluoromethyl)phenyl Tri-n-Butylphosphine–Borane (23)
3.2.9. B-3-(Trifluoromethyl)phenyl Tricyclopentylphosphine–Borane (24)
3.2.10. B-3-(Trifluoromethyl)phenyl Tricyclohexylphosphine–Borane (25)
3.2.11. B-3-(Trifluoromethyl)phenyl Dicyclohexylphosphine–Borane (26)
3.2.12. B-3-(Trifluoromethyl)phenyl Tris(dimethylamino)phosphine–Borane (27)
3.2.13. B-4-(Trifluoromethyl)phenyl Trimethylphosphine–Borane (28)
3.2.14. B-4-(Trifluoromethyl)phenyl Dimethyl(phenyl)phosphine–Borane (29)
3.2.15. B-4-(Trifluoromethyl)phenyl Triethylphosphine–Borane (30)
3.2.16. B-4-(Trifluoromethyl)phenyl Diethyl(phenyl)phosphine–Borane (31)
3.2.17. B-4-(Trifluoromethyl)phenyl Triisopropylphosphine–Borane (32)
3.2.18. B-4-(Trifluoromethyl)phenyl Diisopropyl(phenyl)phosphine–Borane (33)
3.2.19. B-4-(Trifluoromethyl)phenyl Tricyclopropylphosphine–Borane (34)
3.2.20. B-4-(Trifluoromethyl)phenyl Tri-n-butylphosphine–Borane (35)
3.2.21. B-4-(Trifluoromethyl)phenyl Tricyclopentylphosphine–Borane (36)
3.2.22. B-4-(Trifluoromethyl)phenyl Tricyclohexylphosphine–Borane (37)
3.2.23. B-4-(Trifluoromethyl)phenyl Dicyclohexylphosphine–Borane (38)
3.2.24. B-4-(Trifluoromethyl)phenyl Tris(dimethylamino)phosphine–Borane (39)
3.3. Determination of Hydrophobicity (LogP)
3.4. T47D Alkaline Phosphatase Assay for the Evaluation of PR Antagonistic Activity
3.5. PR LBD Competitive Binding Assay
3.6. Docking Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staubitz, A.; Robertson, A.P.; Sloan, M.E.; Manners, I. Amine− and phosphine− borane adducts: New interest in old molecules. Chem. Rev. 2010, 110, 4023–4078. [Google Scholar] [CrossRef]
- Brunel, J.M.; Faure, B.; Maffei, M. Phosphane-boranes: Synthesis, characterization and synthetic applications. Coord. Chem. Rev. 1998, 178, 665–698. [Google Scholar] [CrossRef]
- Paine, R.T.; Noth, H. Recent advances in phosphinoborane chemistry. Chem. Rev. 1995, 95, 343–379. [Google Scholar] [CrossRef]
- Imamoto, T.; Kusumoto, T.; Suzuki, N.; Sato, K. Phosphine oxides and LiAlH4–NaBH4–CeCl3; synthesis and reactions of phosphine-boranes. J. Am. Chem. Soc. 1985, 107, 5301–5302. [Google Scholar] [CrossRef]
- Imamoto, T.; Oshiki, T. Synthesis of Organic phosphorus compounds containing a linear P–B bond chain. Tetrahedron Lett. 1989, 30, 383–384. [Google Scholar] [CrossRef]
- Imamoto, T.; Oshiki, T.; Onozawa, T.; Kusumoto, T.; Sato, K. Synthesis and reactions of phosphine–boranes. Synthesis of new bidentate ligands with homochiral phosphine centers via optically pure phosphine–boranes. J. Am. Chem. Soc. 1990, 112, 5244–5252. [Google Scholar] [CrossRef]
- Li, P.; Sergueeva, Z.A.; Dobrikov, M.; Shaw, B.R. Nucleoside and oligonucleoside boranophosphates: Chemistry and properties. Chem. Rev. 2007, 107, 4746–4796. [Google Scholar] [CrossRef]
- Summers, J.S.; Shaw, B. Boranophosphates as mimics of natural phosphodiesters in DNA. Curr. Med. Chem. 2001, 8, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Schlieve, C.R.; Tam, A.; Nilsson, B.L.; Lieven, C.J.; Raines, R.T.; Levin, L.A. Synthesis and characterization of a novel class of reducing agents that are highly neuroprotective for retinal ganglion cells. Exp. Eye Res. 2006, 83, 1252–1259. [Google Scholar] [CrossRef]
- Remtulla, R.; Das, S.K.; Levin, L.A. Predicting absorption-distribution properties of neuroprotective phosphine-borane compounds using in silico modeling and machine learning. Molecules 2021, 26, 2505. [Google Scholar] [CrossRef]
- Saito, H.; Matsumoto, Y.; Hashimoto, Y.; Fujii, S. Phosphine boranes as less hydrophobic building blocks than alkanes and silanes: Structure-property relationship and estrogen-receptor-modulating potency of 4-phosphinophenol derivatives. Bioorg. Med. Chem. 2020, 28, 115310. [Google Scholar] [CrossRef]
- Miyajima, Y.; Noguchi-Yachide, T.; Ochiai, K.; Fujii, S. Physicochemical characterization of B-hydroxyphenyl phosphine borane derivatives and their evaluation as nuclear estrogen receptor ligands. RSC Med. Chem. 2024, 15, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Aupperlee, M.D.; Smith, K.T.; Kariagina, A.; Haslam, S.Z. Progesterone receptor isoforms A and B: Temporal and spatial differences in expression during murine mammary gland development. Endocrinology 2005, 146, 3577–3588. [Google Scholar] [CrossRef]
- Kolatorova, L.; Vitku, J.; Suchopar, J.; Hill, M.; Parizek, A. Progesterone: A steroid with wide range of effects in physiology as well as human medicine. Int. J. Mol. Sci. 2022, 23, 7989. [Google Scholar] [CrossRef]
- Africander, D.; Verhoog, N.; Hapgood, J.P. Molecular mechanisms of steroid receptor-mediated actions by synthetic progestins used in HRT and contraception. Steroids 2011, 76, 636–652. [Google Scholar] [CrossRef] [PubMed]
- Spitz, I.M. Progesterone antagonists and progesterone receptor modulators: An overview. Steroids 2003, 68, 981–993. [Google Scholar] [CrossRef]
- Islam, M.S.; Afrin, S.; Jones, S.I.; Segars, J. Selective progesterone receptor modulators-mechanisms and therapeutic utility. Endocr. Rev. 2020, 41, bnaa012. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Chodankar, R.R. Selective progesterone receptor modulators in gynaecological therapies. J. Mol. Endocrinol. 2020, 65, T15–T33. [Google Scholar] [CrossRef]
- Singh, S.S.; Belland, L. Contemporary management of uterine fibroids: Focus on emerging medical treatments. Curr. Med. Res. Opin. 2015, 31, 1–12. [Google Scholar] [CrossRef]
- Proietti, C.J.; Cenciarini, M.E.; Elizalde, P.V. Revisiting progesterone receptor (PR) actions in breast cancer: Insights into PR repressive functions. Steroids 2018, 133, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Soria, V.; Gonzalez-Rodriguez, A.; Huerta-Ramos, E.; Usall, J.; Cobo, J.; Bioque, M.; Barbero, J.D.; Garcia-Rizo, C.; Tost, M.; Monreal, J.A.; et al. Targeting hypothalamic-pituitary-adrenal axis hormones and sex steroids for improving cognition in major mood disorders and schizophrenia: A systematic review and narrative synthesis. Psychoneuroendocrinology 2018, 93, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Brisken, C. Progesterone signalling in breast cancer: A neglected hormone coming into the limelight. Nat. Rev. Cancer 2013, 13, 385–396. [Google Scholar] [CrossRef]
- Kim, J.J.; Kurita, T.; Bulun, S.E. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr. Rev. 2013, 34, 130–162. [Google Scholar] [CrossRef]
- Goyeneche, A.A.; Carón, R.W.; Telleria, C.M. Mifepristone inhibits ovarian cancer cell growth in vitro and in vivo. Clin. Cancer Res. 2007, 13, 3370–3379. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Shi, P.; Nie, Z.; Liang, H.; Zhou, Z.; Chen, W.; Chen, H.; Dong, C.; Yang, R.; Liu, S.; et al. Mifepristone suppresses basal triple-negative breast cancer stem cells by down-regulating KLF5 expression. Theranostics 2016, 6, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.; Choi, M.R.; Christov, K.; Ivancic, D.; Khan, S.A. Progesterone receptor antagonism inhibits progestogen-related carcinogenesis and suppresses tumor cell proliferation. Cancer Lett. 2016, 376, 310–317. [Google Scholar]
- Sitruk-Ware, R. Reprint of pharmacological profile of progestins. Maturitas 2008, 61, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Moguilewsky, M.; Philibert, D. RU 38486: Potent antiglucocorticoid activity correlated with strong binding to the cytosolic glucocorticoid receptor followed by an impaired activation. J. Steroid Biochem. 1984, 20, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Jeng, M.H.; Langan-Fahey, S.M.; Jordan, V.C. Estrogenic actions of RU486 in hormone-responsive MCF-7 human breast cancer cells. Endocrinology 1993, 6, 2622–2630. [Google Scholar] [CrossRef]
- Winneker, R.C.; Fensome, A.; Zhang, P.; Yudt, M.R.; McComas, C.C.; Unwalla, R.J. A new generation of progesterone receptor modulators. Steroids 2008, 73, 689–701. [Google Scholar] [CrossRef]
- Zhang, P.; Terefenko, E.A.; Fensome, A.; Wrobel, J.; Winneker, R.; Lundeen, S.; Marschke, K.B.; Zhang, Z. 6-Aryl-1,4-dihydro-benzo[d][1,3]oxazin-2-ones: A novel class of potent, selective, and orally active nonsteroidal progesterone receptor antagonists. J. Med. Chem. 2002, 45, 4379–4382. [Google Scholar] [CrossRef]
- Fensome, A.; Adams, W.R.; Adams, A.L.; Berrodin, T.J.; Cohen, J.; Huselton, C.; Illenberger, A.; Kern, J.C.; Hudak, V.A.; Marella, M.A.; et al. Design, synthesis, and SAR of new pyrrole-oxindole progesterone receptor modulators leading to 5-(7-fluoro-3,3-dimethyl-2-oxo-2,3-dihydro-1H-indol-5-yl)-1-methyl-1H-pyrrole-2-carbonitrile (WAY-255348). J. Med. Chem. 2008, 51, 1861–1873. [Google Scholar] [CrossRef]
- Sakai, H.; Hirano, T.; Mori, S.; Fujii, S.; Masuno, H.; Kinoshita, M.; Kagechika, H.; Tanatani, A. 6-Arylcoumarins as novel nonsteroidal type progesterone antagonists: An example with receptor-binding-dependent fluorescence. J. Med. Chem. 2011, 54, 7055–7065. [Google Scholar] [CrossRef]
- Fujii, S.; Yamada, A.; Nakano, E.; Takeuchi, Y.; Mori, S.; Masuno, H.; Kagechika, H. Design and synthesis of nonsteroidal progesterone receptor antagonists based on C,C′-diphenylcarborane scaffold as a hydrophobic pharmacophore. Eur. J. Med. Chem. 2014, 84, 264–277. [Google Scholar] [CrossRef]
- Fujii, S.; Nakano, E.; Yanagida, N.; Mori, S.; Masuno, H.; Kagechika, H. Development of p-carborane-based nonsteroidal progesterone receptor antagonists. Bioorg. Med. Chem. 2014, 22, 5329–5337. [Google Scholar] [CrossRef]
- Mori, S.; Takeuchi, Y.; Tanatani, A.; Kagechika, H.; Fujii, S. Development of 1, 3-diphenyladamantane derivatives as nonsteroidal progesterone receptor antagonists. Bioorg. Med. Chem. 2015, 23, 803–809. [Google Scholar] [CrossRef]
- Fensome, A.; Bender, R.; Chopra, R.; Cohen, J.; Collins, M.A.; Hudak, V.; Malakian, K.; Lockhead, S.; Olland, A.; Svenson, K.; et al. Synthesis and structure-activity relationship of novel 6-aryl-1,4-dihydrobenzo[d]-[1,3]oxazine-2-thiones as progesterone receptor modulators leading to the potent and selective nonsteroidal progesterone receptor agonist tanaproget. J. Med. Chem. 2005, 48, 5092–5095. [Google Scholar] [CrossRef]
- Collins, M.A.; Hudak, V.; Bender, R.; Fensome, A.; Zhang, P.; Miller, L.; Winneker, R.C.; Zhang, Z.; Zhu, Y.; Cohen, J.; et al. Novel pyrrole-containing progesterone receptor modulators. Bioorg. Med. Chem. Lett. 2004, 14, 2185–2189. [Google Scholar] [CrossRef]
- Yamada, A.; Kazui, Y.; Yoshioka, H.; Tanatani, A.; Mori, S.; Kagechika, H.; Fujii, S. Development of N-(4-phenoxyphenyl) benzenesulfonamide derivatives as novel nonsteroidal progesterone receptor antagonists. ACS Med. Chem. Lett. 2016, 7, 1028–1033. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Mori, S.; Makishima, M.; Fujii, S.; Kagechika, H.; Hashimoto, Y.; Ishikawa, M. Novel nonsteroidal progesterone receptor (PR) antagonists with a phenanthridinone skeleton. ACS Med. Chem. Lett. 2018, 9, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Baban, J.A.; Roberts, B.P. Homolytic reactions of ligated boranes. Part 9. Overall addition of alkanes to electron-deficient alkenes by a radical chain mechanism. J. Chem. Soc. Perkin Trans. 1988, 7, 1195–1200. [Google Scholar] [CrossRef]
- Kawano, Y.; Yamaguchi, K.; Miyake, S.Y.; Kakizawa, T.; Shimoi, M. Investigation of the stability of the M-H-B bond in borane σ complexes [M(CO)5(η1-BH2R⋅L)] and [CpMn(CO)2(η1-BH2R⋅L)](M= Cr, W; L= tertiary amine or phosphine): Substituent and Lewis base effects. Chem. Eur. J. 2007, 13, 6920–6931. [Google Scholar] [CrossRef] [PubMed]
- Leo, A.; Hansch, C.; Elkins, D. Partition coefficients and their uses. Chem. Rev. 1971, 71, 525–616. [Google Scholar] [CrossRef]
- OECD. Guideline for Testing of Chemicals 117, Partition Coefficient (n-octanol/Water), High Performance Liquid Chromatography (HPLC) Method; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Finizio, A.; Vighi, M.; Sandroni, D. Determination of n-octanol/water partition coefficient (Kow) of pesticide critical review and comparison of methods. Chemosphere 1997, 34, 131–161. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Hoekman, D. Exploring QSAR: Hydrophobic, Electronic, and Steric Constants; American Chemical Society: Washington, DC, USA, 1995; Volume 2, ISBN 978-084-122-991-4. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- SwissADME. Available online: http://www.swissadme.ch (accessed on 27 September 2023).
- Moriguchi, I.; Hirono, S.; Liu, Q.; Nakagome, I.; Matsushita, Y. Simple method of calculating octanol/water partition coefficient. Chem. Pharm. Bull. 1992, 40, 127–130. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Nakagome, I.; Hirano, H. Comparison of reliability of log P values for drugs calculated by several methods. Chem. Pharm. Bull. 1994, 42, 976–978. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Wildman, S.A.; Crippen, G.M. Prediction of physicochemical parameters by atomic contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Di Lorenzo, D.; Albertini, A.; Zava, D. Progestin regulation of alkaline phosphatase in the human breast cancer cell line T47D. Cancer Res. 1991, 51, 4470–4475. [Google Scholar] [PubMed]
- Hiraoka, D.; Nakamura, N.; Nishizawa, Y.; Uchida, N.; Noguchi, S.; Matsumoto, K.; Sato, B. Inhibitory and stimulatory effects of glucocorticoid on androgen-induced growth of murine Shionogi carcinoma 115 in vivo and in cell culture. Cancer Res. 1987, 47, 6560–6564. [Google Scholar] [PubMed]
- Thompson, S.K.; Washburn, D.G.; Frazee, J.S.; Madauss, K.P.; Hoang, T.H.; Lapinski, L.; Grygielko, E.T.; Glace, L.E.; Trizna, W.; Williams, S.P.; et al. Rational design of orally-active, pyrrolidine-based progesterone receptor partial agonists. Bioorg. Med. Chem. Lett. 2009, 19, 4777–4780. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]




 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cmpd | R1 | R2 | LogP | MLOGP | Δ(LogP−MLOGP) | WLOGP | Δ(LogP−WLOGP) | |
| 16 | 3-CF3 | Me | Me | 3.65 | 4.26 | −0.61 | 3.47 | 0.18 |
| 17 | Me | Ph | 4.54 | 5.31 | −0.77 | 4.17 | 0.37 | |
| 18 | Et | Et | 4.88 | 5.07 | −0.19 | 4.64 | 0.24 | |
| 19 | Et | Ph | 5.24 | 5.78 | −0.54 | 4.95 | 0.29 | |
| 20 | i-Pr | i-Pr | 5.89 | 5.81 | 0.08 | 5.81 | 0.08 | |
| 21 | i-Pr | Ph | 5.93 | 6.24 | −0.31 | 5.73 | 0.20 | |
| 22 | c-Pr | c-Pr | 5.21 | 5.41 | −0.20 | 4.88 | 0.33 | |
| 23 | n-Bu | n-Bu | 7.34 a | 6.72 | 0.62 | 7.37 | −0.03 | |
| 24 | c-Pen | c-Pen | 7.84 a | 6.75 | 1.09 | 7.41 | 0.43 | |
| 25 | c-Hex | c-Hex | 8.69 a | 7.37 | 1.32 | 8.58 | 0.11 | |
| 26 | c-Hex | H | 7.28 a | 6.10 | 1.18 | 6.45 | 0.83 | |
| 27 | NMe2 | NMe2 | 5.39 | 3.00 | 2.39 | 3.01 | 2.38 | |
| 28 | 4-CF3 | Me | Me | 3.65 | 4.26 | −0.61 | 3.47 | 0.18 |
| 29 | Me | Ph | 4.65 | 5.31 | −0.66 | 4.17 | 0.48 | |
| 30 | Et | Et | 4.99 | 5.07 | −0.08 | 4.64 | 0.35 | |
| 31 | Et | Ph | 5.41 | 5.78 | −0.37 | 4.95 | 0.46 | |
| 32 | i-Pr | i-Pr | 6.06 | 5.81 | 0.25 | 5.81 | 0.25 | |
| 33 | i-Pr | Ph | 6.15 | 6.24 | −0.09 | 5.73 | 0.42 | |
| 34 | c-Pr | c-Pr | 5.32 | 5.41 | −0.09 | 4.88 | 0.44 | |
| 35 | n-Bu | n-Bu | 7.50 a | 6.50 | 1.00 | 6.98 | 0.52 | |
| 36 | c-Pen | c-Pen | 7.95 a | 6.75 | 1.20 | 7.41 | 0.54 | |
| 37 | c-Hex | c-Hex | 8.97 a | 7.37 | 1.60 | 8.58 | 0.39 | |
| 38 | c-Hex | H | 7.34 a | 6.10 | 1.24 | 6.45 | 0.89 | |
| 39 | NMe2 | NMe2 | 5.64 | 3.00 | 2.64 | 3.01 | 2.63 | |
 | ||||||
|---|---|---|---|---|---|---|
| Cmpd | R1 | R2 | LogP | Volume (Å3) a | IC50 (μM) b | |
| 16 | 3-CF3 | Me | Me | 3.65 | 238 | >10 |
| 17 | Me | Ph | 4.54 | 304 | 1.16 ± 0.60 | |
| 18 | Et | Et | 4.88 | 294 | 3.67 ± 3.34 | |
| 19 | Et | Ph | 5.24 | 341 | 1.12 ± 0.22 | |
| 20 | i-Pr | i-Pr | 5.89 | 348 | 1.35 ± 0.47 | |
| 21 | i-Pr | Ph | 5.93 | 389 | 3.35 ± 1.49 | |
| 22 | c-Pr | c-Pr | 5.21 | 328 | 0.65 ± 0.17 | |
| 23 | n-Bu | n-Bu | 7.34 | 404 | >10 | |
| 24 | c-Pen | c-Pen | 7.84 | 424 | 6.64 ± 0.87 | |
| 25 | c-Hex | c-Hex | 8.69 | 474 | >10 | |
| 26 | c-Hex | H | 7.28 | 376 | N.D. c | |
| 27 | NMe2 | NMe2 | 5.39 | 332 | 1.20 ± 0.63 | |
| 28 | 4-CF3 | Me | Me | 3.65 | 238 | >10 |
| 29 | Me | Ph | 4.65 | 304 | 1.61 ± 0.87 | |
| 30 | Et | Et | 4.99 | 294 | 1.76 ± 1.44 | |
| 31 | Et | Ph | 5.41 | 341 | 1.78 ± 0.97 | |
| 32 | i-Pr | i-Pr | 6.06 | 347 | 1.51 ± 0.53 | |
| 33 | i-Pr | Ph | 6.15 | 377 | 2.01 ± 0.58 | |
| 34 | c-Pr | c-Pr | 5.32 | 328 | 0.54 ± 0.09 | |
| 35 | n-Bu | n-Bu | 7.50 | 404 | >10 | |
| 36 | c-Pen | c-Pen | 7.95 | 424 | 2.63 ± 0.72 | |
| 37 | c-Hex | c-Hex | 8.97 | 474 | >10 | |
| 38 | c-Hex | H | 7.34 | 376 | N.D. c | |
| 39 | NMe2 | NMe2 | 5.64 | 332 | 1.40 ± 0.88 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyajima, Y.; Ochiai, K.; Fujii, S. Design, Synthesis, and Evaluation of B-(Trifluoromethyl)phenyl Phosphine–Borane Derivatives as Novel Progesterone Receptor Antagonists. Molecules 2024, 29, 1587. https://doi.org/10.3390/molecules29071587
Miyajima Y, Ochiai K, Fujii S. Design, Synthesis, and Evaluation of B-(Trifluoromethyl)phenyl Phosphine–Borane Derivatives as Novel Progesterone Receptor Antagonists. Molecules. 2024; 29(7):1587. https://doi.org/10.3390/molecules29071587
Chicago/Turabian StyleMiyajima, Yu, Kotaro Ochiai, and Shinya Fujii. 2024. "Design, Synthesis, and Evaluation of B-(Trifluoromethyl)phenyl Phosphine–Borane Derivatives as Novel Progesterone Receptor Antagonists" Molecules 29, no. 7: 1587. https://doi.org/10.3390/molecules29071587