Antimicrobial Activity and Phytochemical Characterization of Baccharis concava Pers., a Native Plant of the Central Chilean Coast
Abstract
:1. Introduction
2. Results
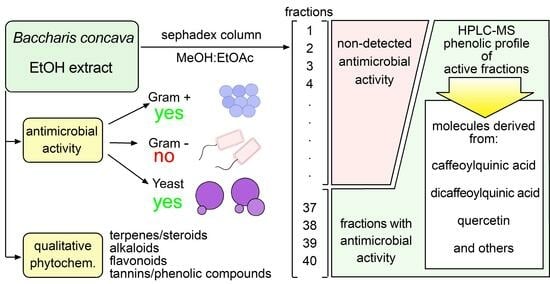
2.1. Alkaloids, Steroids, Terpenes, Flavonoids, and Phenolic Compounds Are Present in the Hydroalcoholic Extract of B. concava
2.2. Hydroalcoholic Extract of B. concava Shows Potent Antimicrobial Effects
2.3. Defective LPS Renders Susceptibility to B. concava Extract in S. Typhimurium
2.4. Column Fractionation of B. concava Extract
2.5. HPLC/Mass Spectrometry Tentative Identification of Phenolic Compounds
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Ethanolic Extract
4.3. Phytochemical Characterization
4.4. Antimicrobial Activity Assays by an Agar Diffusion Test
4.5. Minimum Inhibitory Concentration (MIC)
4.6. Minimum Biocidal Concentration (MBC)
4.7. Sephadex Preparatory Column
4.8. Thin-Layer Chromatography (TLC)
4.9. LC-MS Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooker, W.J.; Arnott, G.A.W. Contributions towards a Flora of South America and the Island of the Pacific. J. Bot. 1840, 3, 19–47. [Google Scholar]
- Ramos Campos, F.; Bressan, J.; Godoy Jasinski, V.C.; Zuccolotto, T.; da Silva, L.E.; Bonancio Cerqueira, L. Baccharis (Asteraceae): Chemical Constituents and Biological Activities. Chem. Biodivers. 2016, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.F.; Heidrich, D.; Fensterseifer, F.; Roso, M.T.; Bruxel, F.; Ethur, E.M.; Hoehne, L.; de Freitas, E.M. Chemical Characterization and Antimicrobial Activity of Baccharis vulneraria Baker Essential Oil against Strains of Microorganisms that Cause Cutaneous Infections. Nat. Prod. Res. 2023, 2023, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rabelo, A.C.; Caldeira Costa, D. A Review of Biological and Pharmacological Activities of Baccharis trimera. Chem. Biol. Interact. 2018, 296, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Ritter, M.R.; Christ, A.L.; de Mello Zevieski, A.; Fülber, M. An Overview of the Cultural and Popular Use of Baccharis. In Baccharis: From Evolutionary and Ecological Aspects to Social Uses and Medicinal Applications; Fernandes, G.W., Oki, Y., Barbosa, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 401–416. ISBN 978-3-030-83511-8. [Google Scholar]
- Houghton, P.J.; Manby, J. Medicinal Plants of the Mapuche. J. Ethnopharmacol. 1985, 13, 89–103. [Google Scholar] [CrossRef]
- Abad, M.; Bermejo, P. Baccharis (Compositae): A Review Update. Arkivoc 2007, 2007, 76–96. [Google Scholar] [CrossRef]
- Desmarchelier, C. Plantas Medicinales Autóctonas de La Argentina—Bases Científicas Para Su Aplicación En Atención Primaria de La Salud; Corpus: Buenos Aires, Argentina, 2015; ISBN 978-987-1860-25-8. [Google Scholar]
- Labbe, C.; Rovirosa, J.; Faini, F.; Mahu, M.; San-Martin, A.; Castillo, M. Secondary Metabolites from Chilean Baccharis Species. J. Nat. Prod. 1986, 49, 517–518. [Google Scholar] [CrossRef]
- Zampini, I.C.; Isla, M.I.; Schmeda-Hirschmann, G. Antimicrobial and antioxidant compounds from the infusion and methanolic extract of Baccharis incarum (WEDD.) PERKINS. J. Chil. Chem. Soc. 2009, 54, 477–481. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Mocan, A.; Sepúlveda, B. High Resolution Metabolite Fingerprinting of the Resin of Baccharis Tola Phil. from the Atacama Desert and Its Antioxidant Capacities. Ind. Crops Prod. 2016, 94, 368–375. [Google Scholar] [CrossRef]
- Carrizo, S.L.; Zampini, I.C.; Sayago, J.E.; Simirgiotis, M.J.; Bórquez, J.; Cuello, A.S.; Isla, M.I. Antifungal Activity of Phytotherapeutic Preparation of Baccharis Species from Argentine Puna against Clinically Relevant Fungi. J. Ethnopharmacol. 2020, 251, 112553. [Google Scholar] [CrossRef]
- Gambaro, V.; Chamy, M.C.; Garbarino, J.A.; San-Martin, A.; Castillo, M. Neo-Clerodane Diterpenoids from Baccharis macraei. Phytochemistry 1986, 25, 2175–2177. [Google Scholar] [CrossRef]
- Zamorano, R.; Aguirre, M.E.; Peña, A.M.D.L.; Cordano, G.; Medina, J.; Timmermann, B. Flavonoids from Baccharis concava Pers. Bol. Soc. Chil. Quim. 1987, 32, 101–103. [Google Scholar]
- Abad, M.J.; Bedoya, L.M.; Bermejo, P. Chapter 14—Essential Oils from the Asteraceae Family Active against Multidrug-Resistant Bacteria. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Rai, M.K., Kon, K.V., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 205–221. ISBN 978-0-12-398539-2. [Google Scholar]
- Santander Meyer, R.d.P. Análisis de los Componentes Químicos y Actividad Antibacteriana de los Aceites Esenciales de dos Plantas Endémicas: Baccharis concava y Haplopappus foliosus. Ph.D. Thesis, Tesis Universidad de Santiago de Chile, Santiago, Chile, 2012. [Google Scholar]
- Plants|Free Full-Text|Polyphenols and Flavonoids Composition, Anti-Inflammatory and Antioxidant Properties of Andean Baccharis macrantha Extracts. Available online: https://www.mdpi.com/2223-7747/11/12/1555 (accessed on 20 March 2024).
- Brighente, I.M.C.; Dias, M.; Verdi, L.G.; Pizzolatti, M.G. Antioxidant Activity and Total Phenolic Content of Some Brazilian Species. Pharm. Biol. 2007, 45, 156–161. [Google Scholar] [CrossRef]
- Cavalaro, R.I.; Fabricio, L.F.d.F.; Vieira, T.M.F.d.S. Ultrasound-Assisted Extraction of Antioxidants from Baccharis dracunculifolia and Green Propolis. Processes 2020, 8, 1530. [Google Scholar] [CrossRef]
- Rodríguez, Ó.E.; Roa, V.P.; Palacios, É.A. Actividad antibacteriana y antioxidante de Baccharis revoluta Kunth. Nova 2016, 14, 57–65. [Google Scholar] [CrossRef]
- Seregina, T.A.; Petrushanko, I.Y.; Shakulov, R.S.; Zaripov, P.I.; Makarov, A.A.; Mitkevich, V.A.; Mironov, A.S. The Inactivation of LPS Biosynthesis Genes in E. coli Cells Leads to Oxidative Stress. Cells 2022, 11, 2667. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.V.; Sortino, M.A.; Ivancovich, J.J.; Pellegrino, J.M.; Favier, L.S.; Raimondi, M.P.; Gattuso, M.A.; Zacchino, S.A. Detection of Synergistic Combinations of Baccharis Extracts with Terbinafine against Trichophyton Rubrum with High Throughput Screening Synergy Assay (HTSS) Followed by 3D Graphs. Behavior of Some of Their Components. Phytomedicine 2013, 20, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Ji, W.; Meng, Q.; Ding, L.; Wang, F.; Dong, J.; Zhou, G.; Wang, B. Measurement and Correlation of the Solubility of Caffeic Acid in Eight Mono and Water + Ethanol Mixed Solvents at Temperatures from (293.15 to 333.15) K. J. Mol. Liq. 2016, 224, 1275–1281. [Google Scholar] [CrossRef]
- Pinho, E.; Soares, G.; Henriques, M. Evaluation of Antibacterial Activity of Caffeic Acid Encapsulated by β-Cyclodextrins. J. Microencapsul. 2015, 32, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Simões-Pires, C.A.; Queiroz, E.F.; Henriques, A.T.; Hostettmann, K. Isolation and On-Line Identification of Anti-Oxidant Compounds from Three Baccharis Species by HPLC-UV-MS/MS with Post-Column Derivatisation. Phytochem. Anal. 2005, 16, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.; Simirgiotis, M.J.; Lima, B.; Paredes, J.D.; Villegas Gabutti, C.M.; Gamarra-Luques, C.; Bórquez, J.; Luna, L.; Wendel, G.H.; Maria, A.O.; et al. Antioxidant, Gastroprotective, Cytotoxic Activities and UHPLC PDA-Q Orbitrap Mass Spectrometry Identification of Metabolites in Baccharis grisebachii Decoction. Molecules 2019, 24, 1085. [Google Scholar] [CrossRef] [PubMed]
- Aboy, A.L.; Apel, M.A.; Debenedetti, S.; Francescato, L.; Rosella, M.A.; Henriques, A.T. Assay of Caffeoylquinic Acids in Baccharis trimera by Reversed-Phase Liquid Chromatography. J. Chromatogr. A 2012, 1219, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.-M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.O.; Freire Pessoa, H.L.; Lira, A.B.; Castillo, Y.P.; de Sousa, D.P. Synthesis, Antibacterial Evaluation, and QSAR of Caffeic Acid Derivatives. J. Chem. 2019, 2019, e3408315. [Google Scholar] [CrossRef]
- Touaibia, M.; Jean-Francois, J.; Doiron, J. Caffeic Acid, A Versatile Pharmacophore: An Overview. Mini-Rev. Med. Chem. 2011, 11, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Chen, H.; Zhong, Q. Nanoencapsulation of Caffeic Acid Phenethyl Ester in Sucrose Fatty Acid Esters to Improve Activities against Cancer Cells. J. Food Eng. 2019, 246, 125–133. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as Encapsulation Agents for Plant Bioactive Compounds. Carbohydr. Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Tambuwala, M.M.; Khan, M.N.; Thompson, P.; McCarron, P.A. Albumin Nano-Encapsulation of Caffeic Acid Phenethyl Ester and Piceatannol Potentiated Its Ability to Modulate HIF and NF-kB Pathways and Improves Therapeutic Outcome in Experimental Colitis. Drug Deliv. Transl. Res. 2019, 9, 14–24. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Jeong, Y.-I.; Kim, E.J.; Lee, K.D.; Choi, S.-H.; Kim, Y.J.; Kim, D.H.; Choi, K.-C. Preparation of Caffeic Acid Phenethyl Ester-Incorporated Nanoparticles and Their Biological Activity. J. Pharm. Sci. 2015, 104, 144–154. [Google Scholar] [CrossRef]
- Rebolledo, V.; Otero, M.C.; Delgado, J.M.; Torres, F.; Herrera, M.; Ríos, M.; Cabañas, M.; Martinez, J.L.; Rodríguez-Díaz, M. Phytochemical Profile and Antioxidant Activity of Extracts of the Peruvian Peppertree Schinus areira L. from Chile. Saudi J. Biol. Sci. 2021, 28, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical Screening and Extraction: A Review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Ndezo Bisso, B.; Njikang Epie Nkwelle, R.; Tchuenguem Tchuenteu, R.; Dzoyem, J.P. Phytochemical Screening, Antioxidant, and Antimicrobial Activities of Seven Underinvestigated Medicinal Plants against Microbial Pathogens. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 1998808. [Google Scholar] [CrossRef] [PubMed]
- Dubale, S.; Kebebe, D.; Zeynudin, A.; Abdissa, N.; Suleman, S. Phytochemical Screening and Antimicrobial Activity Evaluation of Selected Medicinal Plants in Ethiopia. J. Exp. Pharmacol. 2023, 15, 51–62. [Google Scholar] [CrossRef] [PubMed]
- María, R.; Shirley, M.; Xavier, C.; Jaime, S.; David, V.; Rosa, S.; Jodie, D. Preliminary Phytochemical Screening, Total Phenolic Content and Antibacterial Activity of Thirteen Native Species from Guayas Province Ecuador. J. King Saud Univ.-Sci. 2018, 30, 500–505. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, S. Concept of Standardization, Extraction and Pre Phytochemical Screening Strategies for Herbal Drug. J. Pharmacogn. Phytochem. 2014, 2, 115–119. [Google Scholar]
- African Journal of Pure and Applied Chemistry. Volume 4, pp. 206–212, October 2010. Available online: http://www.academicjournals.org/AJPAC (accessed on 21 March 2024).
- Morsy, N. Phytochemical Analysis of Biologically Active Constituents of Medicinal Plants. Main Group Chem. 2014, 13, 7–21. [Google Scholar] [CrossRef]
- Shaikh, J.R.; Patil, M.K. Qualitative Tests for Preliminary Phytochemical Screening: An Overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant Activity of Propolis of Various Geographic Origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Villagra, N.A.; Hidalgo, A.A.; Santiviago, C.A.; Saavedra, C.P.; Mora, G.C. SmvA, and Not AcrB, Is the Major Efflux Pump for Acriflavine and Related Compounds in Salmonella Enterica Serovar Typhimurium. J. Antimicrob. Chemother. 2008, 62, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Mottaghipisheh, J.; Iriti, M. Sephadex® LH-20, Isolation, and Purification of Flavonoids from Plant Species: A Comprehensive Review. Molecules 2020, 25, 4146. [Google Scholar] [CrossRef]
- Kagan, I.A.; Flythe, M.D. Thin-Layer Chromatographic (TLC) Separations and Bioassays of Plant Extracts to Identify Antimicrobial Compounds. J. Vis. Exp. 2014, 85, e51411. [Google Scholar] [CrossRef]



| Assay | Compounds Tested | Positive Results | Result |
|---|---|---|---|
| Dragendorff | alkaloids | red precipitate | + |
| Bornträger | free Anthraquinones | red color in aqueous phase | − |
| Fluorescence under UV | coumarins | blue color under UV light | − |
| Liebermann–Burchard | steroids and terpenes | green–blue or purple–red | + |
| Aluminium chloride | flavonoids | yellow green fluorescence under UV light | + |
| Keller–Killiani | cardiac glycosides | greenish blue color | − |
| Foam formation | saponins | foam production (stands 10 min) | − |
| Ferric chloride | tannins and phenolic | green or dark blue color | + |
| Microorganism | Inhibition Haloes (mm) | MIC-IC50 (mg/mL) | MBC (mg/mL) |
|---|---|---|---|
| S. epidermidis | 30.67 ± 0.58 | 6.95 ± 3.01 | 13.89 ± 6.01 |
| S. aureus | 20.67 ± 0.58 | 2.17 ± 0.75 | 4.34 ± 1.51 |
| B. subtilis | 14.67 ± 1.16 | 13.89 ± 6.01 | 27.78 ± 12.03 |
| B. cereus | 14.67 ± 1.16 | 20.83 ± 0 | 41.67 ± 0 |
| S. pyogenes | 27.33 ± 0.58 | 6.95 ± 3.01 | 13.89 ± 6.01 |
| E. coli | 6 ± 0 | - | - |
| S. Typhimurium | 6 ± 0 | 83.33 ± 0 | 111.11 ± 48.12 |
| K. pneumoniae | 6 ± 0 | - | - |
| A. baumannii | 6 ± 0 | - | - |
| C. albicans | 14.33 ± 0.58 | <27.78 ± 12.03 | 27.78 ± 12.03 |
| C. neoformans | 37.00 ± 0 | 41.67 ± 0 | 83.33 ± 0 |
| S. Typhimurium Strains | Function/Structure Affected | Inhibition Halos (mm) |
|---|---|---|
| S. Typhimurium 14028s WT | Wild-type bacteria | 6 |
| S. Typhimurium ΔrfaC | Synthesis LPS | 20 |
| S. Typhimurium ΔrfaE | Synthesis LPS | 22 |
| S. Typhimurium ΔOmpA | Outer membrane protein/channel | 6 |
| Peak | RT (min) | [M-H]- (m/z) | Fragments MS2 (m/z) | Compound | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 192.1 | 172.4 | 126.3 | 84.4 | 92.3 | 110.4 | Quinic acid |
| 2 | 10.9 | 355.9 | 190.5 | 178.5 | 134.4 | Coumaroylhexaric acid | ||
| 3 | 11.6 | 353.2 | 172.6 | 178.5 | 190.5 | Caffeoylquinic acid | ||
| 11.6 | 593.7 | 473.6 | 503.3 | 407.8 | 575.1 | Apigenin-di-C-hexoside | ||
| 4 | 12.2 | 354.3 | 190.5 | 179.0 | Caffeoylquinic acid | |||
| 5 | 12.8 | 741.6 | 300.0 | 609.2 | 591.3 | 475.1 | 343.1 | Rutin-O-pentoside |
| 6 | 13.3 | 352.2 | 190.5 | 178.4 | Caffeoylquinic acid | |||
| 7 | 14.3 | 609.5 | 300.8 | Quercetin-O-rhamnosyl hexoside | ||||
| 8 | 15.4 | 463.6 | 300.8 | Quercetin-O-hexoside | ||||
| 9 | 15.6 | 477.2 | 300.9 | Quercetin-O-glucuronide | ||||
| 10 | 16.5 | 515.4 | 352.9 | Dicaffeoylquinic acid | ||||
| 11 | 17.6 | 515.1 | 352.9 | 190.8 | 202.8 | 178.7 | Dicaffeoylquinic acid | |
| 12 | 17.8 | 515.1 | 352.9 | Dicaffeoylquinic acid | ||||
| 13 | 18.2 | 515.2 | 352.9 | 190.7 | 178.9 | Dicaffeoylquinic acid | ||
| 14 | 19.0 | 515.1 | 352.9 | Dicaffeoylquinic acid | ||||
| 15 | 19.4 | 516.6 | 352.9 | 202.6 | 335.1 | 190.5 | 172.5 | Dicaffeoylquinic acid |
| 16 | 22.0 | 529.4 | 353.1 | 366.9 | 190.8 | 178.7 | Caffeoyl-feruloylquinic acid | |
| 17 | 24.9 | 677.9 | 515.0 | Dicaffeoylquinic acid-O-hexoside | ||||
| 18 | 33.1 | 269.1 | 224.7 | 148.5 | 200.6 | Apigenin | ||
| 19 | 34.9 | 329.1 | 313.9 | Kaempferol methoxy methyl ether | ||||
| 20 | 37.3 | 300.0 | 283.9 | Kaempferol methyl ether | ||||
| 21 | 43.4 | 313.8 | 297.2 | Kaempferol dimethoxy | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Díaz, M.; Pérez, F.E.; Manosalva, P.M.; Cerda, J.I.; Martínez-Contreras, C.F.; Mora, A.Y.; Villagra, N.A.; Bucarey, S.A.; Barriga, A.; Escobar, J.; et al. Antimicrobial Activity and Phytochemical Characterization of Baccharis concava Pers., a Native Plant of the Central Chilean Coast. Molecules 2024, 29, 1654. https://doi.org/10.3390/molecules29071654
Rodríguez-Díaz M, Pérez FE, Manosalva PM, Cerda JI, Martínez-Contreras CF, Mora AY, Villagra NA, Bucarey SA, Barriga A, Escobar J, et al. Antimicrobial Activity and Phytochemical Characterization of Baccharis concava Pers., a Native Plant of the Central Chilean Coast. Molecules. 2024; 29(7):1654. https://doi.org/10.3390/molecules29071654
Chicago/Turabian StyleRodríguez-Díaz, Maité, Fabián E. Pérez, Paloma M. Manosalva, Juan I. Cerda, Consuelo F. Martínez-Contreras, Aracely Y. Mora, Nicolás A. Villagra, Sergio A. Bucarey, Andrés Barriga, Jorge Escobar, and et al. 2024. "Antimicrobial Activity and Phytochemical Characterization of Baccharis concava Pers., a Native Plant of the Central Chilean Coast" Molecules 29, no. 7: 1654. https://doi.org/10.3390/molecules29071654