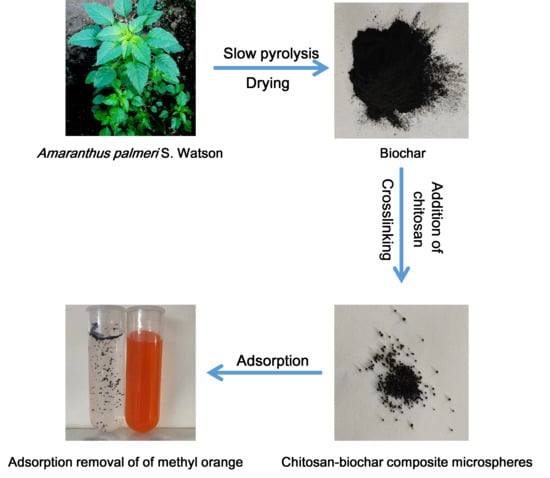
Enhanced Adsorption of Methyl Orange from Aqueous Phase Using Chitosan–Palmer Amaranth Biochar Composite Microspheres
Abstract
:1. Introduction
2. Results and Discussion
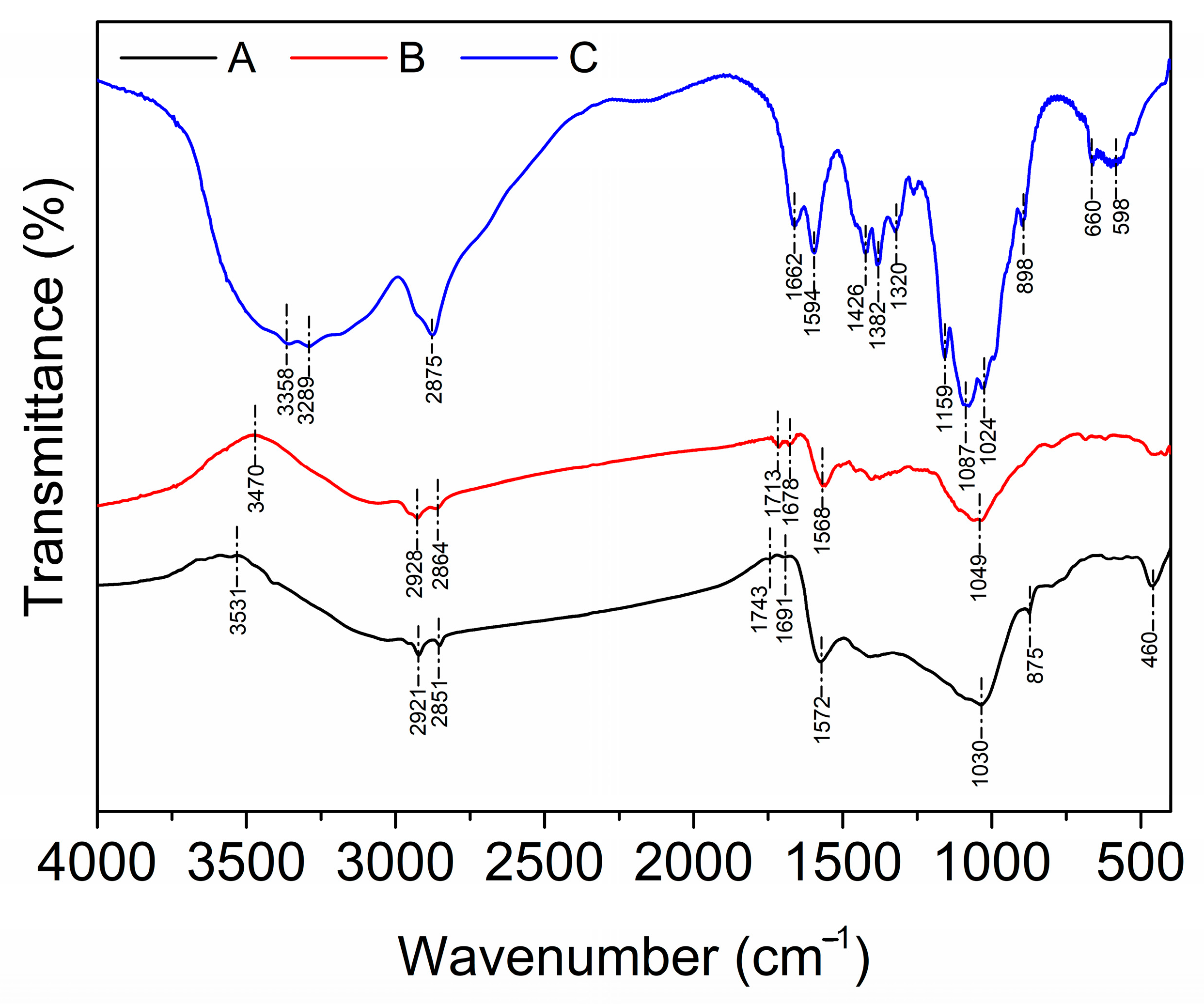
2.1. Characterization Results
2.2. Influent Factors
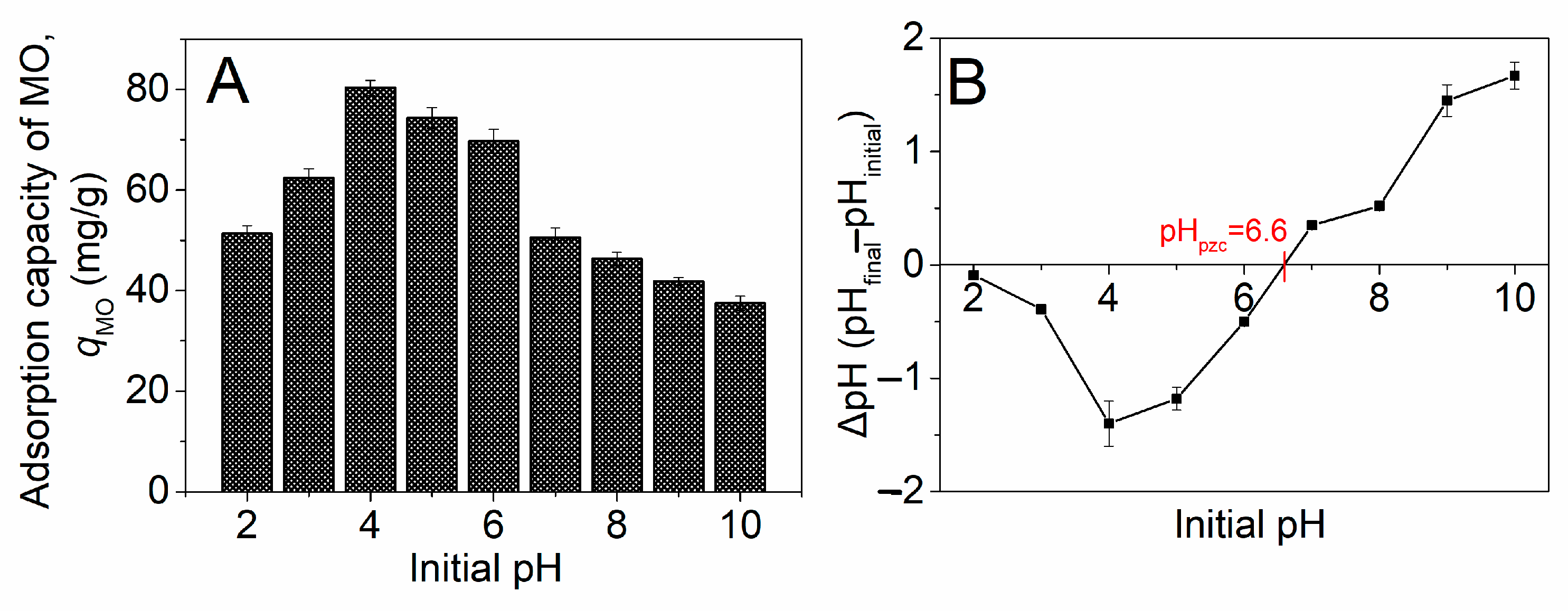
2.2.1. Effect of Initial pH
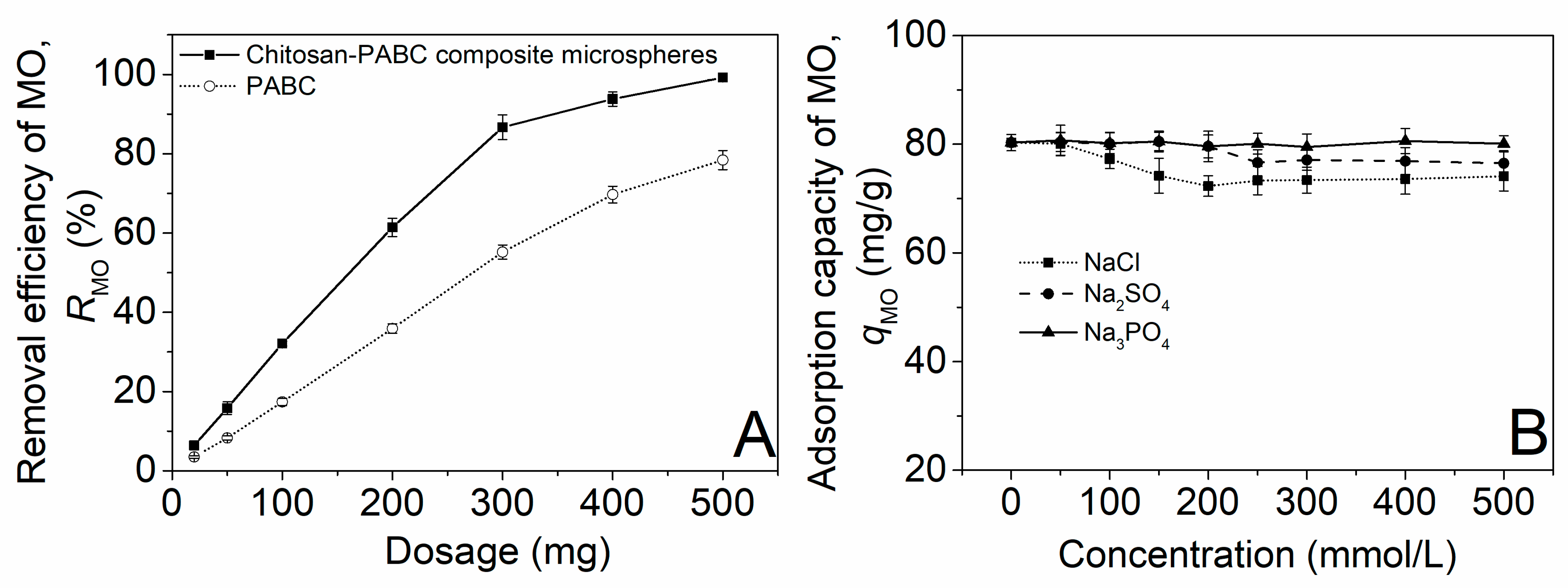
2.2.2. Effect of Dosage
2.2.3. Effects of Salt Type and Concentration
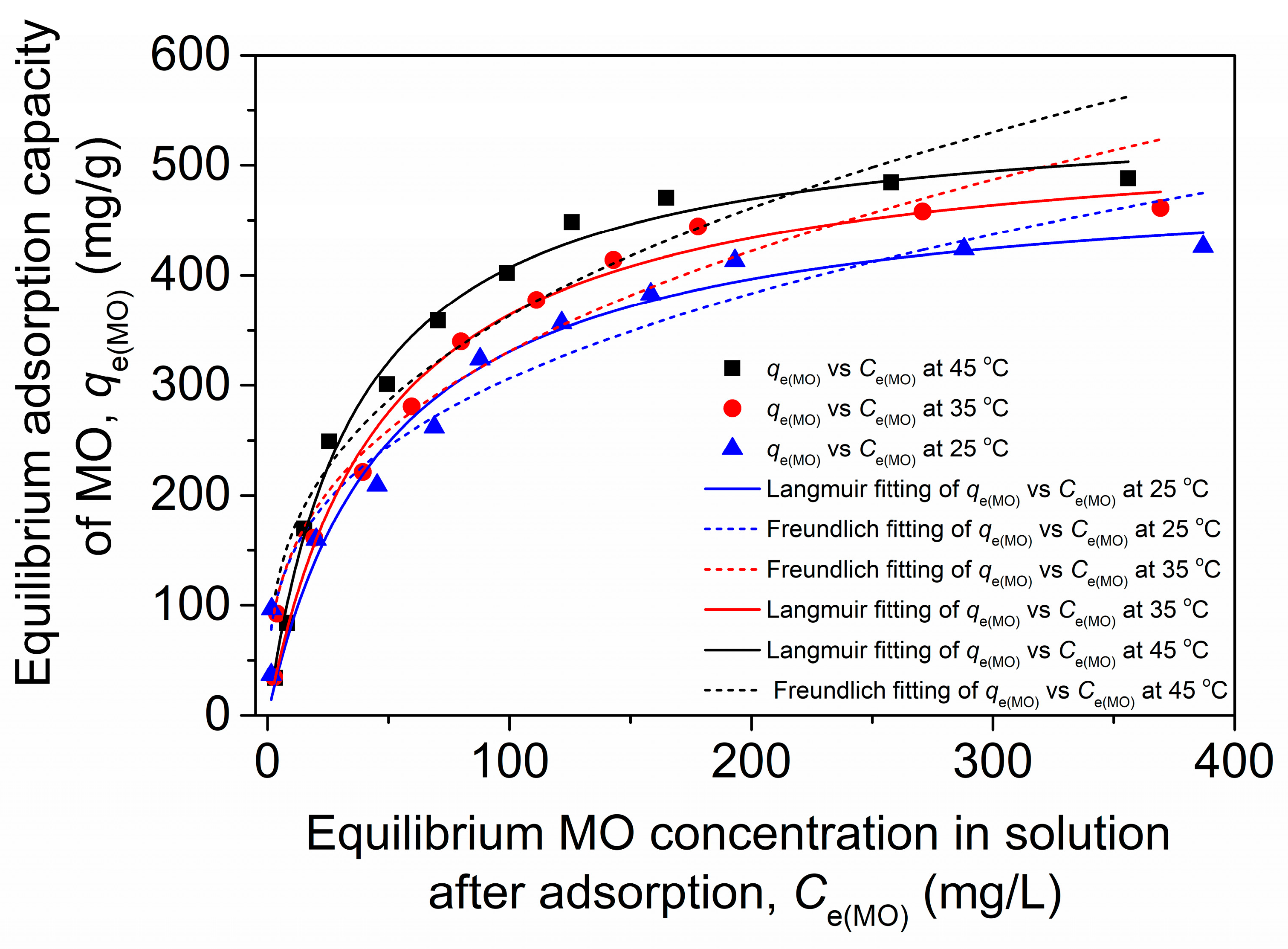
2.3. Adsorption Kinetics, Isotherms and Thermodynamics
2.3.1. Adsorption Kinetics
2.3.2. Adsorption Isotherms
2.3.3. Adsorption Thermodynamics
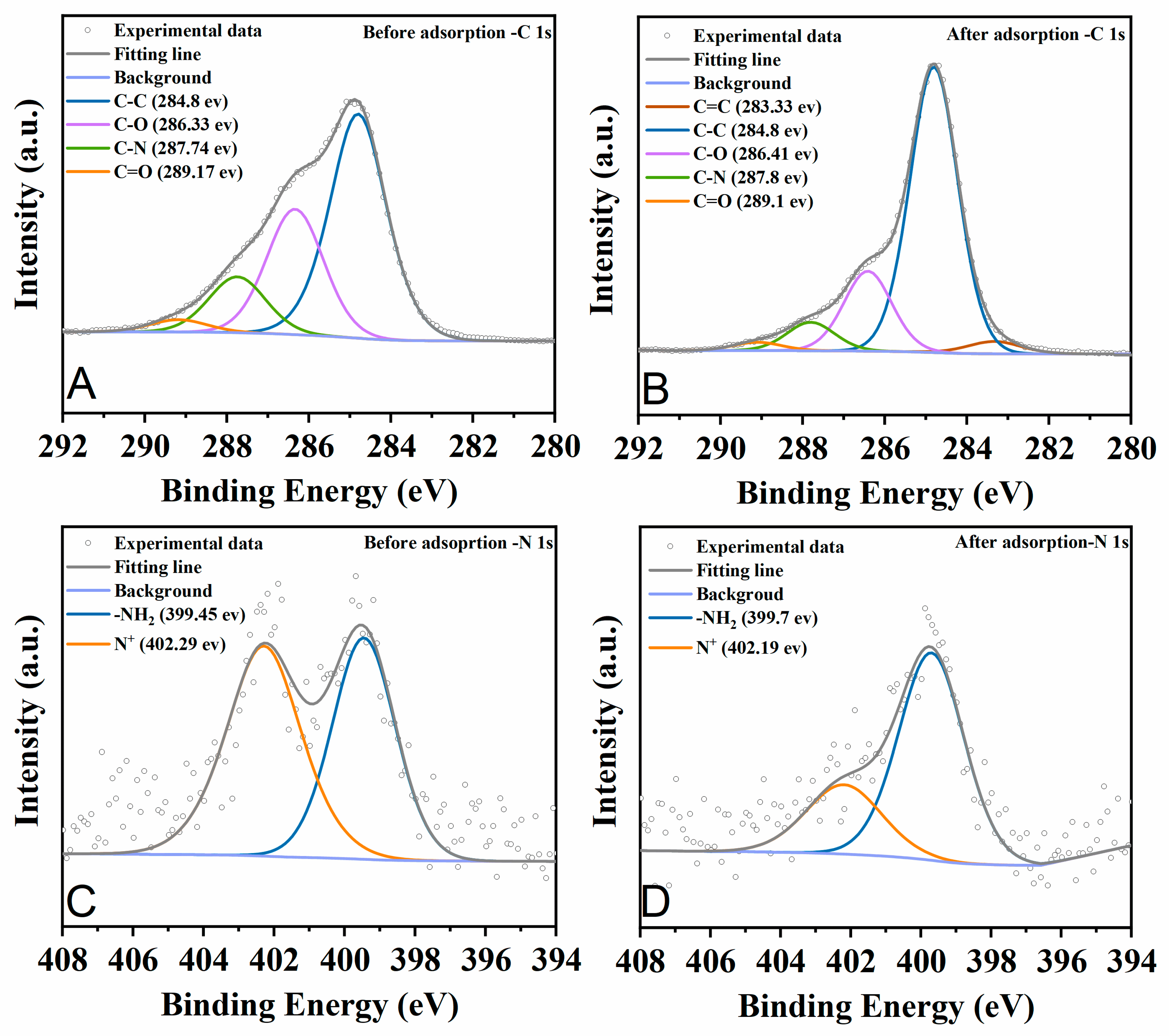
2.4. Adsorption Mechanisms
2.5. Regeneration of Chitosan–PABC Composite Microspheres
3. Materials and Methods
3.1. Materials
3.2. Preparation of PABC
3.3. Preparation of Chitosan–PABC Composite Microspheres
3.4. Characterizations of PABC and Chitosan–PABC Composite Microspheres
3.5. Measurement of MO Concentration
3.6. Batch Adsorption Experiments
3.7. Desorption and Regeneration Studies
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Priyanka, U.; Lens, P.N. Light driven Aspergillus niger-ZnS nanobiohybrids for degradation of methyl orange. Chemosphere 2022, 298, 134162. [Google Scholar] [CrossRef] [PubMed]
- Iwuozor, K.O.; Ighalo, J.O.; Emenike, E.C.; Ogunfowora, L.A.; Igwegbe, C.A. Adsorption of methyl orange: A review on adsorbent performance. Curr. Res. Green Sustain. Chem. 2021, 4, 100179. [Google Scholar] [CrossRef]
- Ismail, M.; Akhtar, K.; Khan, M.I.; Kamal, T.; Khan, M.A.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, toxicity and carcinogenicity of organic dyes and their catalytic bio-remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, A.; Ejsmont, A.; Galarda, A.; Goscianska, J. The outcome of human exposure to environmental contaminants. Importance of water and air purification processes. In Sustainable Materials for Sensing and Remediation of Noxious Pollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 15–37. [Google Scholar]
- Saxena, R.; Saxena, M.; Lochab, A. Recent progress in nanomaterials for adsorptive removal of organic contaminants from wastewater. ChemistrySelect 2020, 5, 335–353. [Google Scholar] [CrossRef]
- Uddin, M.J.; Ampiaw, R.E.; Lee, W. Adsorptive removal of dyes from wastewater using a metal-organic framework: A review. Chemosphere 2021, 284, 131314. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A.; Zohra, S.T.; Ijaz, S.; Iqbal, M.; Iqbal, J.; Bibi, I.; Nouren, S.; Messaoudi, N.E.; Nazir, A. Cellulose-based materials and their adsorptive removal efficiency for dyes: A review. Int. J. Biol. Macromol. 2023, 224, 1337–1355. [Google Scholar] [CrossRef] [PubMed]
- Yudaev, P.; Butorova, I.; Stepanov, G.; Chistyakov, E. Extraction of palladium (ii) with a magnetic sorbent based on polyvinyl alcohol gel, metallic iron, and an environmentally friendly polydentate phosphazene-containing extractant. Gels 2022, 8, 492. [Google Scholar] [CrossRef]
- Yudaev, P.; Semenova, A.; Chistyakov, E. Gel based on modified chitosan for oil spill cleanup. J. Appl. Polym. Sci. 2024, 141, e54838. [Google Scholar] [CrossRef]
- Sayed, N.S.; Ahmed, A.S.; Abdallah, M.H.; Gouda, G.A. ZnO@ activated carbon derived from wood sawdust as adsorbent for removal of methyl red and methyl orange from aqueous solutions. Sci. Rep. 2024, 14, 5384. [Google Scholar] [CrossRef]
- Darwish, A.A.A.; Rashad, M.; AL-Aoh, H.A. Methyl orange adsorption comparison on nanoparticles: Isotherm, kinetics, and thermodynamic studies. Dye. Pigment. 2019, 160, 563–571. [Google Scholar] [CrossRef]
- Birniwa, A.H.; Ali, U.; Jahun, B.M.; Al-dhawi, B.N.S.; Jagaba, A.H. Cobalt oxide doped polyaniline composites for methyl orange adsorption: Optimization through response surface methodology. Case Stud. Chem. Environ. Eng. 2024, 9, 100553. [Google Scholar] [CrossRef]
- Wang, C.; Feng, X.; Shang, S.; Liu, H.; Song, Z.; Zhang, H. Adsorption of methyl orange from aqueous solution with lignin-modified metal-organic frameworks: Selective adsorption and high adsorption capacity. Bioresour. Technol. 2023, 388, 129781. [Google Scholar] [CrossRef] [PubMed]
- Sutar, S.; Patil, P.; Jadhav, J. Recent advances in biochar technology for textile dyes wastewater remediation: A review. Environ. Res. 2022, 209, 112841. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, X.; Wang, D.; Li, P. Adsorption of methyl orange dye onto biochar adsorbent prepared from chicken manure. Water Sci. Technol. 2018, 77, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Loc, N.X.; Tuyen PT, T.; Mai, L.C.; Phuong, D.T.M. Chitosan-modified biochar and unmodified biochar for methyl orange: Adsorption characteristics and mechanism exploration. Toxics 2022, 10, 500. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, J.; Bai, Y.; Gao, J.; Peng, M. Adsorption properties of methyl orange in water by sheep manure biochar. Pol. J. Environ. Stud. 2019, 28, 3791–3797. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.; Lee, Y.G.; Chon, K. Enhanced adsorptive removal of dyes using Mandarin peel biochars via chemical activation with NH4Cl and ZnCl2. Water 2021, 13, 1495. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Zhou, Y.; Zhou, Y.; Igwegbe, C.A.; Anastopoulos, I.; Raji, M.A.; Iwuozor, K.O. A review of pine-based adsorbents for the adsorption of dyes. Biomass-Deriv. Mater. Environ. Appl. 2022, 2022, 319–332. [Google Scholar]
- Aichour, A.; Zaghouane-Boudiaf, H.; Khodja, H.D. Highly removal of anionic dye from aqueous medium using a promising biochar derived from date palm petioles: Characterization, adsorption properties and reuse studies. Arab. J. Chem. 2022, 15, 103542. [Google Scholar] [CrossRef]
- Yan, X.; Sun, Y.; Ma, C.; Kong, X.; Zhang, Y.; Tao, W. Adsorption of anionic and cationic dyes on different biochars. Russ. J. Phys. Chem. A 2021, 95, 2031–2041. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R. Comparison of characteristics of twenty-one types of biochar and their ability to remove multi-heavy metals and methylene blue in solution. Fuel Process. Technol. 2017, 160, 55–63. [Google Scholar] [CrossRef]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci. Total Environ. 2020, 713, 136433. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Florentine, S. A review of the biology, distribution patterns and management of the invasive species Amaranthus palmeri S. Watson (Palmer amaranth): Current and future management challenges. Weed Res. 2022, 62, 113–122. [Google Scholar] [CrossRef]
- Jiao, X.; Long, M.; Li, J.; Yang, Q.; Liu, Z. Reconstructing the invasive history and potential distribution prediction of Amaranthus palmeri in China. Agronomy 2023, 13, 2498. [Google Scholar] [CrossRef]
- Danesh, P.; Niaparast, P.; Ghorbannezhad, P.; Ali, I. Biochar production: Recent developments, applications, and challenges. Fuel 2023, 337, 126889. [Google Scholar] [CrossRef]
- Zhou, Y.; Qin, S.; Verma, S.; Sar, T.; Sarsaiya, S.; Ravindran, B.; Liu, T.; Sindhu, R.; Patel, A.K.; Binod, P.; et al. Production and beneficial impact of biochar for environmental application: A comprehensive review. Bioresour. Technol. 2021, 337, 125451. [Google Scholar] [CrossRef]
- Wang, Q.; Lai, Z.; Mu, J.; Chu, D.; Zang, X. Converting industrial waste cork to biochar as Cu (II) adsorbent via slow pyrolysis. Waste Manag. 2020, 105, 102–109. [Google Scholar] [CrossRef]
- Patra, B.R.; Mukherjee, A.; Nanda, S.; Dalai, A.K. Biochar production, activation and adsorptive applications: A review. Environ. Chem. Lett. 2021, 19, 2237–2259. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Y.; Li, X. Adsorption performance and mechanism of iron-loaded biochar to methyl orange in the presence of Cr6+ from dye wastewater. J. Hazard. Mater. 2021, 415, 125749. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Du, W.; Zhang, M.; Ling, G.; Zhang, P. Chitosan-modified biochar: Preparation, modifications, mechanisms and applications. Int. J. Biol. Macromol. 2022, 209, 31–49. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Y.; Li, R.; Lu, F.; Yang, Y.; Huang, Q.; Deng, D.; Wu, M.; Li, Y. Enhanced reactive brilliant blue removal using chitosan–biochar hydrogel beads. Molecules 2023, 28, 6137. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.H.; Gong, B.; Joseph, S.D.; Marjo, C.E.; Munroe, P.; Rich, A.M. Imaging of mineral-enriched biochar by FTIR, Raman and SEM–EDX. Vib. Spectrosc. 2012, 62, 248–257. [Google Scholar] [CrossRef]
- Nair, R.R.; Mondal, M.M.; Weichgrebe, D. Biochar from co-pyrolysis of urban organic wastes—Investigation of carbon sink potential using ATR-FTIR and TGA. Biomass Convers. Biorefin. 2020, 12, 4729–4743. [Google Scholar] [CrossRef]
- Lago, B.C.; Silva, C.A.; Melo LC, A.; de Morais, E.G. Predicting biochar cation exchange capacity using Fourier transform infrared spectroscopy combined with partial least square regression. Sci. Total Environ. 2021, 794, 148762. [Google Scholar] [CrossRef]
- Duarte, M.L.; Ferreira, M.C.; Marvao, M.R.; Rocha, J. An optimised method to determine the degree of acetylation of chitin and chitosan by FTIR spectroscopy. Int. J. Biol. Macromol. 2002, 31, 1–8. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, S.; He, F.; Liu, Y.; Mao, W.; Guan, Y. Highly efficient and selective capture of heavy metals by poly (acrylic acid) grafted chitosan and biochar composite for wastewater treatment. Chem. Eng. J. 2019, 378, 122215. [Google Scholar] [CrossRef]
- Perera, H.M.; Rajapaksha, A.U.; Liyanage, S.; Ekanayake, A.; Selvasembian, R.; Daverey, A.; Vithanage, M. Enhanced adsorptive removal of hexavalent chromium in aqueous media using chitosan-modified biochar: Synthesis, sorption mechanism, and reusability. Environ. Res. 2023, 231, 115982. [Google Scholar] [CrossRef]
- Dakiky, M.; Khamis, M.; Manasra, A.; Takrouri, K. Effect of surfactants on the thermodynamic properties of methyl orange dye in buffered solutions. Color. Technol. 2002, 118, 191–197. [Google Scholar] [CrossRef]
- Nguyen, X.C.; Nguyen, T.T.H.; Nguyen, T.H.C.; Van Le, Q.; Vo, T.Y.B.; Tran, T.C.P.; La, D.D.; Kumar, G.; VNguyen, K.; Chang, S.W.; et al. Sustainable carbonaceous biochar adsorbents derived from agro-wastes and invasive plants for cation dye adsorption from water. Chemosphere 2021, 282, 131009. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- Ip, A.W.M.; Barford, J.P.; McKay, G. Reactive Black dye adsorption/desorption onto different adsorbents: Effect of salt, surface chemistry, pore size and surface area. J. Colloid Interface Sci. 2009, 337, 32–38. [Google Scholar] [CrossRef]
- Kamiyama, Y.; Israelachvili, J. Effect of pH and salt on the adsorption and interactions of an amphoteric polyelectrolyte. Macromolecules 1992, 25, 5081–5088. [Google Scholar] [CrossRef]
- Tahiruddin, N.S.M.; Aziz, R.A.; Ali, R.; Taib, N.I. Potential of using jackfruit peel (Artocarpus heterophyllus) as green solution for removal of copper (II) and zinc (II) from aqueous solution: Adsorption kinetics, isotherm and thermodynamic studies. J. Environ. Chem. Eng. 2023, 11, 109953. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, S.; Duan, H.; He, J.; Luo, Y. Removal of anionic and cationic dyes using porous chitosan/carboxymethyl cellulose-PEG hydrogels: Optimization, adsorption kinetics, isotherm and thermodynamics studies. Int. J. Biol. Macromol. 2023, 231, 123213. [Google Scholar] [CrossRef]
- Debnath, S.; Das, R. Strong adsorption of CV dye by Ni ferrite nanoparticles for waste water purification: Fits well the pseudo second order kinetic and Freundlich isotherm model. Ceram. Int. 2023, 49, 16199–16215. [Google Scholar] [CrossRef]
- Wang, S.S.; Yan, Y.M.; Hsu, C.H.; Lin, H.P. Waste bamboo-derived biochar and multiporous carbon as adsorbents for methyl orange removal. J. Chin. Chem. Soc. 2023, 70, 1628–1635. [Google Scholar] [CrossRef]
- Li, X.F.; Li, R.X.; Huang, M.M.; Feng, X.Q. Superior adsorption performance of zinc-loaded chitosan biochar for methyl orange dye. Russ. J. Phys. Chem. A 2023, 97, 1582–1596. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Zhang, Z. A versatile EDTA and chitosan bi-functionalized magnetic bamboo biochar for simultaneous removal of methyl orange and heavy metals from complex wastewater. Environ. Pollut. 2022, 293, 118517. [Google Scholar] [CrossRef]
- Kaya, N.; Uzun, Z.Y. Investigation of effectiveness of pine cone biochar activated with KOH for methyl orange adsorption and CO2 capture. Biomass Convers. Biorefinery 2021, 11, 1067–1083. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, Y.; Li, J.; Wang, L.; Wang, X.; Tian, S. Multi-factorial investigation of the effect of biochar of the secondary medicinal residue of snow lotus on the adsorption of two azo dyes, methyl red and methyl orange. Microsc. Res. Tech. 2023, 86, 1416–1442. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gao, J.; Liu, Y.; Zhang, Z.; Zou, M.; Liao, Q.; Shang, J. Removal of methyl orange from water using sulfur-modified nZVI supported on biochar composite. Water Air Soil Pollut. 2018, 229, 355. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Y.; Cha, L. Removal of methyl orange dye using activated biochar derived from pomelo peel wastes: Performance, isotherm, and kinetic studies. J. Dispers. Sci. Technol. 2020, 41, 125–136. [Google Scholar] [CrossRef]
- Hou, Y.; Liang, Y.; Hu, H.; Tao, Y.; Zhou, J.; Cai, J. Facile preparation of multi-porous biochar from lotus biomass for methyl orange removal: Kinetics, isotherms, and regeneration studies. Bioresour. Technol. 2021, 329, 124877. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, W.; Zhang, M.; Zhou, R.; Li, J.; Zhao, W.; Wang, L. Optimize the preparation of Fe3O4-modified magnetic mesoporous biochar and its removal of methyl orange in wastewater. Environ. Monit. Assess. 2021, 193, 179. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, G.; Liu, Q.; Wang, J.; Xia, S.; Xue, Y.; Han, J.; Duanmuc, C.; Zhu, Y. A comprehensive insight into removal of dye and antibiotic based on Mg/Al-LDHs-biochar hybrid materials. Mater. Technol. 2022, 37, 2204–2217. [Google Scholar] [CrossRef]
- Karagöz, S.; Tay, T.; Ucar, S.; Erdem, M. Activated carbons from waste biomass by sulfuric acid activation and their use on methylene blue adsorption. Bioresour. Technol. 2008, 99, 6214–6222. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Guo, S.; Zou, Z.; Chen, Y.; Long, X.; Liu, M.; Li, X.; Tan, J.; Chen, R. Synergistic effect of hydrogen bonding and π-π interaction for enhanced adsorption of rhodamine B from water using corn straw biochar. Environ. Pollut. 2023, 320, 121060. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Johir, M.A.H.; Sun, L.; Asadullah, M.; Belhaj, D. Sorption of hydrophobic organic contaminants on functionalized biochar: Protagonist role of π-π electron-donor-acceptor interactions and hydrogen bonds. J. Hazard. Mater. 2018, 360, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Cheng, J.; Cao, W.; Yang, C.; Chen, J.; Luo, Z. Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 2019, 540, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, X.; Ge, Z.; Bao, Z.; Lin, L.; Chen, Y.; Dai, W.; Sun, Y.; Yuan, H.; Yang, W.; et al. Koh-activated biochar and chitosan composites for efficient adsorption of industrial dye pollutants. Chem. Eng. J. 2024, 486, 150387. [Google Scholar] [CrossRef]
- Ronsse, F.; Van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Salimi, H.; Fattah-Alhosseini, A.; Karbasi, M.; Nikoomanzari, E. Development of WO3-incorporated porous ceramic coating: A key role of WO3 nanoparticle concentration on methylene blue photodegradation upon visible light illumination. Ceram. Int. 2023, 49, 32181–32192. [Google Scholar] [CrossRef]




| Temperature (°C) | 25 | 35 | 45 | |
|---|---|---|---|---|
| Pseudo-first-order kinetic model | qe(MO) (mg/g) | 79.9 | 83.0 | 86.7 |
| K1 (min−1) | 0.0851 | 16.1 | 17.6 | |
| R2 | 0.8618 | 0.08333 | 0.08333 | |
| Pseudo-second-order kinetic model | qe(MO) (mg/g) | 84.0 | 92.9 | 96.3 |
| K2 (g/(mg·min)) | 1.61 × 10−7 | 1.66 × 10−7 | 1.73 × 10−7 | |
| R2 | 0.97149 | 0.98364 | 0.97133 | |
| Intraparticle diffusion model | Kdif (mg/(g·min−1/2)) | 2.20 | 2.30 | 2.31 |
| Θ (mg/g) | 48.3 | 55.6 | 59.3 | |
| R2 | 0.76691 | 0.73244 | 0.75701 | |
| Temperature (°C) | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| KL (L/mg) | qmax(MO) (mg/g) | RL | R2 | KF (mg(1−n) Ln/g) | 1/n | R2 | |
| 25 | 0.0201 | 495.0 | 0.199 | 0.94578 | 68.8 | 0.324 | 0.94482 |
| 35 | 0.0241 | 537.1 | 0.172 | 0.97935 | 65.6 | 0.351 | 0.93311 |
| 45 | 0.0276 | 554.3 | 0.153 | 0.99144 | 74.6 | 0.344 | 0.89581 |
| Adsorbent | qmax(MO) (mg/g) | Reference |
|---|---|---|
| Chicken manure biochar | 39.47 | [16] |
| Chitosan-modified rice husk biochar | 38.75 | [17] |
| Sheep manure biochar | 42.513~45.563 | [18] |
| Biochar derived from date palm petioles | 461 | [21] |
| Waste bamboo-derived biochar | 344.8 | [49] |
| Zinc-loaded chitosan biochar | 120.92 | [50] |
| EDTA and chitosan bi-functionalized magnetic bamboo biochar | 305.4 | [51] |
| Pine cone biochar activated with KOH | 109.5 | [52] |
| Biochar of the secondary medicinal residue of snow lotus | 480~640 | [53] |
| Sulfur-modified nZVI supported on biochar composite | 622.58 | [54] |
| Activated biochar derived from pomelo peel wastes | 163.105 | [55] |
| Multi-porous biochar from lotus biomass | 320 | [56] |
| Fe3O4-modified magnetic mesoporous biochar | 392.06 | [57] |
| Mg/Al-LDHs–biochar hybrid materials | 75.98 | [58] |
| Chitosan–PABC composite microspheres | 495.0~554.3 | This work |
| T (K) | ΔG (KJ/mol) | ΔH (KJ/mol) | ΔS (KJ/(mol·K)) |
|---|---|---|---|
| 298.15 | −7.47 | 12.52 | 0.067 |
| 308.15 | −8.14 | ||
| 318.15 | −8.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Yin, Y.; Zhang, X.; Qian, A.; Pan, X.; Liu, F.; Li, R. Enhanced Adsorption of Methyl Orange from Aqueous Phase Using Chitosan–Palmer Amaranth Biochar Composite Microspheres. Molecules 2024, 29, 1836. https://doi.org/10.3390/molecules29081836
Chen G, Yin Y, Zhang X, Qian A, Pan X, Liu F, Li R. Enhanced Adsorption of Methyl Orange from Aqueous Phase Using Chitosan–Palmer Amaranth Biochar Composite Microspheres. Molecules. 2024; 29(8):1836. https://doi.org/10.3390/molecules29081836
Chicago/Turabian StyleChen, Guiling, Yitong Yin, Xianting Zhang, Andong Qian, Xiaoyang Pan, Fei Liu, and Rui Li. 2024. "Enhanced Adsorption of Methyl Orange from Aqueous Phase Using Chitosan–Palmer Amaranth Biochar Composite Microspheres" Molecules 29, no. 8: 1836. https://doi.org/10.3390/molecules29081836