Preparation and Anti-Icing Properties of Zirconia Superhydrophobic Coating
Abstract
:1. Introduction
2. Results and Discussion
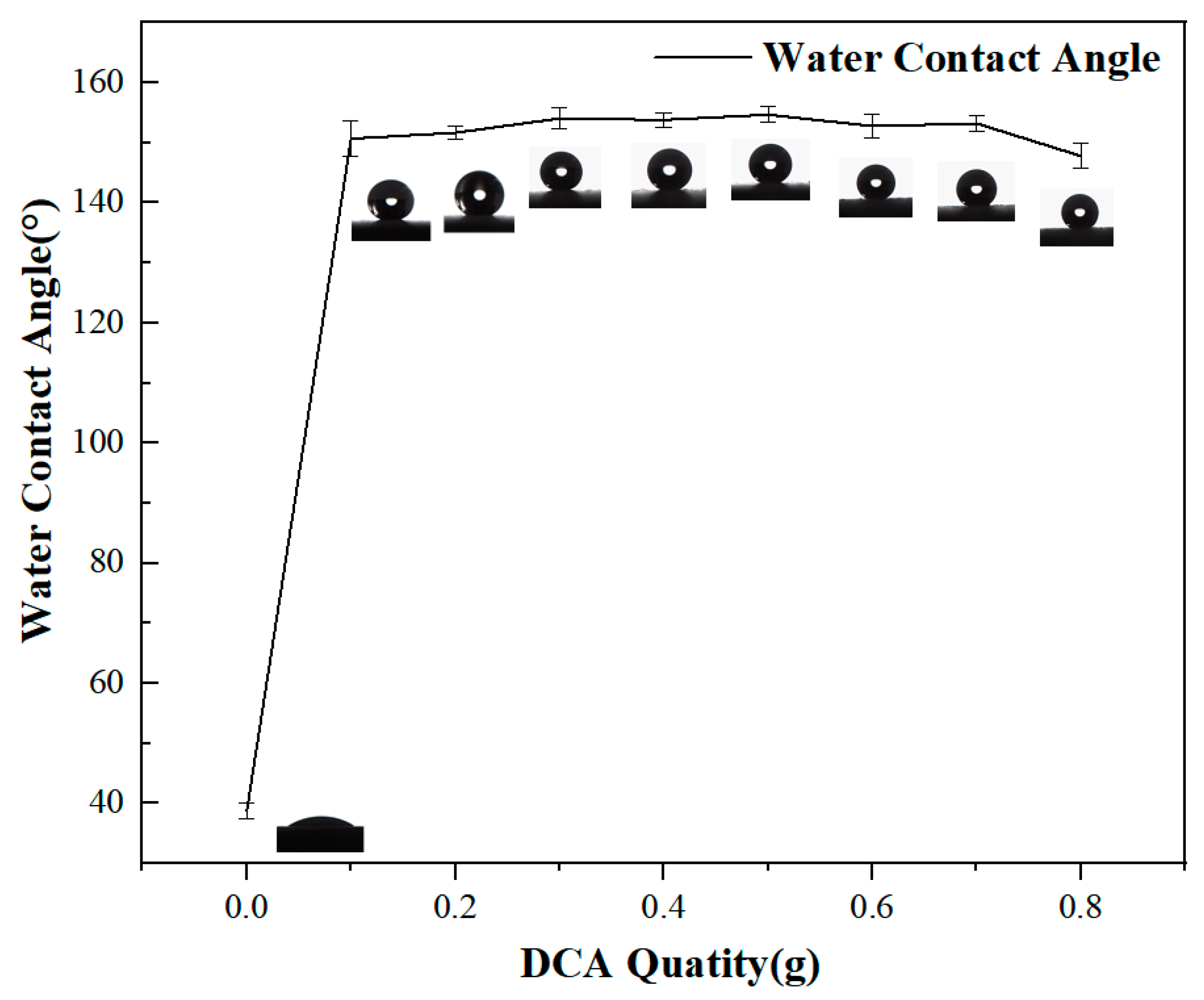
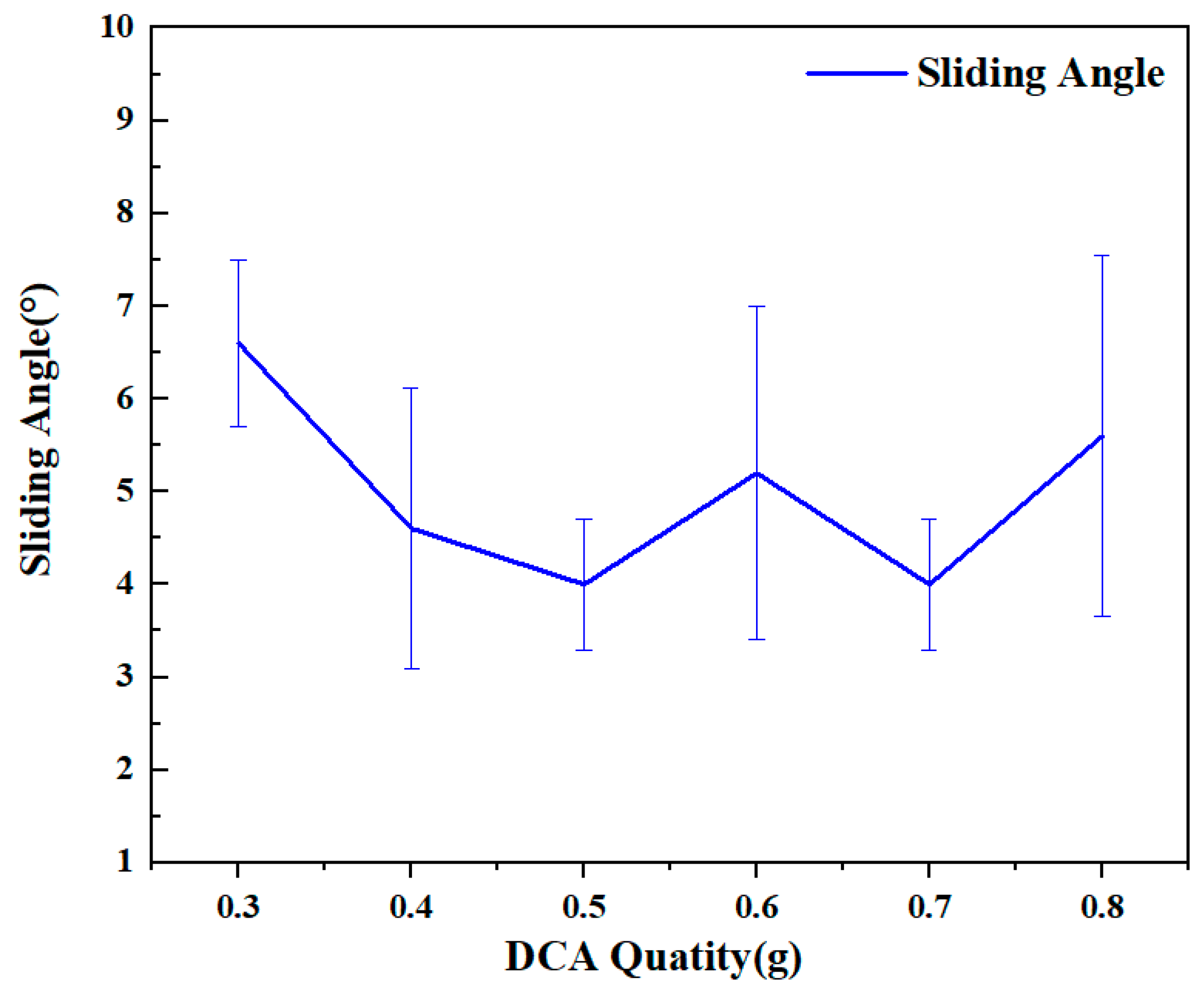
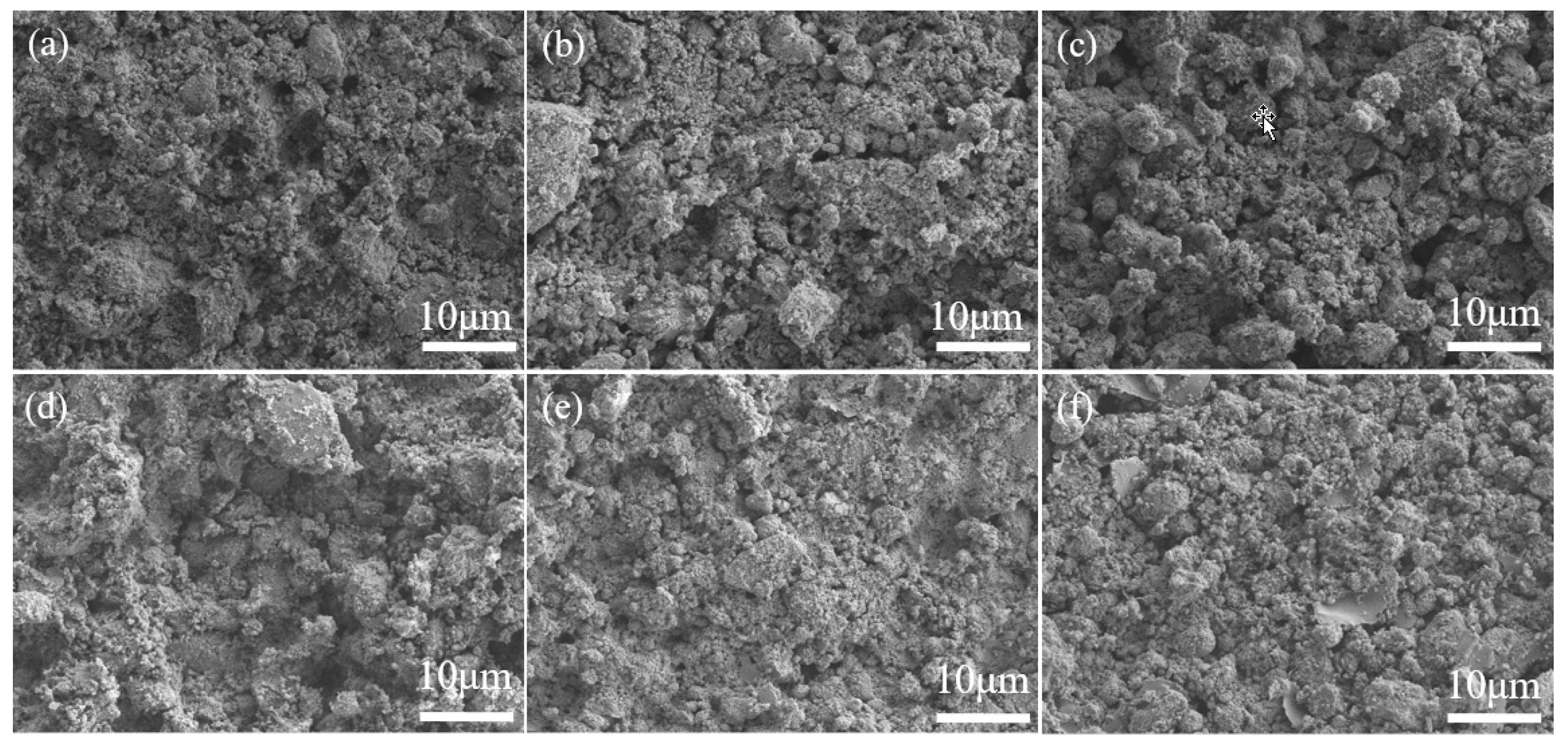
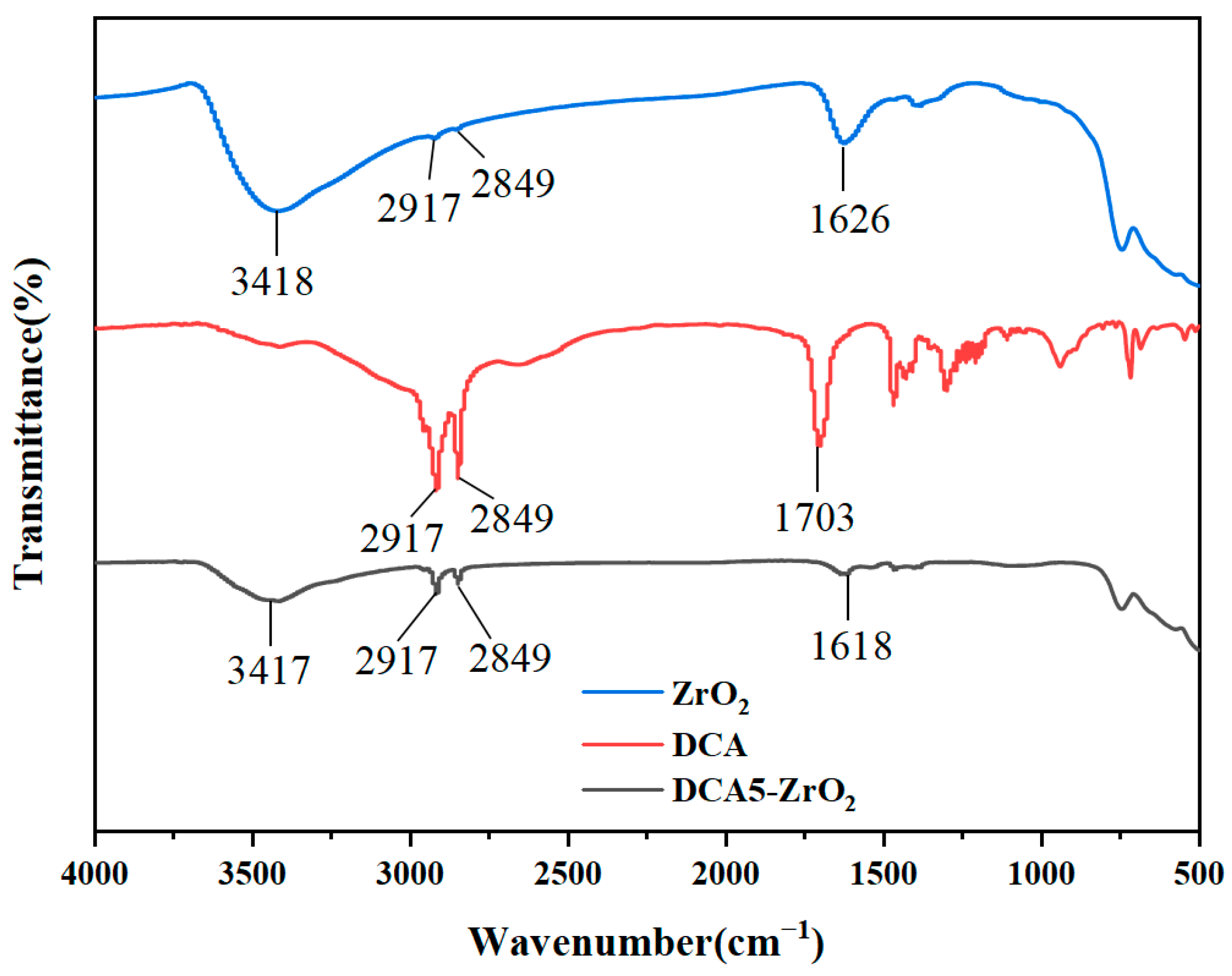
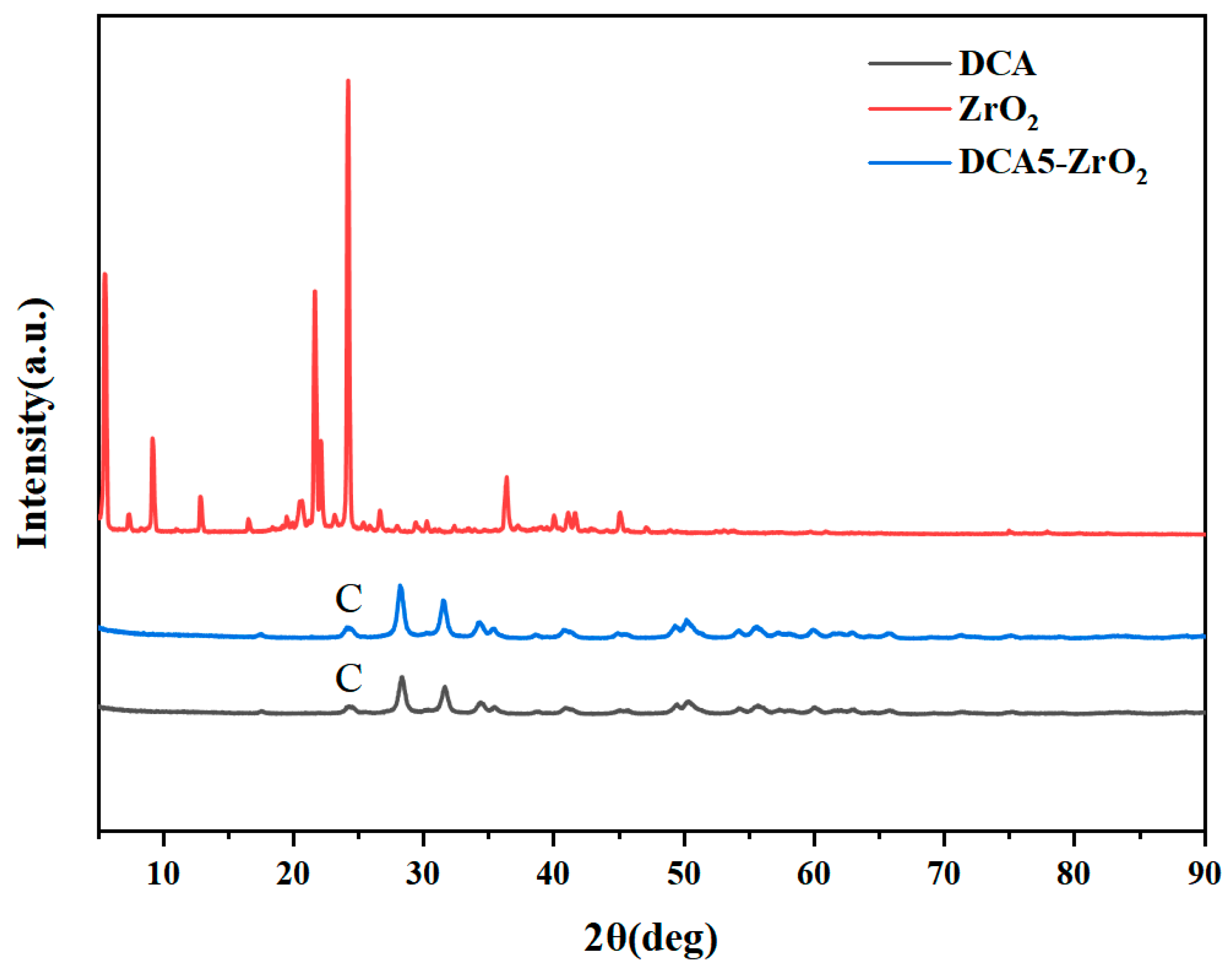
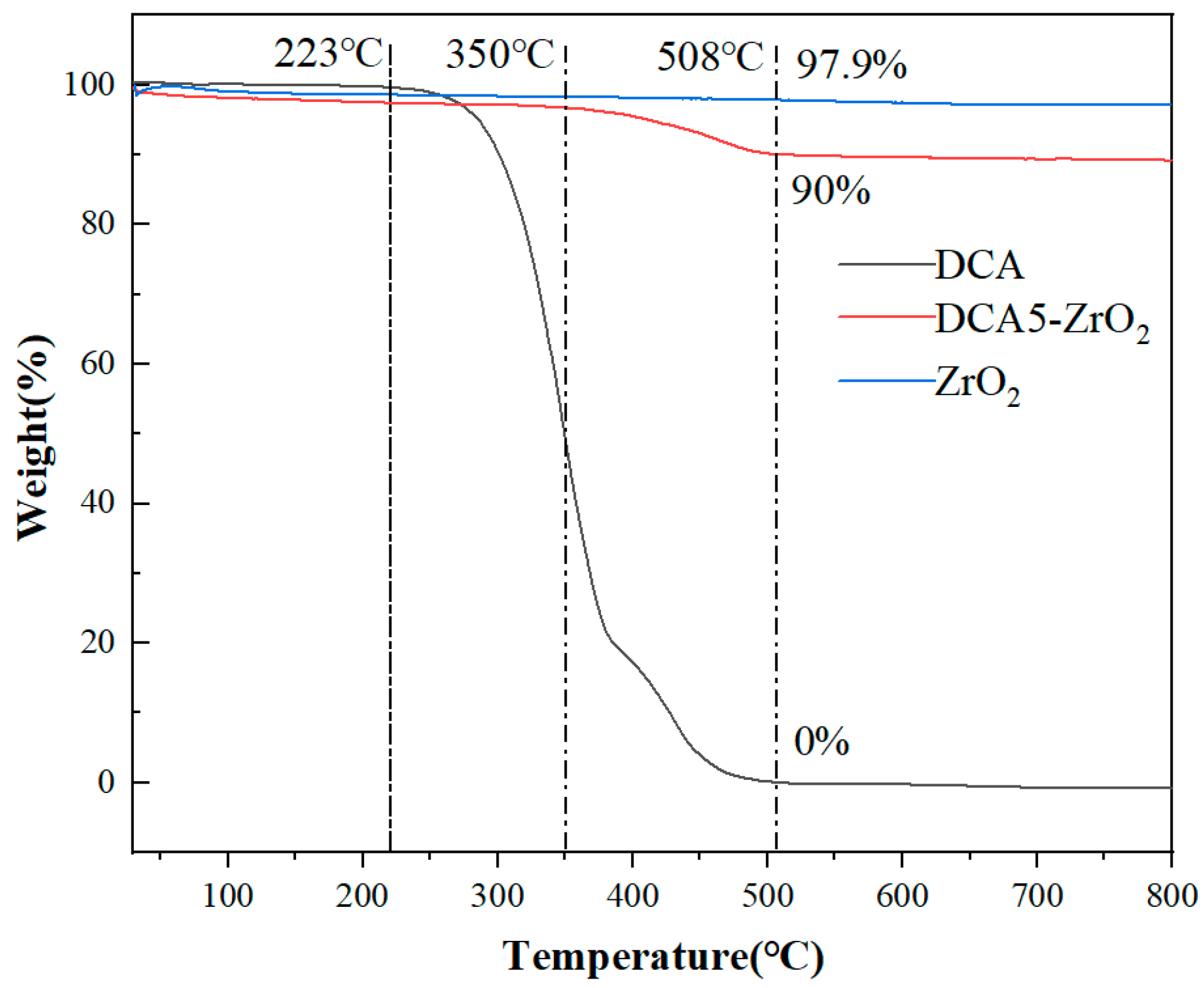
2.1. Surface Preparation and Characterization
2.2. Surface Self-Cleaning Evaluation
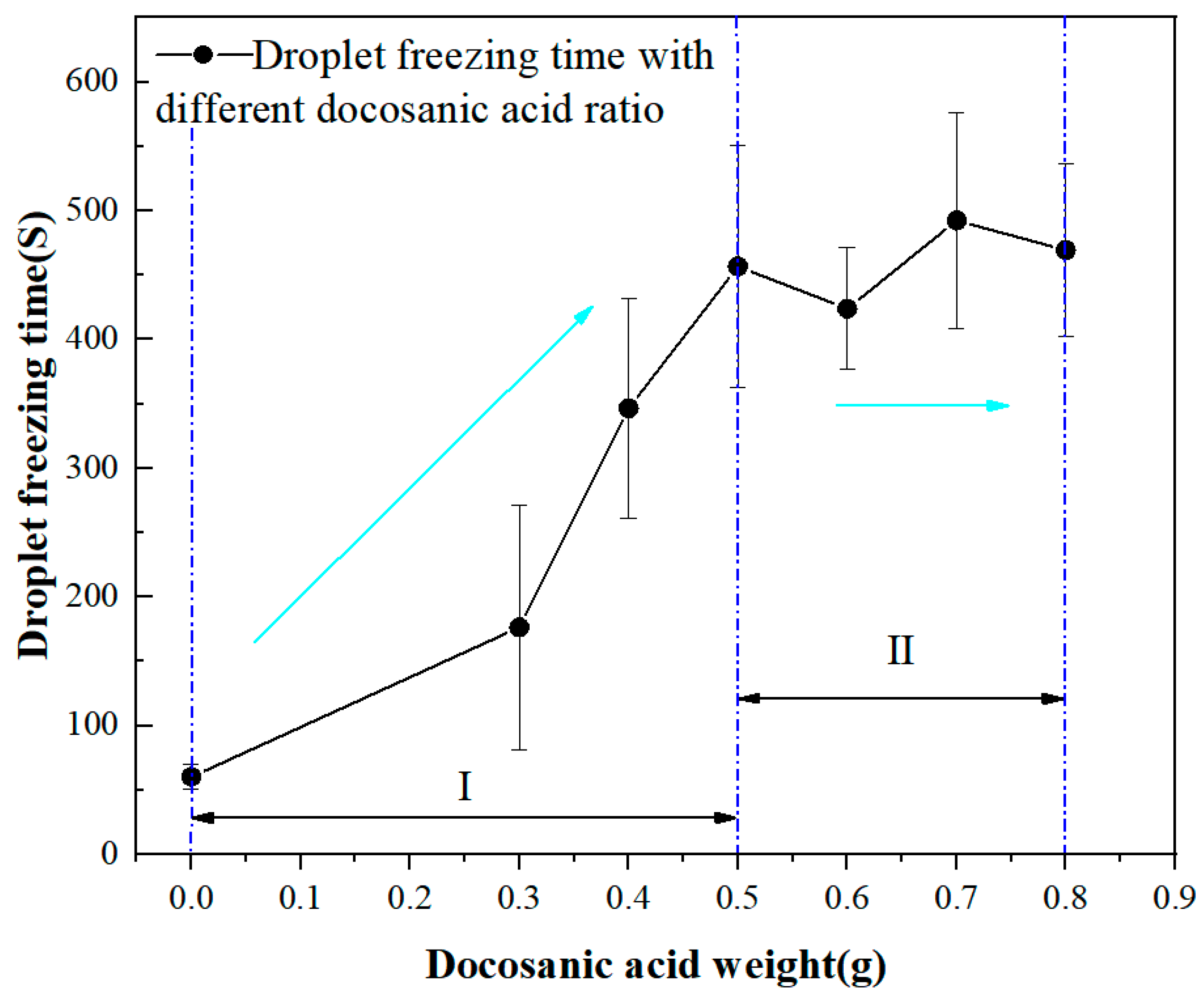
2.3. Droplet Freezing Experiments
3. Experimental Section
3.1. Materials
3.2. Preparation of the Superhydrophobic Surface
3.3. Characterization
3.4. Analysis of Self-Cleaning Performance
3.5. Droplet Freezing Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Lu, Z.C.; Yang, S.; Cheng, H.; Yu, H. Effects of powder size and pre-sintering heating rate on dental recycled zirconia. Zhonghua Kou Qiang Yi Xue Za Zhi = Zhonghua Kouqiang Yixue Zazhi = Chin. J. Stomatol. 2022, 57, 516–522. [Google Scholar]
- Kumar, A.; Kumar, P.; Dhaliwal, A.S. Phase transformation behavior of Ca-doped zirconia sintered at different temperatures. J. Korean Ceram. Soc. 2022, 59, 370–382. [Google Scholar] [CrossRef]
- Anastasaki, A. Failure Modes of Different Zirconia Crown Heights and Materials on Titanium Base Abutments. Master’s Thesis, University Of Alabama At Birmingham, Birmingham, AL, USA, 2023. [Google Scholar]
- Humire, E.N.; Jam, M.; Bjork, E. Investigation of influence of superhydrophilic and superhydrophobic coated aluminum surfaces on frost formation. ASHRAE Trans. 2022, 128, 357–365. [Google Scholar]
- Byun, S.; Jeong, H.; Kim, D.R.; Lee, K.-S. Frost layer growth behavior on ultra-low temperature surface with a superhydrophobic coating. Int. Commun. Heat Mass Transf. 2021, 128, 105641. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S.; Ge, Z.; Li, J.; Xie, J.; Xu, J.; Xie, Z.; Yu, K. Study on Delaying Frost Growth Performance of Micro-Nanostructure Superhydrophobic Copper Surfaces. Pol. J. Environ. Stud. 2022, 32, 943–951. [Google Scholar] [CrossRef]
- Zhang, T.; Deng, J.; Zhang, L.-Z. A photothermal self-healing superhydrophobic coating with anti-frosting and anti-corrosion properties. Prog. Org. Coatings Int. Rev. J. 2023, 180, 107569. [Google Scholar] [CrossRef]
- Sutar, R.S.; Kalel, P.J.; Latthe, S.S.; Kumbhar, D.A.; Xing, R. Superhydrophobic PVC/SiO2 coating for self-cleaning application. Macromol. Symp. 2020, 393, 2000034. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, Y.; Zhang, X.; Gao, G.; Shi, C.; Huang, G.; Li, P.; Kang, Q.; Huang, X.; Wu, G. Self-cleaning of superhydrophobic nanostructured surfaces at low humidity enhanced by vertical electric field. Nano Res. 2022, 15, 4732–4738. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Tao, J.; Liu, S.; Jiang, J.; Xu, Y.; Liu, W.; Li, H. An integrated superhydrophobic anti/de-icing film heater with low energy consumption: Interpenetration behavior of components based on wet-film spraying method. Appl. Therm. Eng. 2023, 223, 120028. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Liang, W.; He, L.; Wang, F.; Zhu, D.; Zhao, H. In Situ Activation of Superhydrophobic Surfaces with Triple Icephobicity at Low Temperatures. ACS Appl. Mater. Interfaces 2022, 14, 49352–49361. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, X.; Xu, Y.; Weng, Z.; Liao, L.; Wang, X. A sprayable superhydrophobic dental protectant with photo-responsive anti-bacterial, acid-resistant, and anti-fouling functions. Nano Res. 2022, 15, 5245–5255. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Tesler, A.B.; Kim, P.; Kolle, S.; Howell, C.; Ahanotu, O.; Aizenberg, J. Extremely durable biofouling-resistant metallic surfaces based on electrodeposited nanoporous tungstite films on steel. Nat. Commun. 2015, 6, 8649. [Google Scholar] [CrossRef]
- Shen, Y.; Li, Y.; He, Y.; Zhang, C.; Xie, C.; Zhang, H.; Han, E. Preparation of high performance superhydrophobic PVDF-PbO2-ZrO2 composite electrode and its application in the degradation of paracetamol and industrial oily wastewater. J. Electroanal. Chem. 2022, 911, 116231. [Google Scholar] [CrossRef]
- Maharana, H.; Basu, A.; Mondal, K. Effect of CTAB on the architecture and hydrophobicity of electrodeposited Cu–ZrO2 nano-cone arrays. Surf. Coatings Technol. 2019, 375, 323–333. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, C.; Chen, A.; Li, Y.; Chen, S.; Sun, D.; Wang, C.; Liu, Z.; Wang, Q.; Huang, J.; et al. Large-scale, abrasion-resistant, and solvent-free superhydrophobic objects fabricated by a selective laser sintering 3D printing strategy. Adv. Sci. 2023, 10, 2207183. [Google Scholar] [CrossRef]
- Chen, Y.; Long, J.; Xie, B.; Kuang, Y.; Chen, X.; Hou, M.; Gao, J.; Liu, H.; He, Y.; Wong, C.-P. One-Step Ultraviolet Laser-Induced Fluorine-Doped Graphene Achieving Superhydrophobic Properties and Its Application in Deicing. ACS Appl. Mater. Interfaces 2022, 14, 4647–4655. [Google Scholar] [CrossRef]
- Yang, X.; Su, J.; Xiong, J.; Wang, H. A self-healing fluorine-free superhydrophobic cotton fabric under heat stimulation. Text. Res. J. 2022, 92, 3049–3059. [Google Scholar] [CrossRef]
- Cheng, Q.-Y.; Guan, C.-S.; Wang, M.; Li, Y.-D.; Zeng, J.-B. Cellulose nanocrystal coated cotton fabric with superhydrophobicity for efficient oil/water separation. Carbohydr. Polym. 2018, 199, 390–396. [Google Scholar] [CrossRef]
- Li, K.; Xu, L.; Yuan, X.; Pan, H.; Wang, L.; Shen, Y.; Li, T.; Li, J. Preparation of self-healing superhydrophobic cotton fabric based on silica aerogel for self-cleaning and oil/water separation. J. Adhes. Sci. Technol. 2023, 37, 2154–2174. [Google Scholar] [CrossRef]
- Gong, A.; Zheng, Y.; Yang, Z.; Guo, X.; Gao, Y.; Li, X. Spray fabrication of superhydrophobic coating on aluminum alloy for corrosion mitigation. Mater. Today Commun. 2021, 26, 101828. [Google Scholar] [CrossRef]
- Yu, X.; Liu, X.; Shi, X.; Zhang, Z.; Wang, H.; Feng, L. SiO2 nanoparticle-based superhydrophobic spray and multi-functional surfaces by a facile and scalable method. Ceram. Int. 2019, 45, 15741–15744. [Google Scholar] [CrossRef]
- Caldona, E.B.; Sibaen, J.W.; Tactay, C.B.; Mendiola, S.L.D.; Abance, C.B.; Añes, M.P.; Serrano, F.D.D.; De Guzman, M.M.S. Preparation of spray-coated surfaces from green-formulated superhydrophobic coatings. SN Appl. Sci. 2019, 1, 1657. [Google Scholar] [CrossRef]
- He, L.; Wang, D.; Ma, T.; Song, J.; Wu, Y.; Li, Y.; Deng, Y.; Zhang, G. Processing and properties of a graphene-reinforced superhydrophobic siloxane. Mater. Des. 2023, 229, 111856. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Z.; Zhou, C.; Lin, Y.; Hou, L.; Shi, L.; Zhong, S. 3D-Printed Robust Dual Superlyophobic Ti-Based Porous Structure for Switchable Oil/Water Emulsion Separations. Adv. Funct. Mater. 2023, 33, 2212262. [Google Scholar] [CrossRef]
- Wang, C.; Tian, F.; Zhang, X. Feasible fabrication of durable superhydrophobic SiO2 coatings with translucency and self-cleaning performance. Mater. Res. Express 2020, 7, 106403. [Google Scholar] [CrossRef]
- Iqbal, A.; Saidu, U.; Adam, F.; Sreekantan, S.; Yahaya, N.; Ahmad, M.N.; Ramalingam, R.J.; Wilson, L.D. Floating ZnO QDs-modified TiO2/LLDPE hybrid polymer film for the effective photodegradation of tetracycline under fluorescent light irradiation: Synthesis and characterisation. Molecules 2021, 26, 2509. [Google Scholar] [CrossRef]
- Xu, P.; Sui, X.; Wang, S.; Liu, G.; Ge, A.; Coyle, T.W.; Mostaghimi, J. Superhydrophobic ceramic coatings with lotus leaf-like hierarchical surface structures deposited via suspension plasma spray process. Surf. Interfaces 2023, 38, 102780. [Google Scholar] [CrossRef]
- Eduok, U. New superhydrophobic and self-cleaning zirconia/polydimethylsiloxane nanocomposite coated cotton fabrics. New J. Chem. 2021, 45, 638–650. [Google Scholar] [CrossRef]
- Bangi, U.K.; Ransing, A.A.; Lee, K.-Y.; Park, H.-H. Self-cleaned zirconia coatings prepared using a co-precursor sol–gel method. Surf. Eng. 2021, 37, 1059–1066. [Google Scholar] [CrossRef]
- Prasanth, V.G.; Prasad, G.; Kiran, T.; Rathore, R.S.; Pathak, M.; Sathiyanarayanan, K.I. Synthesis, spectral characterization and crystal structure of a new precursor [(CH3COCHCOCH3)2Zr{C6H4(N=CHC6H4O)2}] for nano-zirconia: An investigation on the wettability of polyvinylidene fluoride-nano-zirconia composite material. J. Sol-Gel Sci. Techn. 2015, 76, 195–203. [Google Scholar] [CrossRef]
- Yang, J.; Chen, A.; Liu, F.; Gu, L.; Xie, X.; Ding, Z. Hybrid coating of polydimethylsiloxane with nano-ZrO2 on magnesium alloy for superior corrosion resistance. Ceram. Int. 2022, 48, 35280–35289. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, M.-J.; Ouyang, X.; Zhang, A.P.; Tam, H.-Y. 3D μ-printing of polytetrafluoroethylene microstructures: A route to superhydrophobic surfaces and devices. Appl. Mater. Today 2020, 19, 100580. [Google Scholar] [CrossRef]
- Zhang, H.; Gan, J.; Wu, Y.; Wu, Z. Biomimetic high water adhesion superhydrophobic surface via UV nanoimprint lithography. Appl. Surf. Sci. 2023, 633, 157610. [Google Scholar] [CrossRef]
- Yu, M.; Yang, L.; Yan, L.; Wang, T.; Wang, Y.; Qin, Y.; Xiong, L.; Shi, R.; Sun, Q. ZnO nanoparticles coated and stearic acid modified superhydrophobic chitosan film for self-cleaning and oil–water separation. Int. J. Biol. Macromol. 2023, 231, 123293. [Google Scholar] [CrossRef]
- Li, J.; Gao, R.; Wang, Y.; Zhang, T.C.; Yuan, S. Superhydrophobic palmitic acid modified Cu(OH)2/CuS nanocomposite-coated copper foam for efficient separation of oily wastewater. Colloid. Surface. A 2022, 637, 128249. [Google Scholar] [CrossRef]
- Abd-El-Nabey, B.A.; Ashour, M.; Aly, A.M.; Mohamed, M.E. Fabrication of Robust Superhydrophobic Nickel Films on Steel Surface with High Corrosion Resistance, Mechanical and Chemical Stability. J. Eng. Mater. Technol. 2022, 144, 021007. [Google Scholar] [CrossRef]
- Al-Mohammadi, A.-R.; Ibrahim, R.A.; Moustafa, A.H.; Ismaiel, A.A.; Zeid, A.A.; Enan, G. Chemical Constitution and Antimicrobial Activity of Kefir Fermented Beverage. Molecules 2021, 26, 2635. [Google Scholar] [CrossRef] [PubMed]
- Ni, R.; Bhandari, S.; Mitchell, P.R.; Suarez, G.; Patel, N.B.; Lamb, K.; Bisht, K.S.; Merkler, D.J. Synthesis, Quantification, and Characterization of Fatty Acid Amides from In Vitro and In Vivo Sources. Molecules 2021, 26, 2543. [Google Scholar] [CrossRef]
- Tang, W.; Cheng, Y.; Jian, Y.; Sun, Y.; Xiao, J.; Yi, L.; Zhang, H.; Xu, T.; Zhang, Y.; Liu, J.; et al. Synergetic strategy to fabricate superhydrophobic wood by MTMS for improving dimensional stability, durability and self-cleaning ability. Mater. Lett. 2023, 343, 134348. [Google Scholar] [CrossRef]
- Zhu, M.; Yuan, R.; Wang, C.; Gao, Q.; Wang, H.; Qian, H. Fabrication and performance study of a superhydrophobic anti-scaling and anti-corrosion coating. Appl. Surf. Sci. 2023, 615, 156287. [Google Scholar] [CrossRef]
- Pan, Y.; Kong, W.; Bhushan, B.; Zhao, X. Rapid, ultraviolet-induced, reversibly switchable wettability of superhydrophobic/superhydrophilic surfaces. Beilstein J. Nanotechnol. 2019, 10, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Polonskaya, Y.V.; Shramko, V.S.; Morozov, S.V.; Chernyak, E.I.; Chernyavsky, A.M.; Ragino, Y.I. Balance of Fatty Acids and Their Correlations with Parameters of Lipid Metabolism and Markers of Inflammation in Men with Coronary Atherosclerosis. Bull. Exp. Biol. Med. 2017, 164, 33–35. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zheng, H.; Sheng, W.; Hao, X.; Zhang, X. Preparation and Anti-Icing Properties of Zirconia Superhydrophobic Coating. Molecules 2024, 29, 1837. https://doi.org/10.3390/molecules29081837
Zhou J, Zheng H, Sheng W, Hao X, Zhang X. Preparation and Anti-Icing Properties of Zirconia Superhydrophobic Coating. Molecules. 2024; 29(8):1837. https://doi.org/10.3390/molecules29081837
Chicago/Turabian StyleZhou, Jiahui, Haikun Zheng, Wei Sheng, Xiaoru Hao, and Xinmin Zhang. 2024. "Preparation and Anti-Icing Properties of Zirconia Superhydrophobic Coating" Molecules 29, no. 8: 1837. https://doi.org/10.3390/molecules29081837




