Terroir Dynamics: Impact of Vineyard and Canopy Treatment with Chitosan on Anthocyanins, Phenolics, and Volatile and Sensory Profiles of Pinot Noir Wines from South Tyrol
Abstract
:1. Introduction
2. Results and Discussion
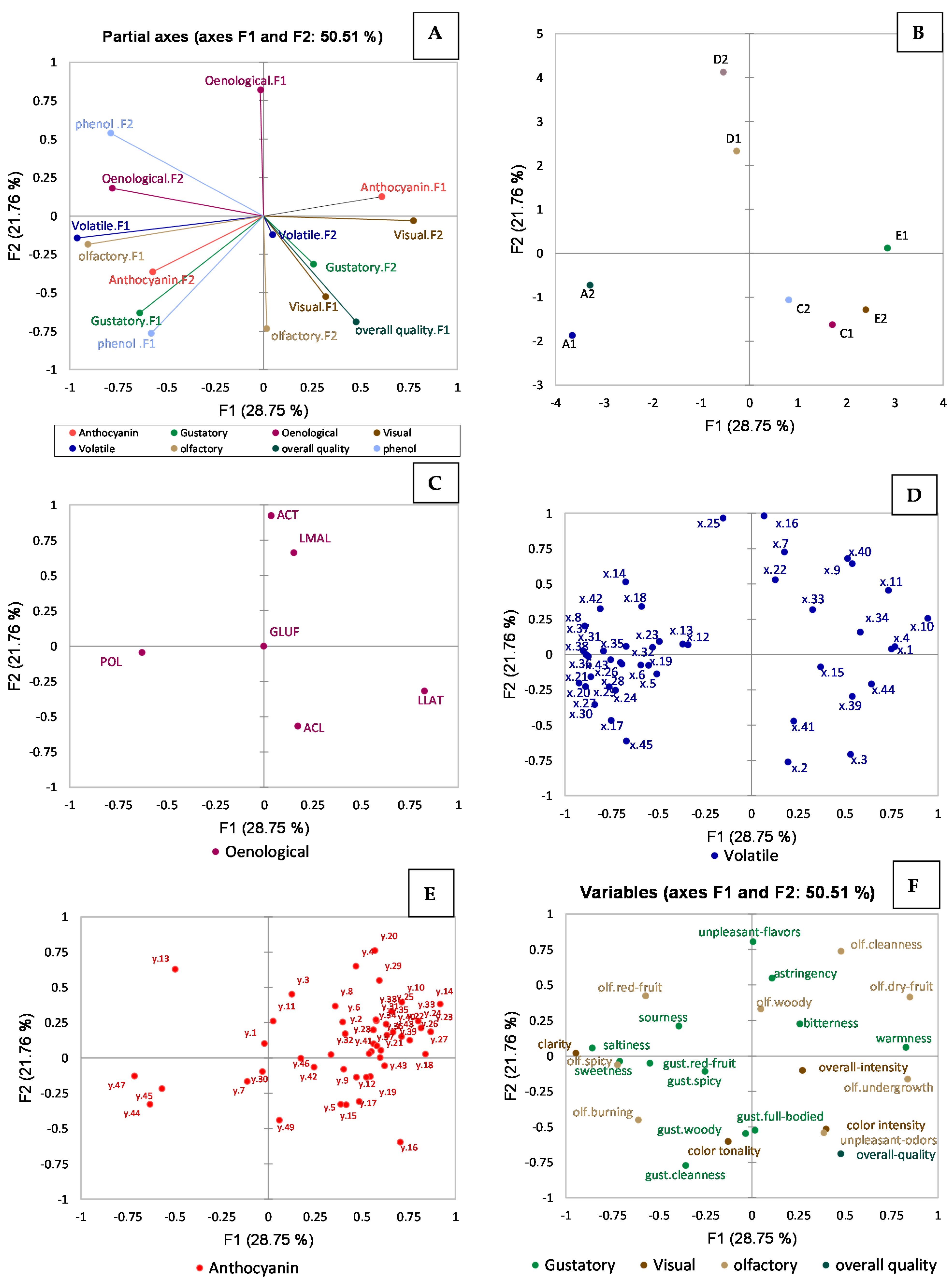
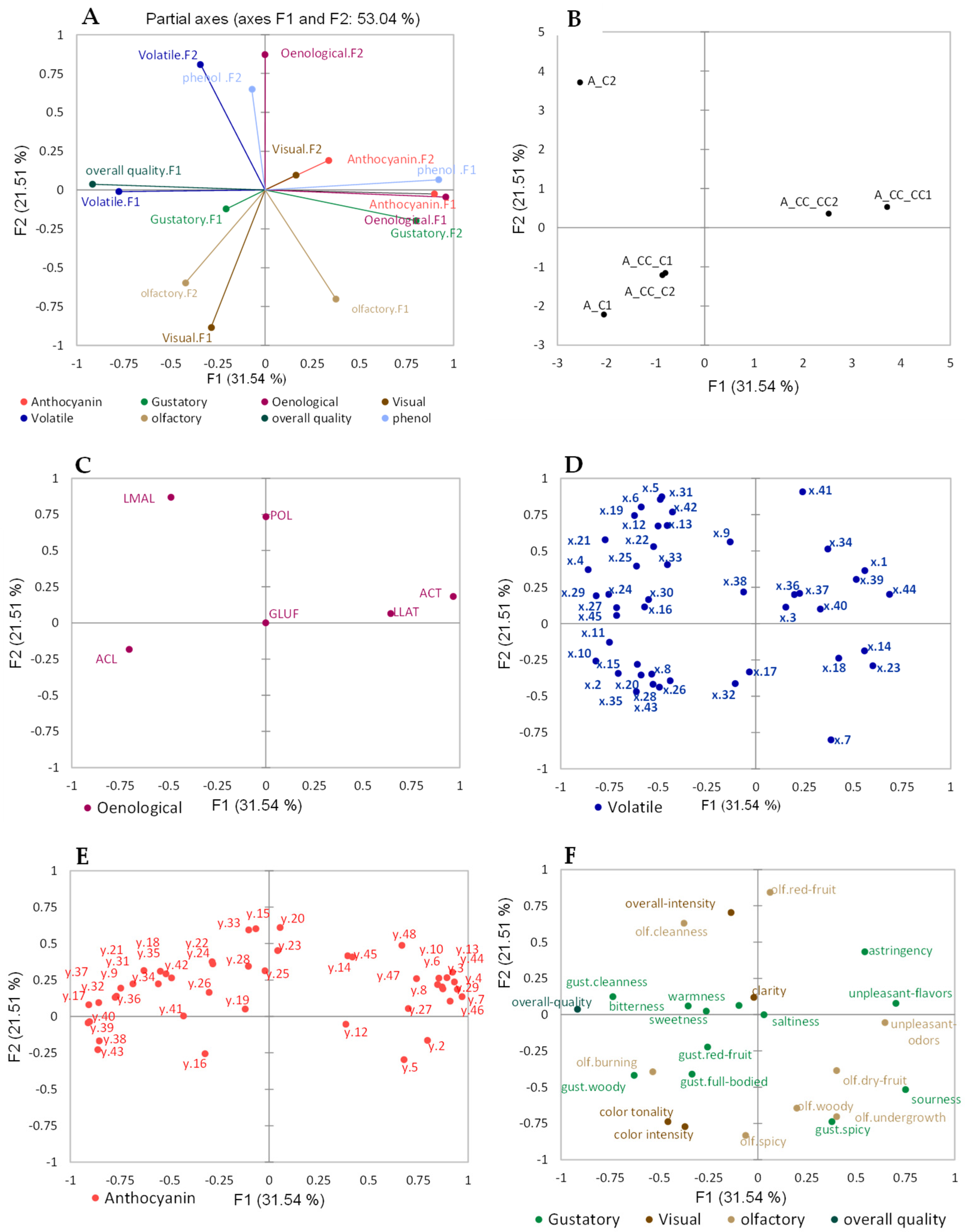
2.1. Multiple Factor Analysis (MFA)
2.1.1. Effect of Vineyard Location
2.1.2. Effect of Chitosan Treatment on Mazzon Vineyard
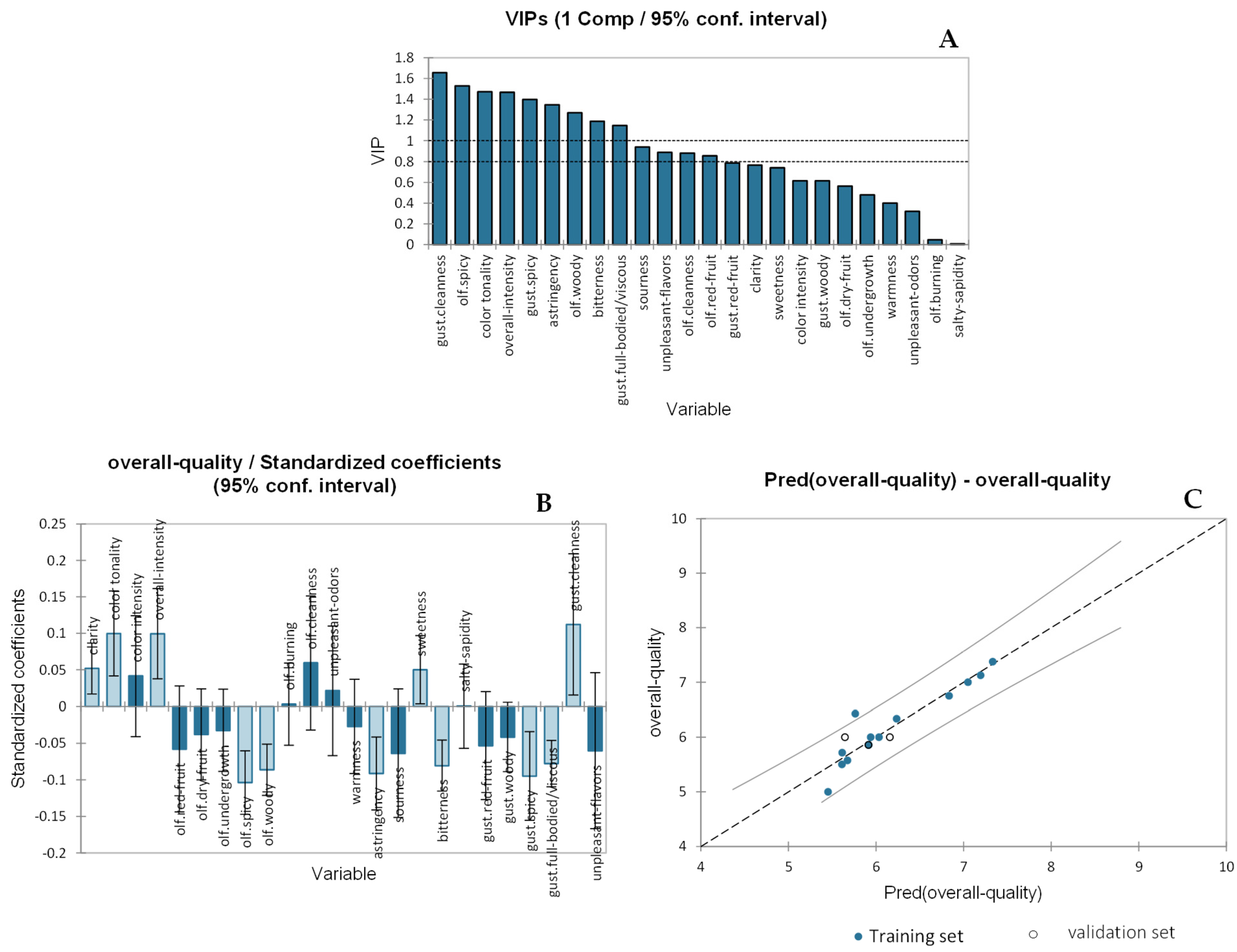
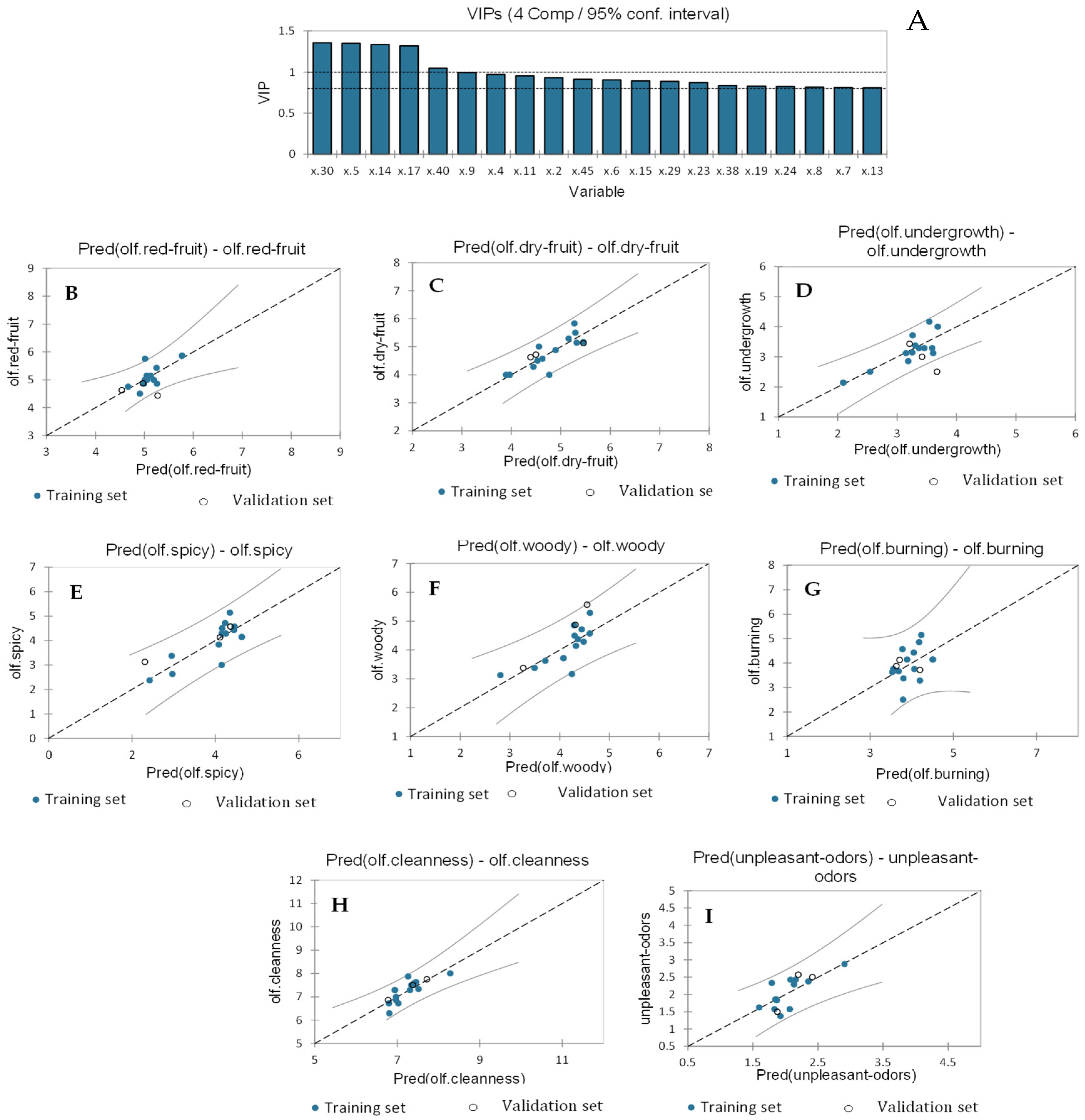
2.2. Regression Models
2.2.1. Partial Least Squares Regression (PLS-R) for the Overall Quality Judgement (OQJ) of Wines
2.2.2. Partial Least Squares Regression to Identify the Volatile Compounds Responsible for the Aroma Sensory Attributes of Wines
2.2.3. Partial Least Squares Regression to Identify Non-Volatile Compounds and Enological Parameters Responsible for the Visual and Gustatory Sensory Attributes of Wines
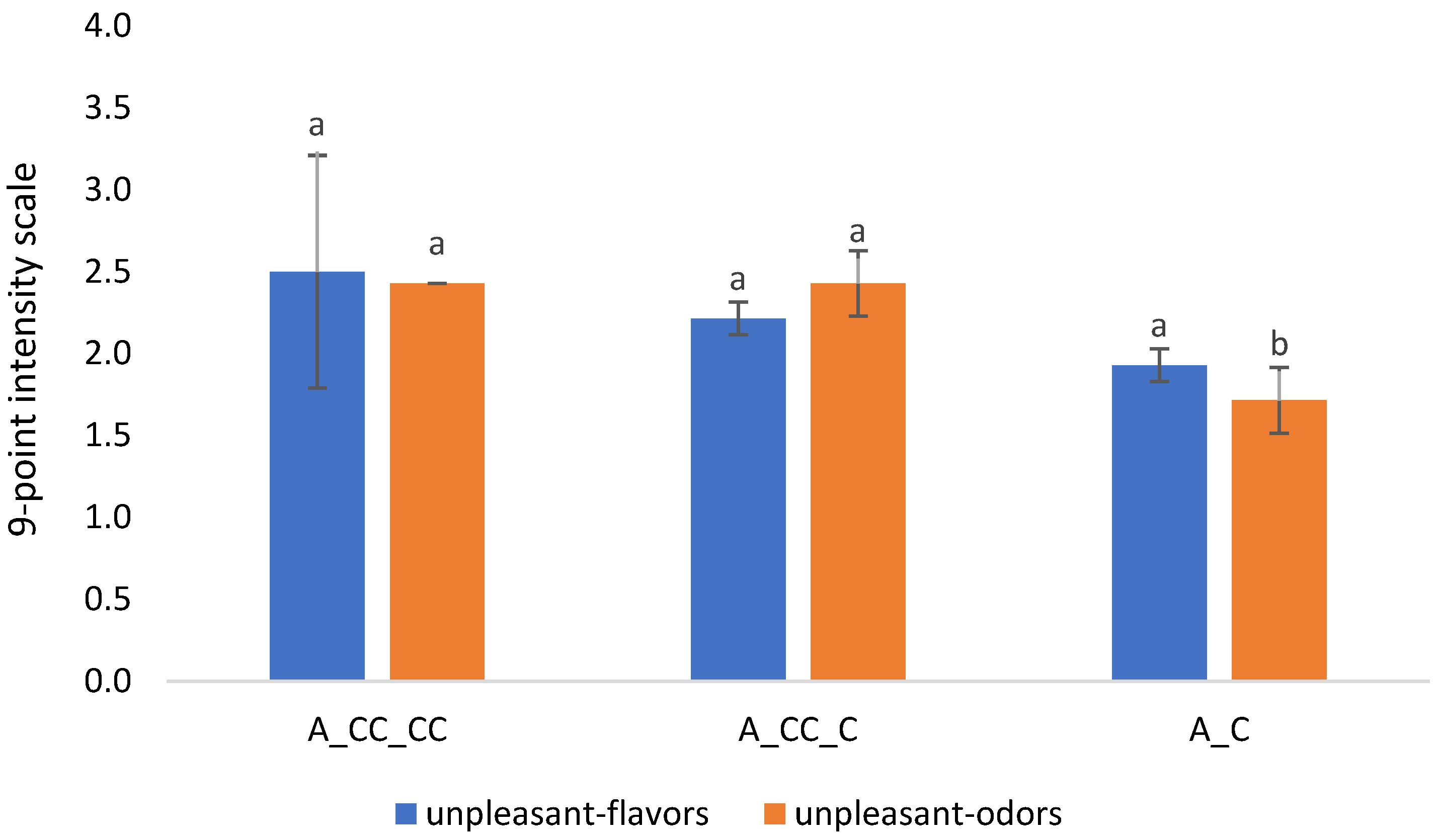
2.3. Analysis of Variance (ANOVA)
2.3.1. Effect of the Vineyards
2.3.2. Effect of Chitosan Treatment on Mazzon Vineyard
3. Materials and Methods
3.1. Vineyards and Treatments on Canopy
3.2. Winemaking
3.3. Analytical and Statistical Methods
3.3.1. General Enological Parameters
3.3.2. HPLC Analysis of Non-Volatile Profile
3.3.3. HS-SPME-GCxGC-ToF/MS
3.3.4. Sensory Analysis
3.3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camera di Commercio di Bolzano. 2020. Available online: https://www.camcom.bz.it/sites/default/files/uploaded_files/Agricoltura/Anbaufl%C3%A4che_nach_Sorten_2019_dt_ital.xlsx (accessed on 11 June 2023).
- OIV. Vineyard Surface Area in European Countries in 2022* (in 1000 Hectares) [Graph]. In Statista. 20 April 2023. Available online: https://www.statista.com/statistics/1247482/vineyard-surface-area-europe (accessed on 27 November 2023).
- Rudari, E. Chemical and Sensory Profiles of South Tyrolean Pinot Noir: Role of Vineyard Location and Treatment with Chitosan on Canopy. Bachelor’s Thesis, Free University of Bolzano-Bozen, Bolzano, Italy, 2023. [Google Scholar]
- Mazzocchi, A. Variability of the Phenolic Components in the Early Winemaking Steps of Pinot Noir. Master’s Thesis, Università degli studi di Padova, Udine, Verona e Libera Università di Bolzano, Padova, Italy, 2020. [Google Scholar]
- Galet, P. Cépages et Vignobles de France; Ministère de la Recherche et de la Technologie: Paris, France, 1990; Volume II. [Google Scholar]
- Geană, E.I.; Ionete, R.E.; Niculescu, V.; Artem, V.; Ranca, A. Changes in polyphenolic content of berry skins from different red grapes cultivars during ripening. Smart Energy Sustain. Environ. 2016, 19, 95. [Google Scholar]
- Boursiquot, J.M.; Charmont, S.; Dufour, M.C.; Moulliet, C.; Ollat, N.; Audeguin, L.; Sereno, C.; Desperrier, J.M.; Jacquet, O.; Lacombe, T.; et al. Catalogue des Variétés et Clones de Vigne Cultivés en France; IFV: Le Grau-du-Roi, France, 2007. [Google Scholar]
- Merkytė, V.; De Matos, A.D.; Longo, E.; Betnga, P.T.; Boselli, E. Cyclic proanthocyanidins in Pinot Noir wine. Ital. J. Food Sci. 2020, 32. [Google Scholar] [CrossRef]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Martins, V.; Soares, B.; Castro, I.; Falco, V. Chitosan application in vineyards (Vitis vinifera L. cv. Tinto Cão) induces accumulation of anthocyanins and other phenolics in berries, mediated by modifications in the transcription of secondary metabolism genes. Int. J. Mol. Sci. 2020, 21, 306. [Google Scholar] [CrossRef]
- Tessarin, P.; Chinnici, F.; Donnini, S.; Liquori, E.; Riponi, C.; Rombolà, A.D. Influence of canopy-applied chitosan on the composition of organic cv. Sangiovese and Cabernet Sauvignon berries and wines. Food Chem. 2016, 210, 512–519. [Google Scholar] [CrossRef]
- Iriti, M.; Vitalini, S.; Di Tommaso, G.; D’Amico, S.; Borgo, M.; Faoro, F. New chitosan formulation prevents grapevine powdery mildew infection and improves polyphenol content and free radical scavenging activity of grape and wine. Aust. J. Grape Wine Res. 2011, 17, 263–269. [Google Scholar] [CrossRef]
- Artem, V.; Antoce, A.O.; Geana, E.I.; Ranca, A. Effect of foliar treatment with chitosan on phenolic composition of῾ Fetească neagră’grapes and wines. Not. Bot. Horti Agrobot. Cluj Napoca 2023, 51, 12920. [Google Scholar] [CrossRef]
- Reglinski, T.; Elmer, P.A.G.; Taylor, J.T.; Wood, P.N.; Hoyte, S.M. Inhibition of Botrytis cinerea growth and suppression of botrytis bunch rot in grapes using chitosan. Plant Pathol. 2010, 59, 882–890. [Google Scholar] [CrossRef]
- Duxbury, M.; Hotter, G.; Reglinski, T.; Sharpe, N. Effect of chitosan and 5- chlorosalicylic acid on total phenolic content of grapes and wine. Am. J. Enol. Vitic. 2004, 55, 191–194. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Zanzotto, A.; Fico, G.; Faoro, F.; Simonetti, P.; Iriti, M. From vineyard to glass: Agrochemicals enhance the melatonin and total polyphenol contents and antiradical activity of red wines. J. Pineal Res. 2011, 51, 278–285. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of grape and wine phenolic content by foliar application to grapevine of three different elicitors: Methyl jasmonate, chitosan, and yeast extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Soares, B.; Goufo, P.; Castro, I.; Cosme, F.; Pinto-Sintra, A.L.; Inês, A.; Oliveira, A.A.; Falco, V. Chitosan upregulates the genes of the ROS pathway and enhances the antioxidant potential of grape (Vitis vinifera L.‘Touriga Franca’and’Tinto Cão’) tissues. Antioxidants 2019, 8, 525. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Singh, R.K.; Gomes, N.; Soares, B.G.; Silva, A.; Falco, V.; Capita, R.; Alonso-Calleja, C.; Pereira, J.E.; Amaral, J.S.; et al. Comparative insight upon chitosan solution and chitosan nanoparticles application on the phenolic content, antioxidant and antimicrobial activities of individual grape components of Sousão variety. Antioxidants 2020, 9, 178. [Google Scholar] [CrossRef]
- Vitalini, S.; Ruggiero, A.; Rapparini, F.; Neri, L.; Tonni, M.; Iriti, M. The application of chitosan and benzothiadiazole in vineyard (Vitis vinifera L. cv Groppello Gentile) changes the aromatic profile and sensory attributes of wine. Food Chem. 2014, 162, 192–205. [Google Scholar] [CrossRef]
- Gürbüz, O.; Rouseff, J.M.; Rouseff, R.L. Comparison of aroma volatiles in commercial Merlot and Cabernet Sauvignon wines using gas chromatography–olfactometry and gas chromatography− mass spectrometry. J. Agric. Food Chem. 2006, 54, 3990–3996. [Google Scholar] [CrossRef]
- Umano, K.; Hagi, Y.; Shibamoto, T. Volatile chemicals identified in extracts from newly hybrid citrus, dekopon (Shiranuhi mandarin Suppl. J.). J. Agric. Food Chem. 2002, 50, 5355–5359. [Google Scholar] [CrossRef] [PubMed]
- Le Guen, S.; Prost, C.; Demaimay, M. Characterization of odorant compounds of mussels (Mytilus edulis) according to their origin using gas chromatography–olfactometry and gas chromatography–mass spectrometry. J. Chromatogr. A 2000, 896, 361–371. [Google Scholar] [CrossRef]
- Tatsuka, K.; Suekane, S.; Sakai, Y.; Sumitani, H. Volatile constituents of kiwi fruit flowers: Simultaneous distillation and extraction versus headspace sampling. J. Agric. Food Chem. 1990, 38, 2176–2180. [Google Scholar] [CrossRef]
- Shimoda, M.; Shiratsuchi, H.; Minegishi, Y.; Osajima, Y. Flavor deterioration of nonfermented coarse-cut sausage during storage. Flavor as a factor of quality for nonfermented sausage. 2. J. Agric. Food Chem. 1993, 41, 946–950. [Google Scholar] [CrossRef]
- Ziegleder, G. Volatile and odorous compounds in unprinted paperboard. Packag. Technol. Sci. Int. J. 1998, 11, 231–239. [Google Scholar] [CrossRef]
- Li, H.; Tao, Y.S.; Wang, H.; Zhang, L. Impact odorants of Chardonnay dry white wine from Changli County (China). Eur. Food Res. Technol. 2008, 227, 287–292. [Google Scholar] [CrossRef]
- Cho, I.H.; Choi, H.K.; Kim, Y.S. Difference in the volatile composition of pine-mushrooms (Tricholoma matsutake Sing.) according to their grades. J. Agric. Food Chem. 2006, 54, 4820–4825. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. Essential oils of the genus Lophostemon (Myrtaceae). Flavour Fragr. J. 2000, 15, 17–20. [Google Scholar] [CrossRef]
- Chyau, C.C.; Ko, P.T.; Chang, C.H.; Mau, J.L. Free and glycosidically bound aroma compounds in lychee (Litchi chinensis Sonn.). Food Chem. 2003, 80, 387–392. [Google Scholar] [CrossRef]
- Umano, K.; Hagi, Y.; Nakahara, K.; Shoji, A.; Shibamoto, T. Volatile constituents of green and ripened pineapple (Ananas comosus [L.] Merr.). J. Agric. Food Chem. 1992, 40, 599–603. [Google Scholar] [CrossRef]
- Chung, H.Y.; Shing Yung, I.K.; Kim, J.S. Comparison of volatile components in dried scallops (Chlamys farreri and Patinopecten yessoensis) prepared by boiling and steaming methods. J. Agric. Food Chem. 2001, 49, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Paniandy, J.C.; Chane-Ming, J.; Pieribattesti, J.C. Chemical composition of the essential oil and headspace solid-phase microextraction of the guava fruit (Psidium guajava L.). J. Essent. Oil Res. 2000, 12, 153–158. [Google Scholar] [CrossRef]
- Ferrari, G.; Lablanquie, O.; Cantagrel, R.; Ledauphin, J.; Payot, T.; Fournier, N.; Guichard, E. Determination of key odorant compounds in freshly distilled cognac using GC-O, GC-MS, and sensory evaluation. J. Agric. Food Chem. 2004, 52, 5670–5676. [Google Scholar] [CrossRef] [PubMed]
- Phi, N.T.L.; Nishiyama, C.; Choi, H.S.; Sawamura, M. Evaluation of characteristic aroma compounds of Citrus natsudaidai Hayata (Natsudaidai) cold-pressed peel oil. Biosci. Biotechnol. Biochem. 2006, 70, 1832–1838. [Google Scholar]
- Shiratsuchi, H.; Shimoda, M.; Imayoshi, K.; Noda, K.; Osajima, Y. Volatile flavor compounds in spray-dried skim milk powder. J. Agric. Food Chem. 1994, 42, 984–988. [Google Scholar] [CrossRef]
- López, R.; Ezpeleta, E.; Sánchez, I.; Cacho, J.; Ferreira, V. Analysis of the aroma intensities of volatile compounds released from mild acid hydrolysates of odourless precursors extracted from Tempranillo and Grenache grapes using gas chromatography-olfactometry. Food Chem. 2004, 88, 95–103. [Google Scholar] [CrossRef]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Sydney, Australia, 2010. [Google Scholar]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
- Pérez-Olivero, S.J.; Pérez-Pont, M.L.; Conde, J.E.; Pérez-Trujillo, J.P. Determination of lactones in wines by headspace solid-phase microextraction and gas chromatography coupled with mass spectrometry. J. Anal. Methods Chem. 2014, 2014, 863019. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Song, Y.Y.; Dang, G.F.; Ye, D.Q.; Gong, X.; Liu, Y.L. Effect of wine closures on the aroma properties of Chardonnay wines after four years of storage. S. Afr. J. Enol. Vitic. 2015, 36, 296–303. [Google Scholar] [CrossRef]
- Pandit, A.; Indurkar, A.; Deshpande, C.; Jain, R.; Dandekar, P. A systematic review of physical techniques for chitosan degradation. Carbohydr. Polym. Technol. Appl. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Liu, S.Q.; Pritchard, G.G.; Hardman, M.J.; Pilone, G.J. Citrulline production and ethyl carbamate (urethane) precursor formation from arginine degradation by wine lactic acid bacteria Leuconostoc oenos and Lactobacillus buchneri. Am. J. Enol. Vitic. 1994, 45, 235–242. [Google Scholar] [CrossRef]
- Du Toit, W.J.; Lambrechts, M.G. The enumeration and identification of acetic acid bacteria from South African red wine fermentations. Int. J. Food Microbiol. 2002, 74, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Cañas, P.M.; Mena-Morales, A.; García-Romero, E. Malolactic fermentation before or during wine aging in barrels. LWT Food Sci. Technol. 2016, 66, 468–474. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Longo, E.; Chiotti, D.; Pedri, U.; Eisenstecken, D.; Sanoll, C.; Robatscher, P.; Boselli, E. Pinot Blanc: Impact of the Winemaking Variables on the Evolution of the Phenolic, Volatile and Sensory Profiles. Foods 2020, 9, 499. [Google Scholar] [CrossRef]
- Poggesi, S.; Merkytė, V.; Longo, E.; Boselli, E. Effects of Microvibrations and Their Damping on the Evolution of Pinot Noir Wine during Bottle Storage. Foods 2022, 11, 2761. [Google Scholar] [CrossRef]
- Poggesi, S.; Darnal, A.; Ceci, A.T.; Longo, E.; Vanzo, L.; Mimmo, T.; Boselli, E. Fusion of 2DGC-MS, HPLC-MS and Sensory Data to Assist Decision-Making in the Marketing of International Monovarietal Chardonnay and Sauvignon Blanc Wines. Foods 2022, 11, 3458. [Google Scholar] [CrossRef] [PubMed]

| Wines | x.2 | x.3 | x.4 | x.8 | x.9 | x.10 | x.14 | x.15 | x.17 | x.18 | x.20 | x.21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 6,675,747 ab | 8,283,127 ab | 335,017 b | 540,694 a | 4,093,904 b | 3,644,990 b | 14,585,331 a | 1,387,724 b | 3,873,619 a | 2,434,583 a | 630,189 a | 3,446,505 a |
| D | 4,404,294 b | 6,303,866 b | 478,534 b | 442,361 ab | 6,755,748 a | 9,455,400 a | 14,190,181 a | 1,102,369 b | 1,453,939 b | 1,991,437 a | 249,516 ab | 1,767,459 b |
| C | 8,159,445 a | 11,139,104 a | 432,010 b | 328,940 ab | 4,504,241 ab | 9,524,711 a | 1,585,906 c | 639,003 b | 2,300,074 ab | 502,922 b | 199,338 b | 1,437,020 b |
| E | 6,713,101 ab | 10,129,613 a | 875,120 a | 147,125 b | 6,785,969 a | 11,889,788 a | 8,042,353 b | 2,687,260 a | 1,597,135 b | 1,729,145 a | 111,249 b | 1,524,666 b |
| Pr > F | 0.032 | 0.010 | 0.002 | 0.031 | 0.025 | 0.007 | 0.001 | 0.007 | 0.032 | 0.011 | 0.023 | 0.020 |
| Wines | x.25 | x.27 | x.29 | x.30 | x.33 | x.36 | x.37 | x.38 | x.39 | x.41 | x.45 | |
| A | 206,836 ab | 533,270 a | 54,788 a | 399,051 a | 3,842,883 b | 23,396,506 a | 11,231,772 a | 584,371,828 a | 655,435 ab | 846,206 b | 125,279 a | |
| D | 585,677 a | 334,621 b | 26,328 ab | 196,123 b | 5,780,058 a | 17,854,483 a | 8,683,904 a | 443,420,094 ab | 557,048 b | 239,291 b | 82,145 b | |
| C | 195,029 b | 385,117 ab | 41,174 ab | 253,891 b | 6,329,581 a | 17,126,232 ab | 8,344,102 a | 417,364,280 b | 610,509 ab | 3,849,583 a | 91,588 ab | |
| E | 183,237 b | 224,939 b | 11,073 b | 156,599 b | 4,108,870 b | 10,000,704 b | 4,803,874 b | 229,152,184 c | 956,862 a | 435,358 b | 94,559 ab | |
| Pr > F | 0.032 | 0.008 | 0.045 | 0.006 | 0.003 | 0.010 | 0.005 | 0.004 | 0.049 | 0.000 | 0.039 |
| Statistic | Comp. 1 | Comp. 2 | Comp. 3 | Comp. 4 |
|---|---|---|---|---|
| Q2 cum | 0.078 | 0.133 | 0.098 | 0.120 |
| R2Y cum | 0.195 | 0.344 | 0.447 | 0.546 |
| R2X cum | 0.531 | 0.687 | 0.834 | 0.916 |
| Statistic | Olfactory Red Fruit | Olfactory Dry Fruit | Olfactory Undergrowth | Olfactory Spiciness | Olfactory Woodiness | Olfactory Burning | Olfactory Cleanness | Unpleasant Odors |
|---|---|---|---|---|---|---|---|---|
| R2 | 0.433 | 0.706 | 0.673 | 0.658 | 0.569 | 0.163 | 0.641 | 0.529 |
| Std. deviation | 0.352 | 0.403 | 0.385 | 0.609 | 0.547 | 0.797 | 0.366 | 0.377 |
| MSE | 0.076 | 0.100 | 0.091 | 0.228 | 0.184 | 0.391 | 0.083 | 0.088 |
| RMSE | 0.276 | 0.316 | 0.302 | 0.478 | 0.429 | 0.626 | 0.287 | 0.296 |
| Statistic | Warmness | Astringency | Sourness | Bitterness | Clarity | Color Tonality | Color Intensity | Overall Intensity |
|---|---|---|---|---|---|---|---|---|
| R2 | 0.719 | 0.797 | 0.778 | 0.814 | 0.925 | 0.721 | 0.818 | 0.520 |
| Std. deviation | 0.364 | 0.312 | 0.272 | 0.309 | 0.205 | 0.274 | 0.387 | 0.398 |
| MSE | 0.066 | 0.049 | 0.037 | 0.048 | 0.021 | 0.038 | 0.075 | 0.079 |
| RMSE | 0.257 | 0.221 | 0.192 | 0.219 | 0.145 | 0.194 | 0.274 | 0.282 |
| Vineyard | Vineyard Code | Vineyard Altitude (m.a.s.l.) | Vineyard Exposure | Treatment on the Canopy |
|---|---|---|---|---|
| Mazzon | A | 350 | Northwest | None |
| Mazzon | A_CC_C | 350 | Northwest | Chitosan once a year: before harvest |
| Mazzon | A_CC_CC | 350 | Northwest | Chitosan three time a year: beginning of flowering, end of veraison, and pre-harvest |
| Aldino | C | 800 | South | None |
| Patone | D | 800 | East | None |
| Eggerhof | E | 1050–1150 | South | None |
| Mazzon, Aldino, Patone, Eggerhof | Wine code 2PN and XPN | / | / | Blend of treated and non-treated wines |
| Sensory Descriptors | Descriptors | Definition |
|---|---|---|
| VISUAL | ||
| Clarity | Absence of particles in suspension | |
| Color tonality | Red garnet or orange/brown | Tonality or shade of color |
| Color intensity | Red garnet or orange/brown | Intensity of color |
| OLFACTORY | ||
| Red fruit | Strawberry, blackberry, raspberry, blackcurrant | Fruit with red or black skin from forest |
| Dried fruits | Strawberry jam, raisin, prune, fig | Jam from strawberry or another red fruit; dry raisin, prune, or fig |
| Undergrowth | Mushroom, wet wood, musk, fern | From forest undergrowth, a complex of different odors from mushroom, wet wood in the forest, or musk |
| Spiciness | Clove, black pepper, anise and liquorice | Different types of spices; clove (strong balsamic odor), black pepper (pungent odor), anise, and liquorice (balsamic odor) |
| Woodiness | Vanilla, oak, and coffee | Vanilla phenolic odor (sweeter, reminiscent of cake), oak (resinous odor), coffee (burnt odor) |
| GUSTATORY | ||
| Warmness | Alcohol | Warm sensation perceived in the mouth due to alcohol |
| Astringency/ tannicity | Tannins or alum | Puckering mouthfeel caused by the tannins, precipitation of saliva, and dry in the mouth |
| Sourness | Acid: citric, lactic, tartaric | Having an acid taste resembling that of vinegar, lemon juice, etc. |
| Sweetness | Sucrose, glucose, fructose | Effect typically from sugar |
| Bitterness | Caffeine | Taste sensation that is peculiarly acrid, like coffee |
| Saltness/sapidity | Salt or glutamate | Has a salty taste |
| Red fruit | Strawberry, blackberry, raspberry, blackcurrant, cherry | Having a strawberry flavor or another flavor from red fruit |
| Woodiness | Vanilla, oak, and coffee | Vanilla phenolic odor (sweeter, reminiscent of cake), oak (resinous odor), coffee (burnt odor) |
| Full-bodied/viscous | High concentration of sugar, oil, or fat | Visual characteristic and chemestasis characteristics; full mouthfeel or sensation from fatty foods |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tchouakeu Betnga, P.F.; Poggesi, S.; Darnal, A.; Longo, E.; Rudari, E.; Boselli, E. Terroir Dynamics: Impact of Vineyard and Canopy Treatment with Chitosan on Anthocyanins, Phenolics, and Volatile and Sensory Profiles of Pinot Noir Wines from South Tyrol. Molecules 2024, 29, 1916. https://doi.org/10.3390/molecules29091916
Tchouakeu Betnga PF, Poggesi S, Darnal A, Longo E, Rudari E, Boselli E. Terroir Dynamics: Impact of Vineyard and Canopy Treatment with Chitosan on Anthocyanins, Phenolics, and Volatile and Sensory Profiles of Pinot Noir Wines from South Tyrol. Molecules. 2024; 29(9):1916. https://doi.org/10.3390/molecules29091916
Chicago/Turabian StyleTchouakeu Betnga, Prudence Fleur, Simone Poggesi, Aakriti Darnal, Edoardo Longo, Elena Rudari, and Emanuele Boselli. 2024. "Terroir Dynamics: Impact of Vineyard and Canopy Treatment with Chitosan on Anthocyanins, Phenolics, and Volatile and Sensory Profiles of Pinot Noir Wines from South Tyrol" Molecules 29, no. 9: 1916. https://doi.org/10.3390/molecules29091916






