Synthetical Application of Alkyl 2-isothiocyanatocarboxylates. A Simple Synthesis of 5-Substituted-3-amino-2-thioxo-4-imidazolidinones (3-Amino-2-thiohydantoins)
Abstract
:Introduction
Results and discussion
Experimental
General metod for preparation of isothiocyanates 1b-i
Ethoxycarbonylmethylthiosemicarbazide (2b)
Methoxycarbonyl-t-butylmethylthiosemicarbazide (2d)
3-Aminothiohydantoin (3b)
3-Aminothiohydantoins 3; General procedure
Acknowledgements
References and Notes
- Uota, H.; Takai, A. Japanese Patent 1733, 1955. Chem.Abstr. 1957, 51, 3668h.
- Jacobsen, N.; Toelberg, J. A simple synthesis of 3-substituted 1-amino-2-thioxo-4-imidazolidinones (1-amino-thiohydantoins). Synthesis 1986, 559–561. [Google Scholar] [CrossRef]
- Pilgram, K.H.; Kollmeyer, W.D.; Skiles, R.D.; Wittsell, L.E. Synthesis and Chemistry of Agrochemicals; ACS Symposium series; Comstock, M.J., Ed.; American Chemical Society, 1987; Chapter 4, Synthesis and herbicidal properties of N-(benzilideneamino), N-(benzilamino), and N-(phenylazo) heterocycles; pp. 36–53. [Google Scholar]
- Thorwart, W.; Gebert, V.; Schleuerbach, R.; Bartlett, R. Ger.Offen. DE 3,702,757, 1987. Chem.Abstr. 1989, 110, 8223k.
- Floch, L.; Kosik, M.; Kosik, M. Study of the thermal properties of 1,4-disubstituted thiosemicarbazides. J.Thermal Analysis 1991, 2377–2382, ibid. 1992, 38, 2417 (Errata ). [Google Scholar] [CrossRef]
- Gut, J.; Novacek, A.; Fiedler, P. Reaction of six-membered cyclic hydrazides with aromatic aldehydes. Coll.Czechoslov.Chem.Commun. 1968, 2087–2096. [Google Scholar] [CrossRef]
- Gante, J.; Lautsch, W. Über semicarbazino-(4)-essigsäure- und thiosemicarbazino-(4)-essigsäurederivate. Chem.Ber. 1964, 97, 994–1001. [Google Scholar] [CrossRef]
- Veverka, M.; Marchalin, M. Addition-cyclization reactions of ethyl isothiocyanatoacetate with carboxylic acid hydrazides. Coll. Czechoslov. Chem. Commun. 1987, 52, 113–119. [Google Scholar] [CrossRef]
- Glushkov, V. A.; Mashevskaya, I. V.; Sokol, V. I.; Feshina, E. V.; Majorova, O. A. Syntez i struktura 5,5-dialkyl-3-arylamino-2-tiogitantoinov. Khim. Geterotsikl. Soedin. 1997, 898–903. [Google Scholar]
- Böehme, F.; Martin, F.; Strahl, J. Synthesen von derivativen des 3-amino-2-thiohydantoins. Arch.Pharm. (Weinheim, Ger). 1980, 313, 10–15. [Google Scholar]
- Floch, L.; Kovac, S. Synthesis and properties of alkyl isothiocyanatocarboxylates. Coll. Czechoslov. Chem. Commun. 1975, 40, 2845–2854. [Google Scholar] [CrossRef]
- Sample Availability: Available from the authors.
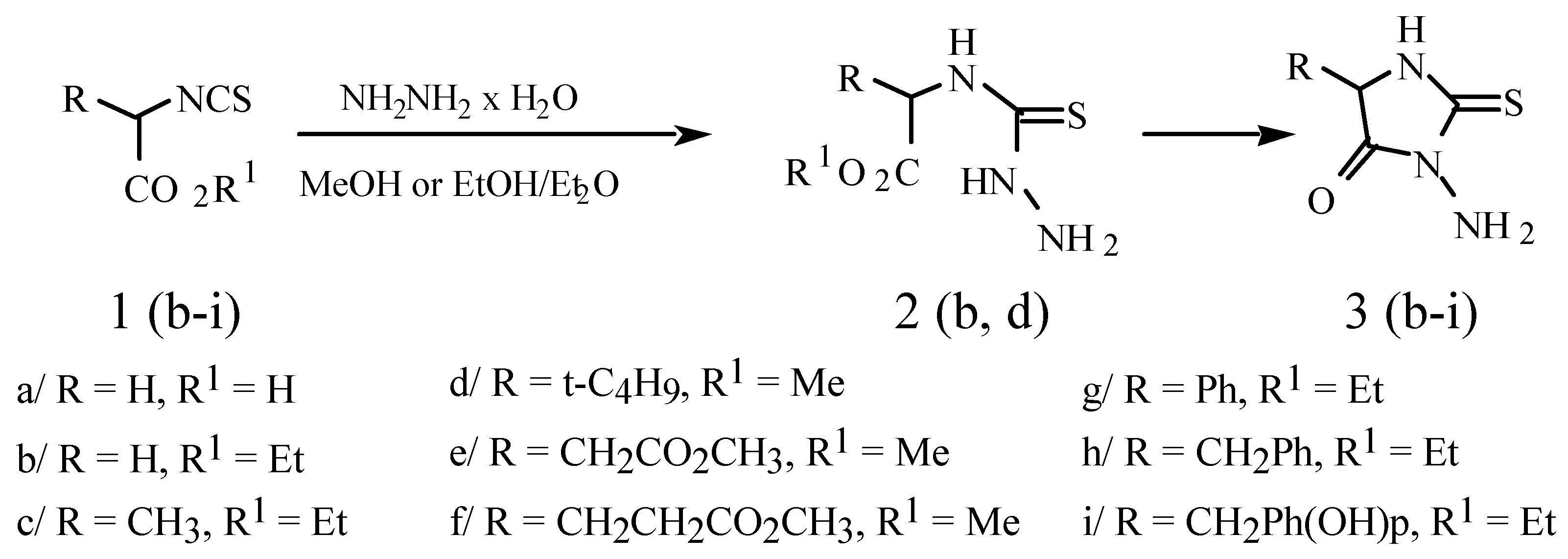
| Product | [α]22 (g /100ml) | IR (cm-1) | 1H NMR (CDCl3/TMS) δ (ppm), J (Hz) | 13C NMR (CDCl3/TMS) δ (ppm) |
| n - hexane | ||||
| 1dc | ± | 2081, 1750b | 1.06 (s, 9H, (CH3)3C), 3.81 (s, 3H, OCH3), 3.96 (s, 1H, H-2) | 26.5 (CH3), 36.8 (C(CH3)3), 68.9 (OCH3), 136.1 (NCS), 167.8 (C=O) |
| 1e | -26.2° (0,29) | 2054, 1740a | 2.98 (dd, J = 11.2, 5.5, H-3a), 3.04 (dd, 1H, J = 11.2, 5.5, H-3b), 3.75 (s, 3H, OCH3), 3.85 (s, 3H, OCH3), 4.75 (dd, 1H, 2 x J = 4.9, H-2) | 36.9 (C-3), 52.0, 53.2 (2xOCH3), 55.1 (C-2), 139.0 (NCS), 167.4, 168.9 (2xC=0) |
| 1f | -32.4° (0.37) | 2085, 1740b | 2.16 (m, 1H, H-3a), 2.30 (m, 1H, H-3b), 2.53 (m, 2H, H-4), 3.71 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 4.44 (dd, 1H, 2 x J = 4.9, H-2) | 28.2 (C-4), 29.4 (C-3), 51.6, 53.0 (2xOCH3), 58.2 (C-2), 137.8 (NCS), 166.2, 171.9 (2xC=O) |
| 1g | ± | 2052, 1749 | 1.25 (t, 3H, J = 7.1, OCH2CH3), 4.21 (q, 2H, J = 7.1, OCH2CH3, 5.26 (s, 1H, H-2 ), 7.42-7.98 (m, 5Harom.) | 14.0 (OCH2CH3), 62.9 (OCH2), 62.9 (C-2), 126.8, 129.1, 129.3 (Carom.), 131.0 (C-1’arom.), 139.8 (NCS), 167.4 (C=O) |
| 1h | ± | 2110, 2170, 1742, 1732a | 1.27 (t, 3H, J = 7.2, OCH2CH3), 3.23 (dd, 1H, J = 4.8, 13.7, H-3a), 3.11 (dd, 1H, J = 8.2, 13.7, H- 3b), 4.22 (q, 2H, J = 7.2, OCH2CH3), 4.44 (dd, 1H, 2 x J = 4.8, H-2), 7.21-7.35 (m, 5H, Harom) | 14.1 (OCH2CH3), 39.7 (C-3), 60.9 (C-2), 62.6 (OCH2), 127.6, 128.8, 129.4, (3xCarom.), 135.1 (C-1’arom.), 138.0 (NCS), 167.9 (C=O) |
| 1i | -35.3° (0.45) | 2064, 1760, 1749a | 1.29 (t, 3H, J = 7.2, OCH2CH3), 3.07 (dd, 1H, J = 7.8, 14.0, H-3a), 3.17 (dd, 1H, J = 4.8, 14.0, H-3b), 4.21 (q, 2H, 2 x J = 7.2, OCH2CH3), 4.41 (dd, 1H, 2 x J = 4.7, H-2), 6.79 (d, 2H, J = 8.4, Harom), 7.09 (d, 2H, J = 8.4, Harom.) | 14.1 (OCH2CH3), 38.9 (C-3), 61.1 (C-2), 62.7 (OCH2), 115.7 (C-2’,6’), 127.1 (C-1’), 130.7 (C-3’,5’arom.), 137.9 (NCS), 155.1 (C-4’), 168.1 (C=O) |
| Product | [α]22 (g / 100 ml) | UV (MeOH) λm (nM) | IR (cm-1) | 1H NMR (DMSO-d6/TMS) δ (ppm), J (Hz) | 13C NMR (DMSO d6/TMS) δ (ppm) | |
| C-2,4,5 | others | |||||
| 3b | - | 239.5 (3.84) | 3283, 3179, 3135, 1742, 1514 | 4.08 (s, 2H, CH2), 4.96 (s, 2H, NH2), 10.06 (bs, 1H, NH) | 183.55 169.42 46.99 | _ |
| 3c | +3.7° (0.4) MeOH | 234.0 (3.48) 264.0 (3.97) | 3304, 3240, 3210, 1741, 1507 | 1.46 (d, 3H, J = 7.1, CH3), 4.09 (dq, 1H , J = 7.1, 1.3, CH), 9.65 (bs, 1H, NH) | 182.63 172.47 53.09 | 16.21 |
| 3d | + | _ | _ | 0.94 (s, 9H, (CH3)3C)), 3.73 (s, 2H, NH2), 3.89 (s, 1H, CH), 10.27 (bs, 1H, NH) | 183.10 170.49 65.59 | 34.91 25.27 |
| 3e | -25.4° (0.45) 4% HCl | 235.8 (3.85) 264.8 (4.35) | 3360, 3230, 1744, 1729 | 2.81 (d, 2H, J = 5.1, CH2), 3.60 (s, 3H, OCH3), 4.43 (dd, 1H, 2 x J = 5.4, CH), 4.98 (s, 2H, NH2), 10.18 (bs, 1H, NH) | 183.40 170.73 53.75 | 169.23 51.74 34.41 |
| 3f | -25.3° (0.27) 4% HCl | 235.8 (3.83) 264.6 (4.32) | 3343, 3240, 3196, 1740, 1732, 1501 | 1.9 (m, 2H, CCH2), 2.43 (m, 2H, CH2CO), 3.60 (s, 3 H, OCH3), 4.23 (dd, 1H, 2 x J = 5.4, CH), 4.98 (s, 2H, NH2), 10.29 (bs, 1H, NH) | 183.0 172.25 56.29 | 171.35 51.44 28.46 26.04 |
| 3g | + | 270.0 (4.17) | 3324, 3248, 3162, 1757, 1518 | 5.08 (s, 2H, NH), 5.38 (s, 1H, CH), 7.45 - 7.26 (m, 5H, Harom.), 10.70 (bs, 1H, NH) | 183.30 170.22 60.59 | 134.40 128.76 127.57 126.88 |
| 3h | + | 238.0 (3.78) 266.0 (4.25) | 3308, 3235, 3179, 1736, 1510 | 3.05 (dd, 1H, J = 14.1, 6.1, CHH), 3.21 (dd, 1H, J =14.3, 4.4, CHH), 3.30 (s, 2H, NH2), 4.31 (dd, 1H, J = 6.1, 4.3, CH), 7.20-7.43 (m, 5H, H arom.), 9.90 (bs, 1H, NH) | 182.66 170.78 58.16 | 134.91 129.4 128.11 126.77 35.83 |
| 3i | +12.0° (0.14) 4% HCl | 226.7 (4.04) 266.2 (4.26) | 3435, 3299, 3237, 3177, 1724, 1514 | 2.47 (m, 2H, CH2), 4.42 (m, 1H, CH), 4.83 (s, 1H, NH2), 6.60 (d, 2H, J = 8.5, Harom. ), 6.92 (d, 2H, J = 8.4, H arom.), 9.28 (s, 1H, OH), 10.25 (bs, 1H, NH) | 182.61 170.90 58.48 | 156.09 130.42 124.80 114.96 35.02 |
| Product | mp (°C) Yield (%) | Moleculare Formula a M.W. | MS m/z (%) |
| 3b | 159 – 160 80 | C3H5N3OS 131.15 | 131 (M+., 100), 103 (47), 74 (20), 73 (5), 72 (11), 60 (6), 55 (4), 47 (5), 45 (10), 43 (13), 34 (41), 33 (14) |
| 3c | 161 – 163 70 | C4H7N3OS 145.18 | 145 (M+., 100), 117 (27), 86 (17), 74 (13), 60 (8), 44 (56) |
| 3e | 152 – 154 72 | C6H9N3O3S 203.22 | 203 (M+., 100), 175 (13), 172 (14), 171 (6), 144 (20), 143 (16), 130 (11), 116 (5), 113 (30), 112 (9), 102 (96), 101 (25), 86 (22), 85 (29), 75 (24), 74 (39), 70 (39), 60 (26), 59 (42), 58 (8), 57 (16), 55 (34), 54 (5), 43 (46) |
| 3f | 160 – 61 75 | C7H11N3O3S 217.34 | 217 (M+., 100), 186 (18), 185 (49), 158 (5), 144 (11), 143 (15), 126 (11), 116 (11), 114 (5), 102 (8), 98 (17), 84 (60), 75 (9), 74 (12), 59 (5), 56 (13), 55 (13) |
| 3g | 157 – 158 80 | C9H9N3OS 207.25 | 207 (M+., 100), 205 (7), 179 (20), 148 (47), 147 (27), 118 (14), 106 (58), 104 (14), 102 (5), 91 (8), 90 (15), 89 (5), 79 (8), 77 (10), 74 (15), 51 (6) |
| 3h | 234 – 235 75 | C10H11N3OS 221.28 | 221 (M+., 61), 193 (26), 163 (5), 162 (5), 131 (7), 130 (13), 128 (13), 121 (6), 120 (64), 119 (5), 118 (5), 117 (17), 116 (20), 106 (45), 104 (13), 103 (16), 102 (12), 92 (23), 91 (100), 89 (5), 86 (5), 78 (8), 77 (17), 75 (18), 65 (23), 63 (6), 51 (12), 50 (6) |
| 3i | 210 – 212 64 | C10H11N3O2S 237.27 | 237 (M+., 12), 131 (10), 122 (9), 108 (9), 107 (100), 77 (5) |
© 1999 by the authors. Reproduction of this article, by any means, is permitted for noncommercial purposes.
Share and Cite
Floch, L.; Oremus, V.; Kovac, M. Synthetical Application of Alkyl 2-isothiocyanatocarboxylates. A Simple Synthesis of 5-Substituted-3-amino-2-thioxo-4-imidazolidinones (3-Amino-2-thiohydantoins). Molecules 1999, 4, 279-286. https://doi.org/10.3390/41000279
Floch L, Oremus V, Kovac M. Synthetical Application of Alkyl 2-isothiocyanatocarboxylates. A Simple Synthesis of 5-Substituted-3-amino-2-thioxo-4-imidazolidinones (3-Amino-2-thiohydantoins). Molecules. 1999; 4(10):279-286. https://doi.org/10.3390/41000279
Chicago/Turabian StyleFloch, Lubomir, Vladimir Oremus, and Martin Kovac. 1999. "Synthetical Application of Alkyl 2-isothiocyanatocarboxylates. A Simple Synthesis of 5-Substituted-3-amino-2-thioxo-4-imidazolidinones (3-Amino-2-thiohydantoins)" Molecules 4, no. 10: 279-286. https://doi.org/10.3390/41000279
APA StyleFloch, L., Oremus, V., & Kovac, M. (1999). Synthetical Application of Alkyl 2-isothiocyanatocarboxylates. A Simple Synthesis of 5-Substituted-3-amino-2-thioxo-4-imidazolidinones (3-Amino-2-thiohydantoins). Molecules, 4(10), 279-286. https://doi.org/10.3390/41000279




