Synthesis of Novel 1-Substituted and 1,9-Disubstituted-1,2,3,4-tetrahydro-9H-Carbazole Derivatives as Potential Anticancer Agents
Abstract
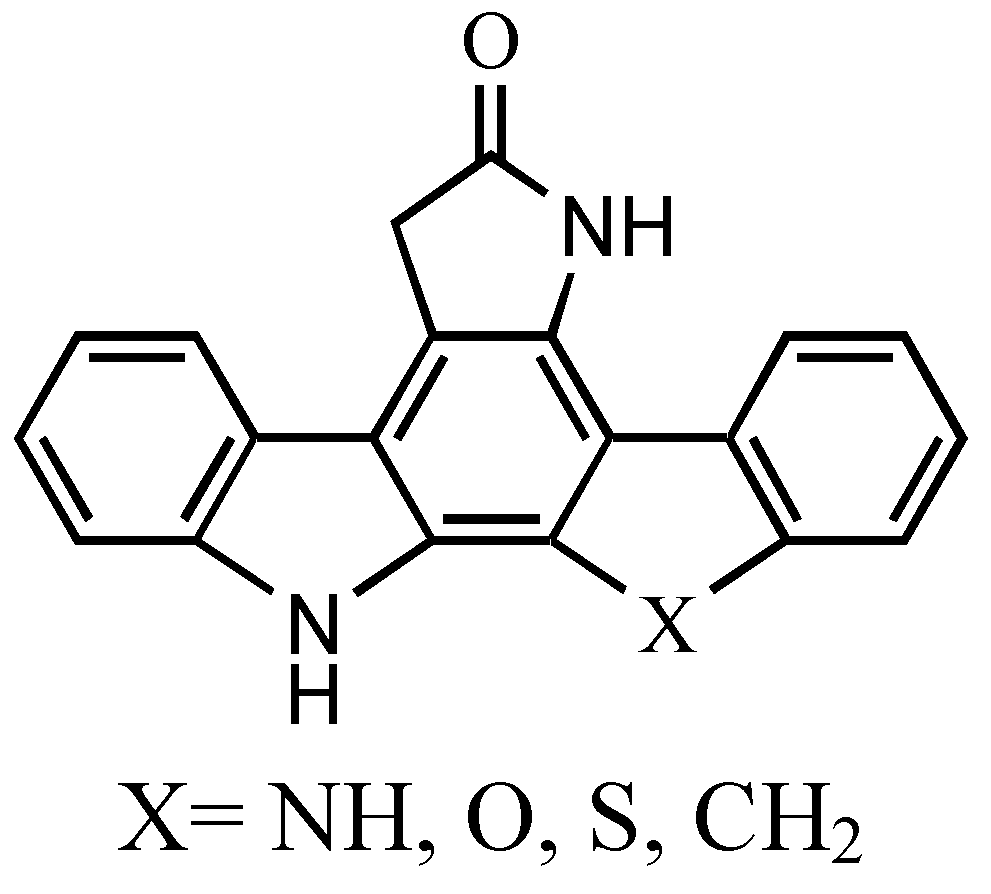
:Introduction
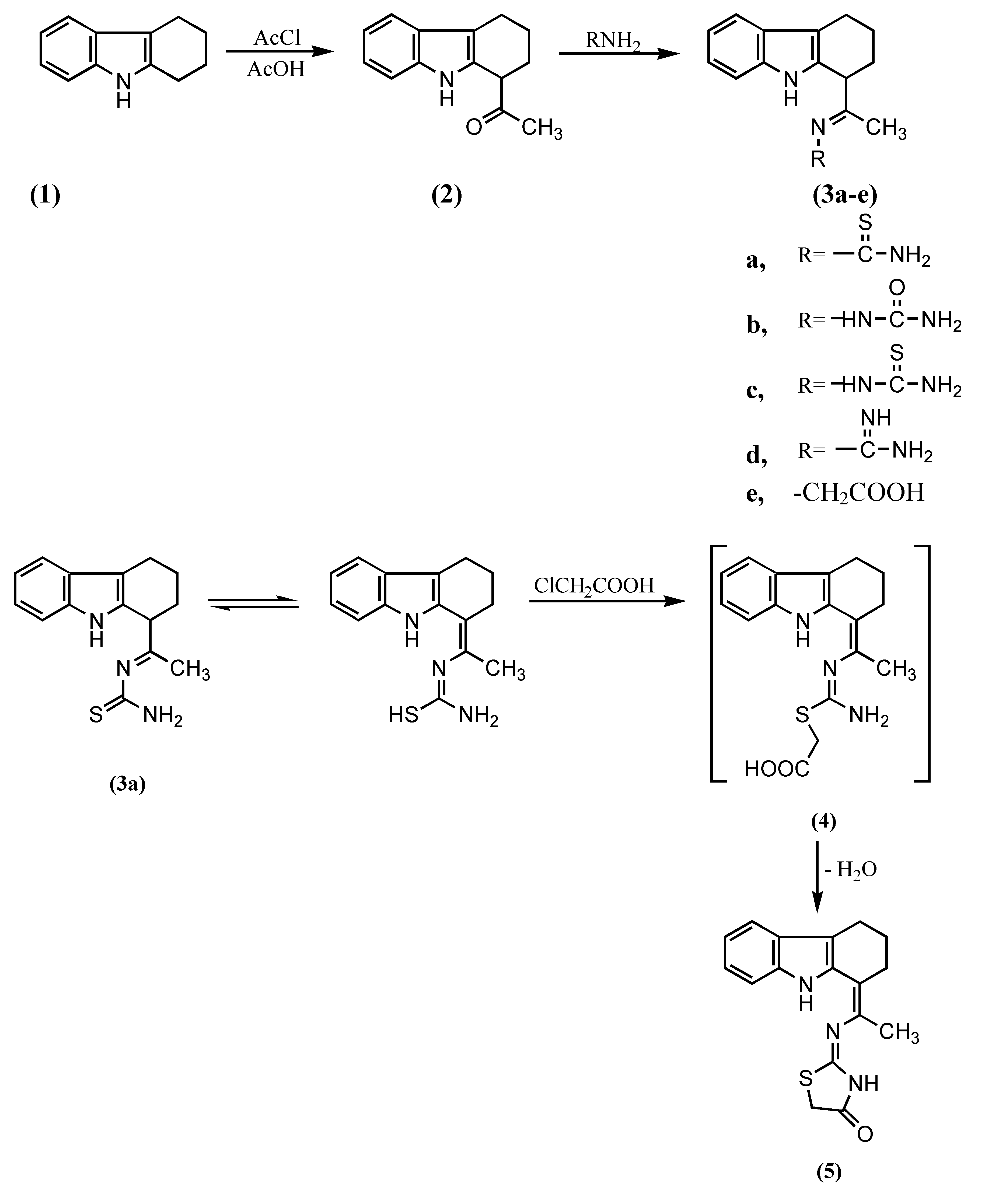
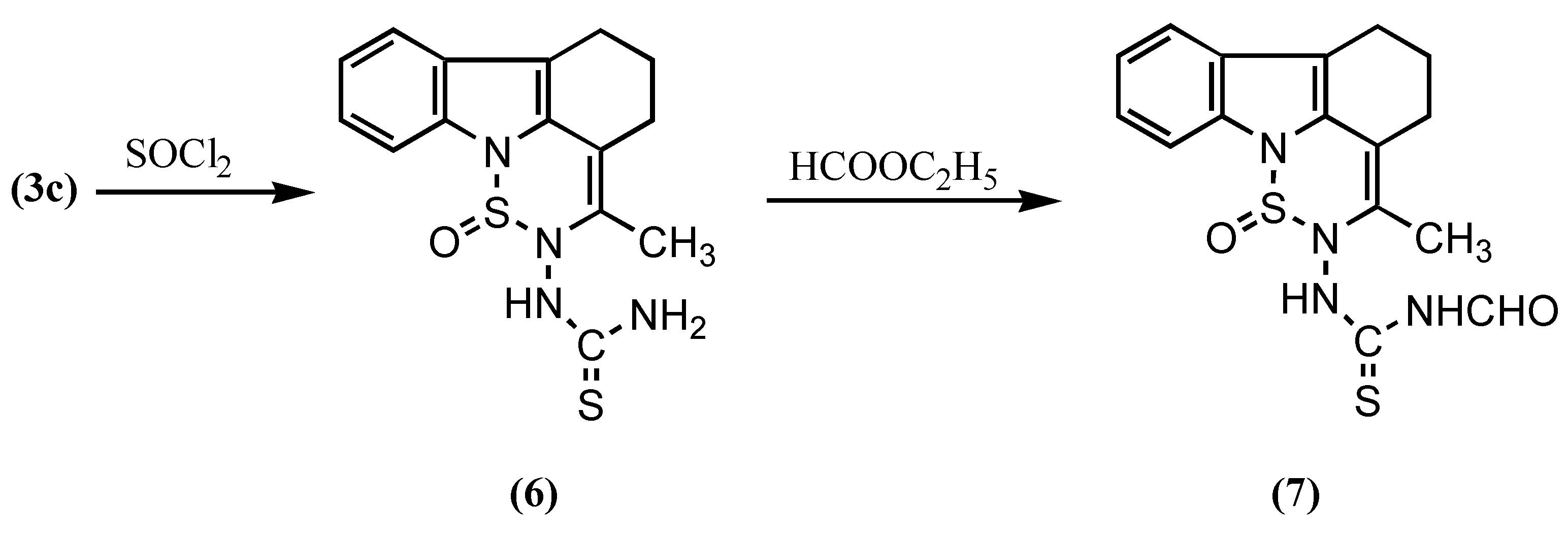
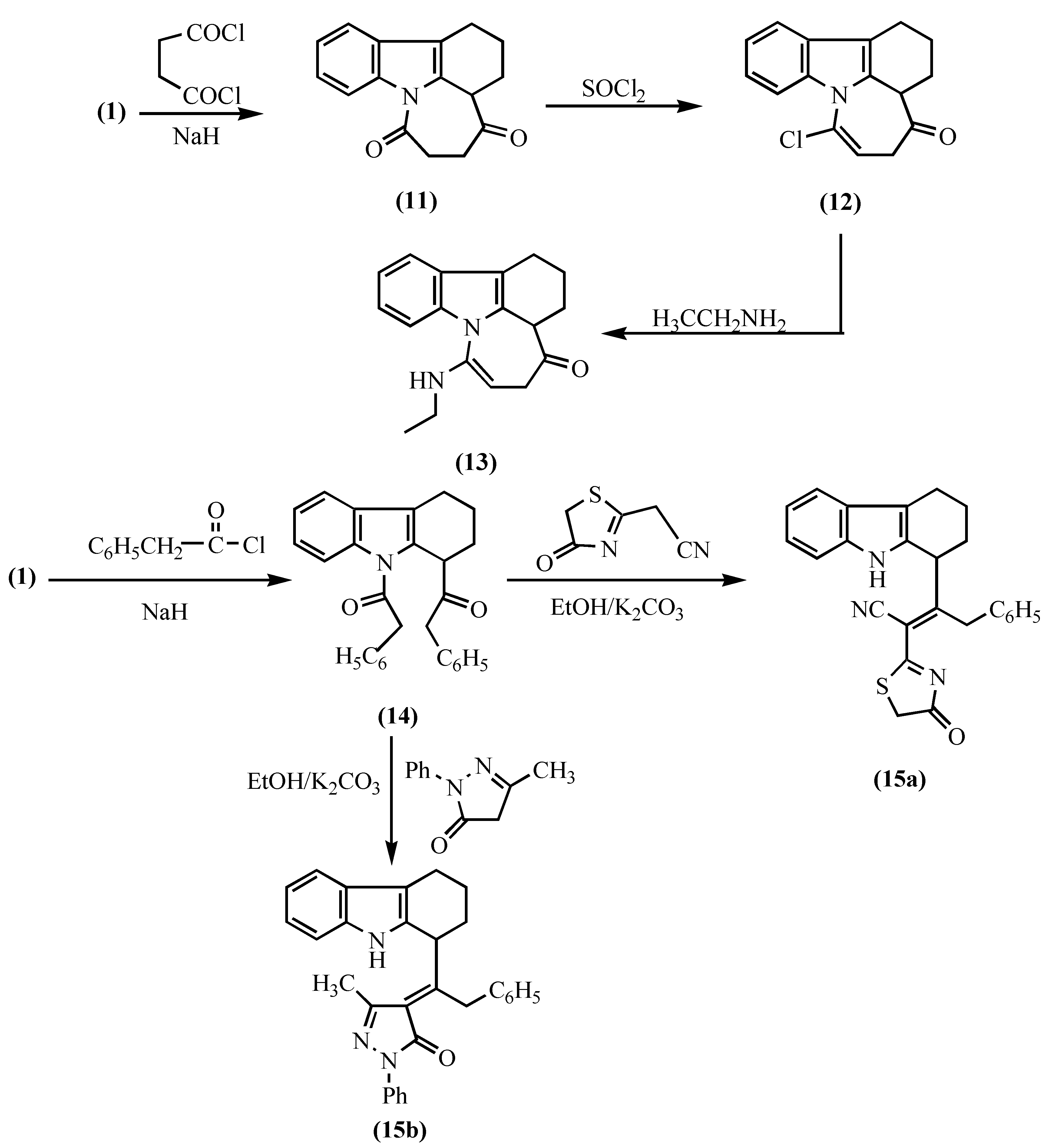
Results and Discussion
EXPERIMENTAL
General
Pharmacological Study: Cytotoxic assay
References
- St. Georgiev, V. New Synthetic Immunomodulating Agents. Trends Pharmacol, Sci 1988, 9, 446–451. [Google Scholar]
- Hudkins, R. L.; Diebold, J. L.; Angeles, T. S.; Knight, E., Jr. J. Med. Chem. 1997, 40, 2994–2996.
- Svoboda, G. H. Lloydia 1961, 24, 173. Nable, R. L.; Beer, C. T.; Cutts, J. H.; Ann, N. Y. Acad. Sci. 1958, 76, 3, 882.
- Dalton, L.K.; Demerac, S.; Elmes, C.; Loder, J.W.; Swan, J.M.; Teitei, T. Aust. J. Chem. 1967, 20, 2715.
- Creasy, W. A. Vinca Alkaloids and Colchicine. In Handbook of Experimental Pharmacology; Sartarelli, A.C., Johns, D.J., Eds.; Springer Verlag: New York, 1975; Volume 39, pp. 670–674. [Google Scholar]
- Auclair, C. Arch. Biophys. 1987, 1, 259. Suffness, M.; Cardell, G. A. The Alkaloids; Brassi, A., Ed.; Academic Press: New York, 1985; Volume 25, p. 1. [Google Scholar]
- Dug, B.; Auclair, C.; Meunier, B. Cancer Res. 1986, 46, 3828. Meunier, G.; de Montauzon, D.; Bernadou, J.; Grassy, G.; Bonnafous, M.; Cros, S.; Meunier, B. Mol. Pharmacol. 1988, 33, 93.
- Ding, L.; Gasas, C.; Etemad-Moghadam, G.; Cros, S.; Meunier, B. New J. Chem. 1990, 14, 421.
- Ziman, S.D. J. Heterocyclic Chem. 1979, 16, 5, 895–96.
- Finar, I.L. Heterocyclic CompoundsLongman Group Ltd.: London, 6th ed; 1973; vol. 1, p. 846. [Google Scholar]
- Ismail, M. M. F. Az. J. Pharm. Sci. 1999, 23, 82–99.
- Sample Availability: Not available
| Compd No. | M.P.* °C | Yield % | Formula (M. Wt) | Elemental analyses Calculated/ Found, % | ||||
| C | H | N | Cl | S | ||||
| 2 | 122a | 59 | C14H15NO (213) | 78.87 78.77 | 7.04 7.10 | 6.57 6.49 | 11.81 11.71 | |
| 3a | 190b | 60 | C15H17N3S (271.37) | 66.38 66.21 | 6.31 6.11 | 15.48 15.32 | ||
| 3b | 140b | 65 | C15H18N4O (270.31) | 66.64 66.51 | 6.71 6.66 | 20.72 20.63 | ||
| 3c | 160b | 62 | C15H18N4S (286.39) | 62.90 62.81 | 6.33 6.25 | 19.56 19.46 | 11.19 11.09 | |
| 3d | 240c | 66 | C15H18N4 (254.32) | 70.83 70.71 | 7.13 7.03 | 22.03 21.97 | ||
| 3e | 170b | 50 | C16H18N2O2 (270.3) | 70.03 71.00 | 6.66 6.57 | 10.36 10.42 | ||
| 5 | 135b | 55 | C17H17N3OS (311.38) | 65.51 65.49 | 5.46 5.50 | 13.49 13.38 | 10.28 10.26 | |
| 6 | 180b | 57 | C15H16N4OS2 (332.42) | 54.19 54.01 | 4.85 4.69 | 16.85 16.72 | 19.29 19.15 | |
| 7 | 220c | 50 | C16H16N4O2S2 (360.3) | 53.33 53.29 | 4.47 4.39 | 15.55 15.44 | 17.76 17.66 | |
| 8 | 190b | 64 | C18H19N3O4 (341.31) | 63.33 63.11 | 5.61 5.51 | 12.31 12.08 | ||
| 9 | 100d | 65 | C20H18N2O (302.35) | 79.44 79.31 | 6.00 5.95 | 9.26 9.16 | ||
| 10 | 155b | 55 | C20H18N4 (314) | 76.43 76.26 | 5.73 5.71 | 17.83 17.88 | ||
| 11 | 160b | 53 | C16H15NO2 (253.26) | 75.87 75.77 | 5.97 5.85 | 5.53 5.49 | ||
| 12 | 210b | 63 | C16H14NOCl (271.72) | 70.71 70.63 | 5.19 5.00 | 5.15 5.03 | 13.04 13.00 | |
| 13 | 140b | 69 | C18H20N2O (280.34) | 77.11 77.00 | 7.19 7.13 | 9.99 9.82 | ||
| 14 | 180b | 50 | C28H25NO2 (407.48) | 82.53 82.39 | 6.18 6.08 | 3.44 3.34 | ||
| 15a | 100e | 61 | C25H21N3OS (411.42) | 72.98 72.82 | 5.15 5.09 | 10.21 10.12 | ||
| 15b | 160b | 57 | C30H27N3O (445.52) | 80.87 80.79 | 6.11 6.01 | 9.43 9.31 | ||
| Compd. No. | νmax./cm-1 |
|---|---|
| 2 | 3440 (NH), 2990 (CH-aliphatic), 1691 (C=O) |
| 3a | 3400, 3370, 3240 (NH, NH2), , 2890 (CH aliphatic), 1650 (C=N), 1340 (C=S) |
| 3b | 3430, 3310, 3250 (NH, NH2), 1680 (C=O), 1625 (C=N) |
| 3c | 3430, 3300, 3220 (NH, NH2), 1640 (C=N), 1342 (C=S) |
| 3d | 3450, 3320, 3200 (NH, NH2), 1620 (C=N) |
| 3e | 3400-2800 (br. NH + OH), 1705 (C=O), 1630 (C=N) |
| 5 | broad band around 3396 (2NH), 1688 (C=O), 1639 (C=N) |
| 6 | 3340, 3300, 3200 (NH, NH2), 1340 (C=S) |
| 7 | 3370, 3250 (2NH), 1660 (C=O), 1340 (C=S) |
| 8 | 3500-2600 (NH + OH), 1710 (C=O, carboxylic), 1680 (C=O), 1650 (C=N) |
| 9 | 3398 (NH), 2990 (CH-aliphatic), 1660 (C=O), 1640 (C=N) |
| 10 | 3450, 3270 (2NH), 2890 (CH-aliphatic), 1640 (2C=N) |
| 11 | 2985 (CH-aliphatic), 1720 (br. 2C=O) |
| 12 | 2980 (CH-aliphatic), 1720 (C=O) |
| 13 | 3410 (NH), 2990 (CH aliphatic), 1720 (C=O) |
| 14 | 2985 (CH aliphatic), broad band around 1700 (2C=O) |
| 15a | 3415 (NH), 2220 (C≡N), 1680 (C=O), 1625 (C=N) |
| 15b | 3410 (NH), 1680 (C=O), 1635 (C=N) |
| Compd. No. (Solvent) | δ (ppm) |
|---|---|
| 2 (CDCl3) | 1.8-1.9 (m, 4H, 2CH2-cyclo), 2.2-2.3 (m, 3H, CH2 + CH-cyclo), 2.8 (s, 3H, COCH3), 7.2-7.5 (m. 4H, Ar-H), 10.20 (s, 1H, NH; exchangeable) |
| 3a (CDCl3) | 1.8-1.9 (m, 4H, 2CH2-cyclo), 2.2-2.4 (m, 3H, CH2 + CH-cyclo), 2.7 (s, 3H, CH3), 5.6 (s, 2H, NH2; exchangeable), 7.2-7.6 (m. 4H, Ar-H), 10.20 (s, 1H, NH; exchangeable) |
| 3c (CDCl3) | 1.8-1.9 (m, 4H, 2CH2-cyclo), 2.2-2.4 (m, 3H, CH2 + CH-cyclo), 2.7 (s, 3H, CH3), 5.6 (s, 2H, NH2; exchangeable),7.1-7.5 (m, 4H, Ar-H), 9.1(s, 1H, NH, exchangeable), 10.25(s,1H, NH; exchangeable) |
| 3e (CDCl3) | 1.85-1.95 (m, 4H, 2CH2-cyclo), 2.2-2.35 (m, 3H, CH2 + CH-cyclo), 3.7 (s,2H, CH2), 7.2-7.45 (m, 4H, Ar-H), 10.2 (s, 1H, NH; exchangeable), 10.6 (s,1H, OH, exchangeable) |
| 5 (CDCl3) | 1.75-1.9 (m, 4H, 2CH2-cyclo), 2.2-2.3 (m, 2H, CH2-cyclo), 2.7 (s, 3H, CH3),3.9 (s, 2H, CH2), 6.9-7.4 (m, 4H, Ar-H), 9.2 (s, 1H, NH, thiazolidine; exchangeable), 10.2 (s, 1H, NH; exchangeable) |
| 8 (CDCl3) | 1.75-1.85 (m, 4H, 2CH2-cyclo), 2.2-2.35 (m, 3H, CH2 + CH-cyclo), 2.6 (s,3H, CH3), 4.6 (s, 2H, CH2), 6.85-7.3 (m, 4H, Ar-H), 9.1 (s, 1H, NH, exchangeable), 10.20 (s, 1H, NH; exchangeable), 10.6 (s, 1H, OH; exchangeable). |
| 9 (CDCl3) | 1.8-1.9 (m, 4H, 2CH2-cyclo), 2.15-2.4 (m, 3H, CH2 + CH-cyclo), 6.8-7.3 (dd,2H, H-olefinic), 7.4-8.3 (m, 8H, Ar-H), 10.2 (s, 1H, NH; exchangeable) |
| 15b (DMSO-d6) | 1.75-1.85 (m, 4H, 2CH2-cyclo), 2.2-2.45 (m, 3H, CH2 + CH-cyclo), 3.0 (s, 3H, CH3), 3.9 (s, 2H, CH2), 7.2-7.6 (m, 14H, Ar-H), 10.2 (s, 1H, NH; exchangeable) |
| Compd. No. | m/e |
|---|---|
| 3e | 270 (M+, 1.0%), 255 (M-15 (CH3); 1.0%), 226 (M-44 (CO2); 1.5%), 211 (7.59 (CO2+ CH3); 5.7%), 191 (10.3%), 167 (16.9%), 149 (35.2%), 125 (20.2%), 111 (31.4%), 97 (13.6%), 57 (100%) |
| 5 | 296 (M-15 (CH3); 0.5%), 247 (0.5%), 229 (0.5%), 167 (11.9%), 154 (3.9 %), 143 (100%), 127 (4.98%), 116 (3.16%), 115 (9.8%), 85 (7.45%), 77 (4.2%) |
| 7 | 360 (M+, 1.0%), 344 (M-16 (0); 0.7%), 341 (0.5%), 269 (1.3%), 267 (2.64%), 235 (2.0 %), 217 (1.5%), 199 (11.86%), 170 (1.19%), 167 (41%), 154 (2.4%), 139 (8.6%), 127 (3.6%), 115 (7.4%), 105 (11.3%), 91 (17.2%), 77 (23.4%), 64 (100%) |
| 8 | 341 (M+, 0.5%), 297 (M-44 (CO2); 0.5%), 278 (0.5%), 217 (0.5%), 187 (18.8%), 171 (89.2 %), 143 (100%), 115 (11.9%), 83 (5.9%), 77 (8.9%) |
| 10 | 314 (M+, 0.5%), 299 (0.1%), 277 (0.3%), 261 (0.5%), 249 (1.0%), 230 (0.5 %), 215 (100%), 210 (0.5%), 191 (1.0%), 189 (1.0%), 171 (90.5%), 170 (26.86%), 143 (10.0%), 115 (13.49%), 77 (5.6%) |
| 11 | 253 (M+, 0.5%), 225 (M-28 (CO); 0.5%), 197 (M-56 (2CO); 0.5%), 184 (0.6%), 171 (10%), 159 (1.4 %), 145 (1.4%), 133 (1.6%), 115 (15.3%), 101 (89.8%), 74 (62.5%), 55 (100%) |
| 12 | 271 (M+, 1.5%), 273 (M+2 (Cl isotope); 0.5%),243 (M-28 (CO); 1.2%), 236 (M-35 (Cl), 1.2%), 213 (22.5%), 171 (66.5 %), 168 (38.3%), 143 (100%), 128 (24.3%), 115 (33.4%), 97 (24.2%) |
| 13 | 280 (M+, 0.5%), 252 (M-28 (CO); 0.5%), 236 (M-44 (C2H5NH); 0.5%), 231(0.5%), 186 (0.5%), 150 (1.0 %), 128 (2.5%), 102 (66.8%), 94 (15%), 86 (100%), 84 (85.0%) |
| 14 | 407 (M+, 0.7%), 288 (0.8%), 279 (33.1%), 255 (26.1%), 227 (11.3%), 209 (48.3%), 194 (58.6%), 167 (53.2%), 149(100%), 111 (18.8%), 97 (28.7%), 91 (65.9%), 77 (5.24%) |
| 15a | 411 (M+, 0.5%), 383 (M-28 (CO); 0.5%), 354 (1.16%), 310 (0.5%), 289 (4.6%), 236 (8.4 %), 200 (0.7%), 171 (8.2%), 140 (100%), 112 (10.4%), 91 (33.7) |
| Compound No. | ED50 |
|---|---|
| 2 | 30 |
| 3a | 42 |
| 3c | 38 |
| 6 | 37 |
| 7 | 35 |
| 14 | 29 |
| 15a | 26 |
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes
Share and Cite
Shmeiss, N.A.M.M.; Ismail, M.M.F.; Soliman, A.M.; El-Diwani, H.I. Synthesis of Novel 1-Substituted and 1,9-Disubstituted-1,2,3,4-tetrahydro-9H-Carbazole Derivatives as Potential Anticancer Agents. Molecules 2000, 5, 1101-1112. https://doi.org/10.3390/51001101
Shmeiss NAMM, Ismail MMF, Soliman AM, El-Diwani HI. Synthesis of Novel 1-Substituted and 1,9-Disubstituted-1,2,3,4-tetrahydro-9H-Carbazole Derivatives as Potential Anticancer Agents. Molecules. 2000; 5(10):1101-1112. https://doi.org/10.3390/51001101
Chicago/Turabian StyleShmeiss, N. A. M. M., M. M.F. Ismail, A. M. Soliman, and H. I. El-Diwani. 2000. "Synthesis of Novel 1-Substituted and 1,9-Disubstituted-1,2,3,4-tetrahydro-9H-Carbazole Derivatives as Potential Anticancer Agents" Molecules 5, no. 10: 1101-1112. https://doi.org/10.3390/51001101
APA StyleShmeiss, N. A. M. M., Ismail, M. M. F., Soliman, A. M., & El-Diwani, H. I. (2000). Synthesis of Novel 1-Substituted and 1,9-Disubstituted-1,2,3,4-tetrahydro-9H-Carbazole Derivatives as Potential Anticancer Agents. Molecules, 5(10), 1101-1112. https://doi.org/10.3390/51001101




