Synthesis of Some 2, 6-Disubstituted 4-Amidopyridines and -Thioamidopyridines, and Their Antimycobacterial and Photosynthesis-Inhibiting Activity
Abstract
:Introduction
Results and Discussion
Experimental
General
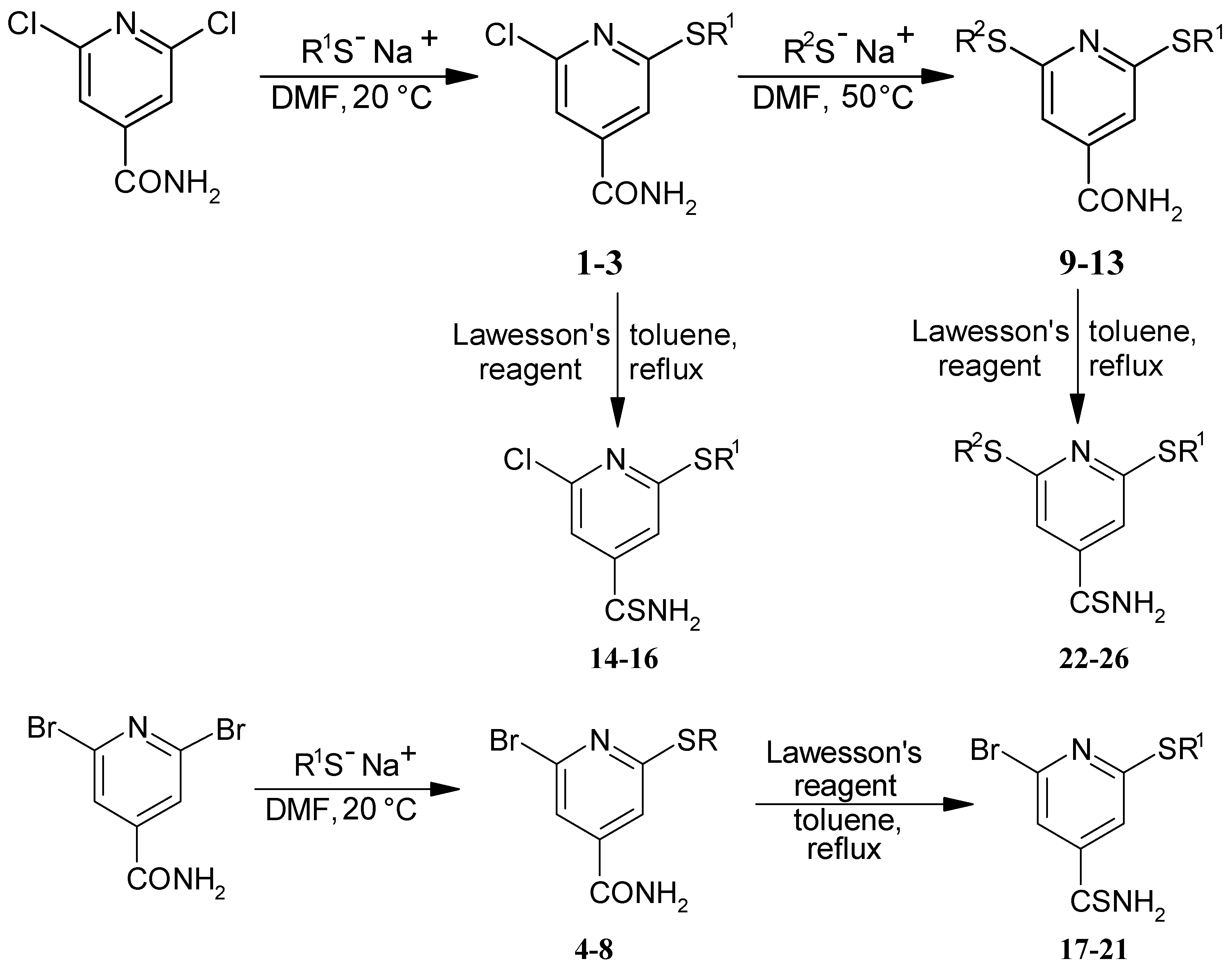
Synthesis of 2-halogeno-6-alkysulfanyl-4-amidopyridines 1-8
Synthesis of 2-alkylsulfanyl-6-hexylsulfanyl-4-amidopyridines 9-13
Synthesis of 2,6-disubstituted 4-thioamidopyridines 14-26
Biological assays
Acknowledgements
References and Notes
- Raviglione, M. C.; Snider, D. E.; Kochi, A. Global epidemiology of tuberculosis: morbidity and mortality of a worldwide epidemic. J. Am. Med. Ass. 1995, 273, 220–226. [Google Scholar] [CrossRef]
- Houston, S.; Fanning, A. Current and potential treatment of tuberculosis. Drugs 1994, 48, 689–708. [Google Scholar] [CrossRef] [PubMed]
- Buschauer, A. Pharmakotherapie der Tuberkulose: Wirkmechanismen und Resistenzen. Pharm. Z. 1997, 142, 11–25. [Google Scholar]
- Miletin, M.; Hartl, J.; Odlerova, Z.; Machacek, M. Synthesis of some 2,6-bis(alkylthio)-4-pyridinecarboxamides and carbothioamides and their antimycobacterial and antialgal activity. Pharmazie 1997, 52, 558–559. [Google Scholar]
- Waisser, K.; Klimesova, V.; Odlerova, Z. Relationships between the chemical structure of substances and their antimycobacterial activity to atypical strains. VII. 2-Alkythio-4-pyridinecarbothioamides. Folia Pharm. Univ. Carol. 1996, 20, 59–62. [Google Scholar]
- Klimesova, V.; Otcenasek, M.; Waisser, K. Potential antifungal agents. Synthesis and activity of 2-alkylthiopyridine-4-carbothioamides. Eur. J. Med. Chem. 1996, 31, 389–395. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Klimesova, V.; Waisser, K. Effect of 2-alkylthio-4-pyridinecarbothio-amides on photosynthetic electron transport in spinach chloroplasts. Collect. Czech Chem. Commun. 1997, 62, 516–520. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Klimesova, V.; Waisser, K. Relationships between photosynthesis-inhibiting activity and lipophilicity of 2-alkylthio-4-pyridinecarbothiamides. In Sbornik abstrakt prispevku. 50. sjezd chemických spolecnosti, Zlin, 8.-11.9.1997; Vydavatelstvi University Palackeho: Olomouc, 1997; p. 75. [Google Scholar]
- Levelt, W.H.; Wibaut, J.P. 2,6-Dibromopyridine-4-carboxylic acid, 2,6-dichloropyridine-4-carboxylic acid and some their derivatives. Rec. Trav. Chim. Pays-Bas 1929, 48, 466–473. [Google Scholar] [CrossRef]
- Kralova, K.; Sersen, F.; Loos, D.; Miletin, M. Photosynthesis-inhibiting and antimycobacterial activity of 6-substituted 2-alkylsulfanyl-4-pyridinecarboxamides. Folia Pharm. Univ. Carol. 1998, 23 Suppl., 79–80. [Google Scholar]
- Samples Availability: Available from the authors.
| Compd. | Formula | R1, | X | M. p. °C | Calculated / Found | ||||
|---|---|---|---|---|---|---|---|---|---|
| M. w. | R2 | Yield % | % C | % H | % N | % S | % Cl(Br) | ||
| 1 | C8H9ClN2OS | C2H5, | O | 162-163 | 44.34 | 4.19 | 12.93 | 14.80 | 16.36 |
| (216.7) | Cl | 75 | 44.21 | 4.12 | 13.11 | 14.69 | 16.51 | ||
| 2 | C9H11ClN2OS | C3H7, | O | 112-113 | 46.85 | 4.81 | 12.14 | 13.90 | 15.37 |
| (230.7) | Cl | 76 | 46.65 | 4.73 | 12.35 | 13.62 | 15.50 | ||
| 3 | C12H17ClN2OS | C6H13, | O | 129-131 | 52.84 | 6.28 | 10.27 | 11.75 | 13.00 |
| (272.8) | Cl | 72 | 52.76 | 6.21 | 10.39 | 11.64 | 13.14 | ||
| 4 | C7H7BrN2OS | CH3, | O | 178-180 | 34.02 | 2.86 | 11.34 | 12.97 | 32.34 |
| (247.1) | Br | 70 | 33.96 | 2.78 | 11.46 | 12.89 | 32.48 | ||
| 5 | C8H9BrN2OS | C2H5, | O | 160-162 | 36.80 | 3.47 | 10.73 | 12.28 | 30.60 |
| (261.1) | Br | 73 | 36.66 | 3.35 | 10.85 | 12.18 | 30.76 | ||
| 6 | C10H13BrN2OS | C4H9, | O | 113-115 | 41.53 | 4.53 | 9.69 | 11.09 | 27.63 |
| (289.2) | Br | 71 | 41.31 | 4.41 | 9.90 | 10.81 | 27.81 | ||
| 7 | C13H19BrN2OS | C7H15, | O | 120-122 | 47.13 | 5.78 | 8.46 | 9.68 | 24.12 |
| (331.3) | Br | 68 | 47.01 | 5.71 | 8.57 | 9.58 | 24.26 | ||
| 8 | C14H21BrN2OS | C8H17, | O | 123-125 | 48.70 | 6.13 | 8.11 | 9.28 | 23.14 |
| (345.3) | Br | 65 | 48.43 | 6.01 | 8.31 | 9.11 | 23.26 | ||
| 9 | C14H22N2OS2 | C6H13, | O | 83-84 | 56.34 | 7.43 | 9.39 | 21.48 | - |
| (298.5) | SC2H5 | 75 | 56.29 | 7.41 | 9.45 | 21.43 | |||
| 10 | C16H26N2OS2 | C6H13, | O | 86-88 | 58.86 | 8.03 | 8.58 | 19.64 | - |
| (326.5) | SC4H9 | 72 | 58.93 | 8.09 | 8.51 | 19.71 | |||
| 11 | C17H28N2OS2 | C6H13, | O | 109-111 | 59.96 | 8.29 | 8.23 | 18.83 | - |
| (340.6) | SC5H11 | 70 | 60.05 | 8.34 | 8.17 | 18.92 | |||
| 12 | C19H32N2OS2 | C6H13, | O | 106-108 | 61.91 | 8.75 | 7.60 | 17.40 | - |
| (368.6) | SC7H15 | 67 | 61.93 | 8.72 | 7.55 | 17.43 | |||
| 13 | C20H34N2OS2 | C6H13, | O | 96-98 | 62.78 | 8.96 | 7.32 | 16.76 | - |
| (382.6) | SC8H17 | 64 | 62.67 | 8.90 | 7.38 | 16.67 | |||
| 14 | C8H9ClN2S2 | C2H5, | S | 80-81 | 41.28 | 3.90 | 12.04 | 27.55 | 15.23 |
| (232.8) | Cl | 89 | 41.16 | 3.81 | 12.17 | 27.41 | 15.38 | ||
| 15 | C9H11ClN2S2 | C3H7, | S | oil | 43.81 | 4.49 | 11.35 | 25.98 | 14.37 |
| (246.8) | Cl | 87 | 43.73 | 4.45 | 11.43 | 25.87 | 14.49 | ||
| 16 | C12H17ClN2S2 | C6H13, | S | 45-47 | 49.90 | 5.93 | 9.70 | 22.20 | 12.27 |
| (288.9) | Cl | 91 | 49.69 | 5.78 | 9.81 | 22.01 | 12.42 | ||
| 17 | C7H7BrN2S2 | CH3, | S | 127-129 | 31.95 | 2.68 | 10.64 | 24.36 | 30.36 |
| (263.2) | Br | 85 | 31.82 | 2.61 | 10.51 | 24.22 | 30.48 | ||
| 18 | C8H9BrN2S2 | C2H5, | S | 107-108 | 34.66 | 3.27 | 10.11 | 23.13 | 28.83 |
| (277.2) | Br | 92 | 34.58 | 3.22 | 10.03 | 22.96 | 28.98 | ||
| 19 | C10H13BrN2S2 | C4H9, | S | 53-55 | 39.35 | 4.29 | 9.18 | 21.01 | 26.18 |
| (305.3) | Br | 90 | 39.15 | 4.20 | 9.07 | 20.89 | 26.39 | ||
| 20 | C13H19BrN2S2 | C7H15, | S | 43-45 | 44.96 | 5.51 | 8.07 | 18.46 | 23.01 |
| (347.3) | Br | 89 | 44.78 | 5.47 | 8.15 | 18.27 | 23.27 | ||
| 21 | C14H21BrN2S2 | C8H17, | S | 44-46 | 46.53 | 5.86 | 7.75 | 17.74 | 22.11 |
| (361.4) | Br | 91 | 46.31 | 5.77 | 7.92 | 17.59 | 22.35 | ||
| 22 | C14H22N2S3 | C6H13, | S | oil | 53.46 | 7.05 | 8.91 | 30.58 | - |
| (314.5) | SC2H5 | 90 | 53.41 | 7.02 | 9.03 | 30.41 | |||
| 23 | C16H26N2S3 | C6H13, | S | 58-60 | 56.10 | 7.65 | 8.18 | 28.08 | - |
| (342.6) | SC4H9 | 91 | 56.25 | 7.71 | 8.03 | 28.23 | |||
| 24 | C17H28N2S3 | C6H13, | S | 62-63 | 57.26 | 7.91 | 7.86 | 26.97 | - |
| (356.6) | SC5H11 | 89 | 57.02 | 7.78 | 8.05 | 26.72 | |||
| 25 | C19H32N2S3 | C6H13, | S | 71-73 | 59.33 | 8.39 | 7.28 | 25.00 | - |
| (384.7) | SC7H15 | 89 | 59.47 | 8.42 | 7.15 | 24.78 | |||
| 26 | C20H34N2S3 | C6H13, | S | 62-64 | 60.25 | 8.60 | 7.03 | 24.12 | - |
| (398.7) | SC8H17 | 87 | 60.03 | 8.47 | 7.25 | 23.91 | |||
| Compd. | IR, (cm-1) | 1H NMR, δ (ppm) |
|---|---|---|
| 1 | 3019, 2972 (CH aliph.) 1690 (C=O) | 7.38 d, J = 1, 1 H, arom.; 7.27 d, J = 1, 1 H, arom.; 6.09 bs, 1 H, NH; 5.90 bs, 1 H, NH; 3.18 q, J = 7, 2 H, SCH2; 1.37 t, J = 7, 3 H, CH3 |
| 2 | 3013, 2968 (CH aliph.) 1689 (C=O) | 7.38 d, J = 1, 1 H, arom.; 7.27 d, J = 1, 1 H, arom.; 6.53 bs, 1 H, NH; 6.42 bs, 1 H, NH; 3.14 t, J = 7, 2 H, SCH2; 1.73 sext, J = 7, 2 H, CH2; 1.02 t, J = 7, 3 H, CH3 |
| 3 | 3014, 2959, 2931 (CH aliph.) 1689 (C=O) | 7.38 d, J = 1, 1 H, arom.; 7.27 d, J = 1, 1 H, arom.; 6.19 bs, 2 H, NH2; 3.15 t, J = 7, 2 H, SCH2; 1.69-1.26 m, 4 H, (CH2)2; 0.87 t, J = 7, 3 H, CH3 |
| 4 | 3019, 2970 (CH aliph.) 1696 (C=O) | 7.44-7.46 m, 2 H, arom.; 6.15 bs, 1 H, NH; 5.75 bs, 1 H, NH; 2.60 s, 3 H, CH3 |
| 5 | 3018, 2969, 2936 (CH aliph.) 1695 (C=O) | 7.40-7.42 m, 2 H, arom.; 6.06 bs, 1 H, NH; 5.88 bs, 1 H, NH; 3.18 q, J = 7, 2 H, SCH2; 1.37 t, J = 7, 3 H, CH3 |
| 6 | 3014, 2962, 2933 (CH aliph.) 1694 (C=O) | 7.40-7.42 m, 2 H, arom.; 6.24 bs, 2 H, NH2; 3.14 t, J = 7, 2 H, SCH2; 1.73 m, 2 H, CH2; 1.44 m, 2 H, CH2; 0.92 dist. t, J = 5, 3 H, CH3 |
| 7 | 3014, 2958, 2930 (CH aliph.) 1690 (C=O) | 7.40-7.42 m, 2 H, arom.; 6.19 bs, 2 H, NH2; 3.14 t, J = 7, 2 H, SCH2; 1.70-1.25 m, 10 H, (CH2)5; 0.87 dist. t, J = 5, 3 H, CH3 |
| 8 | 3013, 2957, 2929 (CH aliph.) 1689 (C=O) | 7.39-7.41 m, 2 H, arom.; 6.14 bs, 2 H, NH2; 3.14 t, J = 7, 2 H, SCH2; 1.70-1.26 m, 12 H, (CH2)6; 0.87 dist. t, J = 5, 3 H, CH3 |
| 9 | 3013, 2960, 2931 (CH aliph.) 1686 (C=O) | 7.14 s, 2 H, arom.; 6.32 bs, 2 H, NH2; 3.21 q overlapping with 3.17 t, 4 H both, 2 × SCH2; 1.1 - 1.9 m, 8 H, 4 × CH2; 1.38 t, J = 7, 3 H, SCH2CH3; 0.90 dist. t, J = 5, 3 H, CH3 |
| 10 | 3010, 2961, 2932 (CH aliph.) 1686 (C=O) | 7.14 s, 2 H, arom.; 6.03 bs, 2 H, NH2; 3.20 t, J = 7, 4 H, 2 × SCH2; 1.1 -1.9 m, 12 H, 6 × CH2; 0.95 t, J = 6, 3 H, S(CH2)3CH3; 0.90 dist. t, 1686 (C=O) J = 5, 3 H, CH3 |
| 11 | 3010, 2960, 2931 (CH aliph.) 1686 (C=O) | 7.14 s, 2 H, arom.; 6.14 bs, 2 H, NH2; 3.19 t, J = 7, 4 H, 2 × SCH2; 1.1 - 1.9 m, 14 H, 7 × CH2; 0.90 dist. t, J = 5, 6 H, 2 × CH3 |
| 12 | 3009, 2959, 2930 (CH aliph.) 1686 (C=O) | 7.14 s, 2 H, arom.; 6.02 bs, 2 H, NH2; 3.19 t, J = 7, 4 H, 2 × SCH2; 1.1 -1.9 m, 18 H, 9 × CH2; 0.90 dist. t, J = 5, 6 H, 2 × CH3 |
| 13 | 3010, 2959, 2929 (CH aliph.) 1686 (C=O) | 7.14 s, 2 H, arom.; 6.20 bs, 2 H, NH2; 3.19 t, J = 7, 4 H, 2 × SCH2; 1.1 -1.9 m, 20 H, 10 × CH2; 0.90 dist. t, J = 5, 6 H, 2 × CH3 |
| 14 | 2991, 2931, 2874 (CH aliph.) 1603 (C=O) | 7.64 bs, 1 H, NH; 7.37 d, J = 1, 1 H, arom.; 7.29 d, J = 1, 1 H, arom.; 7.3 bs, 1 H, NH; 3.18 q, J = 7, 2 H, SCH2; 1.38 t, J = 7, 3 H, CH3 |
| 15 | 2996, 2968, 2934 (CH aliph.) 1603 (C=O) | 7.7 bs, 1 H, NH; 7.38 d, J = 1, 1 H, arom.; 7.29 d, J = 1, 1 H, arom.; 7.2 bs, 1 H, NH; 3.17 t, J = 7, 2 H, SCH2; 1.74 sext, J = 7, 2 H, CH2; 1.05 t, J = 7, 3 H, CH3 |
| 16 | 2996, 2959, 2931 (CH aliph.) 1603 (C=O) | 7.82 bs, 1 H, NH; 7.36 d, J = 1, 1 H, arom.; 7.28 d, J = 1, 1 H, arom.; 7.3 bs, 1 H, NH; 3.17 t, J = 7, 2 H, SCH2; 1.69-1.26 m, 8 H, (CH2)4; 0.90 dist. t , J = 5, 3 H, CH3 |
| 17 | 3001, 2932 (CH aliph.) 1603 (C=O) | 7.76 bs, 1 H, NH; 7.40 d, J = 1, 1 H, arom.; 7.29 d, J = 1, 1 H, arom.; 7.26 bs, 1 H, NH; 2.59 s, 3 H, CH3 |
| 18 | 2992, 2932 (CH aliph.) 1603 (C=O) | 7.79 bs, 1 H, NH; 7.39 d, J = 1, 1 H, arom.; 7.29 d, J = 1, 1 H, arom.; 7.26 bs, 1 H, NH; 3.19 q, J = 7, 2 H, SCH2; 1.38 t, J = 7, 3 H, CH3 |
| 19 | 2999, 2962, 2933 (CH aliph.) 1603 (C=O) | 7.88 bs, 1 H, NH; 7.37 d, J = 1, 1 H, arom.; 7.29 d, J = 1, 1 H, arom.; 7.3 bs, 1 H, NH; 3.18 t, J = 7, 2 H, SCH2; 1.25-1.86, 4 H, (CH2)2; 0.95 t, J = 6, 3 H, CH3 |
| 20 | 2997, 2958, 2929 (CH aliph.) 1603 (C=O) | 7.85 bs, 1 H, NH; 7.38 d, J = 1, 1 H, arom.; 7.29 d, J = 1, 1 H, arom.; 7.3 bs, 1 H, NH; 3.17 t, J = 7, 2 H, SCH2; 1.25-1.86, 10 H, (CH2)5; 0.89 dist. t, J = 5, 3 H, CH3 |
| 21 | 2998, 2957, 2928 (CH aliph.) 1603 (C=O) | 7.77 bs, 1 H, NH; 7.37 d, J = 1, 1 H, arom.; 7.28 d, J = 1, 1 H, arom.; 7.26 bs, 1 H, NH; 3.16 t, J = 7, 2 H, SCH2; 1.2-1.8 m, 12 H, (CH2)6; 0.88 dist. t, J = 5, 3 H, CH3 |
| 22 | 3004, 2960, 2930 (CH aliph.) 1601 (C=O) | 7.13 s, 2H, arom.; 6.1 bs, 1 H, NH; 5.8 bs, 1 H, NH; 3.20 q overlapping with 3.19 t, 4 H both, 2 × SCH2; 1.2 -1.8 m, 8 H, 4 × CH2; 1.34 t, J = 7, 3 H, SCH2CH3; 0.90 dist. t, J = 5, 3 H, CH3 |
| 23 | 2996, 2961, 2931 (CH aliph.) 1601 (C=O) | 7.76 bs, 1 H, NH; 7.13 s, 2H, arom.; 7.2 bs, 1 H, NH; 3.19 t, J = 7, 4 H, 2 × SCH2; 1.1-1.9 m, 12 H, 6 × CH2; 0.95 t, J = 6, 3 H, S(CH2)3CH3; 0.90 dist. t, J = 5, 3 H, CH3 |
| 24 | 2995, 2960, 2931 (CH aliph.) 1601 (C=O) | 7.76 bs, 1 H, NH; 7.13 s, 2H, arom.; 7.2 bs, 1 H, NH; 3.19 t, J = 7, 4 H, 2 × SCH2; 1.1-1.9 m, 14 H, 7 × CH2; 0.90 dist. t, J = 5, 6 H, 2 × CH3 |
| 25 | 2996, 2959, 2930 (CH aliph.) 1601 (C=O) | 7.61 bs, 1 H, NH; 7.13 s, 2H, arom.; 7.2 bs, 1 H, NH; 3.19 t, J = 7, 4 H, 2 × SCH2; 1.1-1.9 m, 18 H, 9 × CH2; 0.90 dist. t, J = 5, 6 H, 2 × CH3 |
| 26 | 2995, 2958, 2929 (CH aliph.) 1601 (C=O) | 7.66 bs, 1 H, NH; 7.13 s, 2H, arom.; 7.2 bs, 1 H, NH; 3.18 t, J = 7, 4 H, 2 × SCH2; 1.1-1.9 m, 20 H, 10 × CH2; 0.90 dist. t, J = 5, 6 H, 2 × CH3 |
| Compd. | MIC (μmol.dm-3) | IC50 (μmol.dm-3) | calculated | |||
|---|---|---|---|---|---|---|
| tuberculosis H37Rv | M. kansasii PKG8 | M. avium 80/72 | M. fortuitum 1021 | spinach chloroplasts | logP | |
| 1 | 500 | 1000 | 1000 | 1000 | 101.5 | 1.96 ± 0.38 |
| 2 | - | - | - | - | 58.4 | 2.49 ± 0.38 |
| 3 | - | - | - | - | 10.2 | 4.08 ± 0.38 |
| 4 | >1000 | >1000 | >1000 | >1000 | 76.7 | 1.52 ± 0.42 |
| 5 | - | - | - | - | 34.2 | 2.06 ± 0.42 |
| 6 | - | - | - | - | 10.6 | 3.12 ± 0.42 |
| 7 | 250 | 250 | >1000 | >1000 | 5.9 | 4.71 ± 0.42 |
| 8 | - | - | - | - | - | 5.24 ± 0.42 |
| 9 | 60 | 60 | 250 | 250 | 9.1 | 5.00 ± 0.41 |
| 10 | - | - | - | - | 203.5 | 6.06 ± 0.41 |
| 11 | - | - | - | - | 249.3 | 6.59 ± 0.41 |
| 12 | 1000 | >1000 | >1000 | >1000 | 543.6 | 7.66 ± 0.41 |
| 13 | - | - | - | - | 258.8 | 8.19 ± 0.41 |
| 14 | 125 | 250 | 250 | 500 | 104.8 | 2.73 ± 0.41 |
| 15 | 125 | 125 | 250 | 500 | 9.3 | 3.27 ± 0.41 |
| 16 | 60 | 60 | 60 | 250 | 29.8 | 4.86 ± 0.41 |
| 17 | 500 | 500 | 500 | 1000 | 187.7 | 2.34 ± 0.48 |
| 18 | 250 | 250 | 500 | 500 | 19.6 | 2.87 ± 0.48 |
| 19 | 125 | 250 | 250 | 500 | 20.9 | 3.93 ± 0.48 |
| 20 | 60 | 60 | 125 | 500 | 61.0 | 5.52 ± 0.48 |
| 21 | 125 | 125 | 250 | 500 | 105.1 | 6.06 ± 0.48 |
| 22 | 30 | 60 | 60 | 250 | - | 5.75 ± 0.47 |
| 23 | 30 | 125 | 125 | 125 | 99.7 | 6.81 ± 0.47 |
| 24 | 30 | 250 | 125 | 125 | 157.5 | 7.34 ± 0.47 |
| 25 | 60 | 250 | 250 | 500 | - | 8.41 ± 0.47 |
| 26 | 125 | 250 | 250 | 500 | - | 8.94 ± 0.47 |
| isoniazid | 7 | 250 | 1000 | 1000 | - | -0.89 ± 0.24 |
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Miletin, M.; Hartl, J.; Dolezal, M.; Odlerova, Z.; Kralova, K.; Machacek, M. Synthesis of Some 2, 6-Disubstituted 4-Amidopyridines and -Thioamidopyridines, and Their Antimycobacterial and Photosynthesis-Inhibiting Activity. Molecules 2000, 5, 208-218. https://doi.org/10.3390/50300208
Miletin M, Hartl J, Dolezal M, Odlerova Z, Kralova K, Machacek M. Synthesis of Some 2, 6-Disubstituted 4-Amidopyridines and -Thioamidopyridines, and Their Antimycobacterial and Photosynthesis-Inhibiting Activity. Molecules. 2000; 5(3):208-218. https://doi.org/10.3390/50300208
Chicago/Turabian StyleMiletin, Miroslav, Jiri Hartl, Martin Dolezal, Z. Odlerova, K. Kralova, and Milos Machacek. 2000. "Synthesis of Some 2, 6-Disubstituted 4-Amidopyridines and -Thioamidopyridines, and Their Antimycobacterial and Photosynthesis-Inhibiting Activity" Molecules 5, no. 3: 208-218. https://doi.org/10.3390/50300208





