Synthesis and Bioactivity of Teasterone and Typhasterol Analogs
Abstract
:Introduction

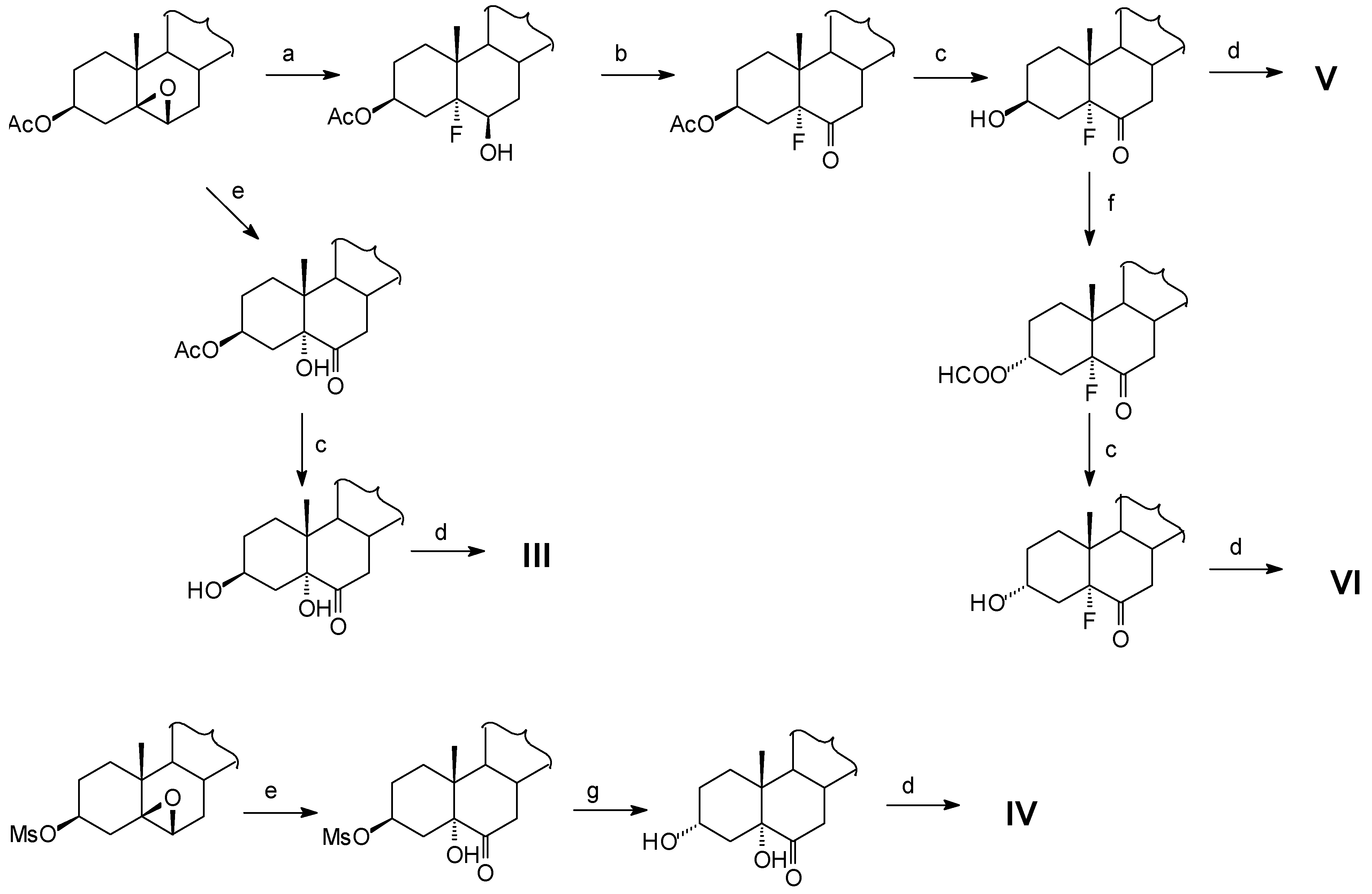
Results and Discussion
Acknowledgements:
References and Notes
- Clouse, S.; Sasse, J. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427. [PubMed]
- Kamuro, Y.; Takatsuto, S. Brassinosteroids: Steroidal Plant Hormones; Sakurai, A., Yokota, T., Clouse, S., Eds.; Springer-Verlag: Tokyo, 1999; pp. 223–241. [Google Scholar]
- Brosa, C.; Zamora, I.; Terricabras, E.; Soca, L.; Peracaula, R.; Rodríguez, C. Lipids 1997, 32, 12, 1341.
- Takatsuto, S.; Ikekewa, N. J. Chem. Soc.Perkin Trans I: 1984, 439.
- Wada, K.; Marumo, S.; Abe, H.; Morishita, T.; Nakamura, K.; Uchishama, M.; Mori, K. Agric. Biol. Chem. 1984, 48(3), 719.
Share and Cite
Ramírez, J.A.; Mancusso, R.; Sarno, S.; Galagovsky, L.R. Synthesis and Bioactivity of Teasterone and Typhasterol Analogs. Molecules 2000, 5, 367-369. https://doi.org/10.3390/50300367
Ramírez JA, Mancusso R, Sarno S, Galagovsky LR. Synthesis and Bioactivity of Teasterone and Typhasterol Analogs. Molecules. 2000; 5(3):367-369. https://doi.org/10.3390/50300367
Chicago/Turabian StyleRamírez, Javier A., Romina Mancusso, Silvina Sarno, and Lydia R. Galagovsky. 2000. "Synthesis and Bioactivity of Teasterone and Typhasterol Analogs" Molecules 5, no. 3: 367-369. https://doi.org/10.3390/50300367


