Insecticidal Activity of Cyanohydrin and Monoterpenoid Compounds
Abstract
:Introduction
Results and Discussion
Experimental
Acknowledgments
References and Notes
- Ku, H.S. Potential industrial applications of allelochemicals and their problems. In Allelochemicals: Role in Agriculture and Forestry; ACS Symposium Series #330; American Chemical Society: Washington, DC, 1987; pp. 449–454. [Google Scholar]
- Peterson, C.J.; Tsao, R.; Coats, J.R. Naturally occurring cyanohydrins, analogues and derivatives as potential fumigants. Pest Management Science. (in press).
- Lee, S.; Tsao, R.; Coats, J.R. Influence of dietary applied monoterpenoids and derivatives on survival and growth of the European corn borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 1999, 92, 56–67. [Google Scholar] [CrossRef]
- Rice, P.J.; Coats, J.R. Insecticidal properties of monoterpenoid derivatives to the house fly (Diptera: Muscidae) and the red flour beetle (Coleoptera: Tenebrionidae). Pestic. Sci. 1994, 41, 195–202. [Google Scholar] [CrossRef]
- Tsao, R.; Lee, S.; Rice, P.J.; Jensen, C.; Coats, J.R. Monoterpenoids and their synthetic derivatives as leads for new insect-control agents. In Synthesis and Chemistry of Agrochemicals IV; Feynes, J.G., Basarab, G.S., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, 1995; pp. 312–324. [Google Scholar]
- Tewe, O.O.; Iyayi, E.A. Cyanogenic glycosides. In Toxicants of Plant Origin, vol. II; Cheeke, P.R., Ed.; CRC Press: Boca Raton, FL, 1989; pp. 43–60. [Google Scholar]
- Wise, M.L.; Croteau, R. Monoterpene biosynthesis. In Comprehensive Natural Products Chemistry, Vol. 2; Barton, D., Nakanishi, K., Meth-Cohn, O., Eds.; Elsevier: Amsterdam, 1999; pp. 97–153. [Google Scholar]
- SAS Institute. Ultrix SAS, Version 6.09 SAS User’s Guide; SAS Institute: Cary, NC, 1991. [Google Scholar]
- Tsao, R.; Reuber, M.; Johnson, L.; Coats, J.R. Insecticidal toxicities of glucosinolate–containing extracts from crambe seeds. J. Agric. Entomol. 1996, 13, 109–120. [Google Scholar]
- Samples Availability: Available from the authors.
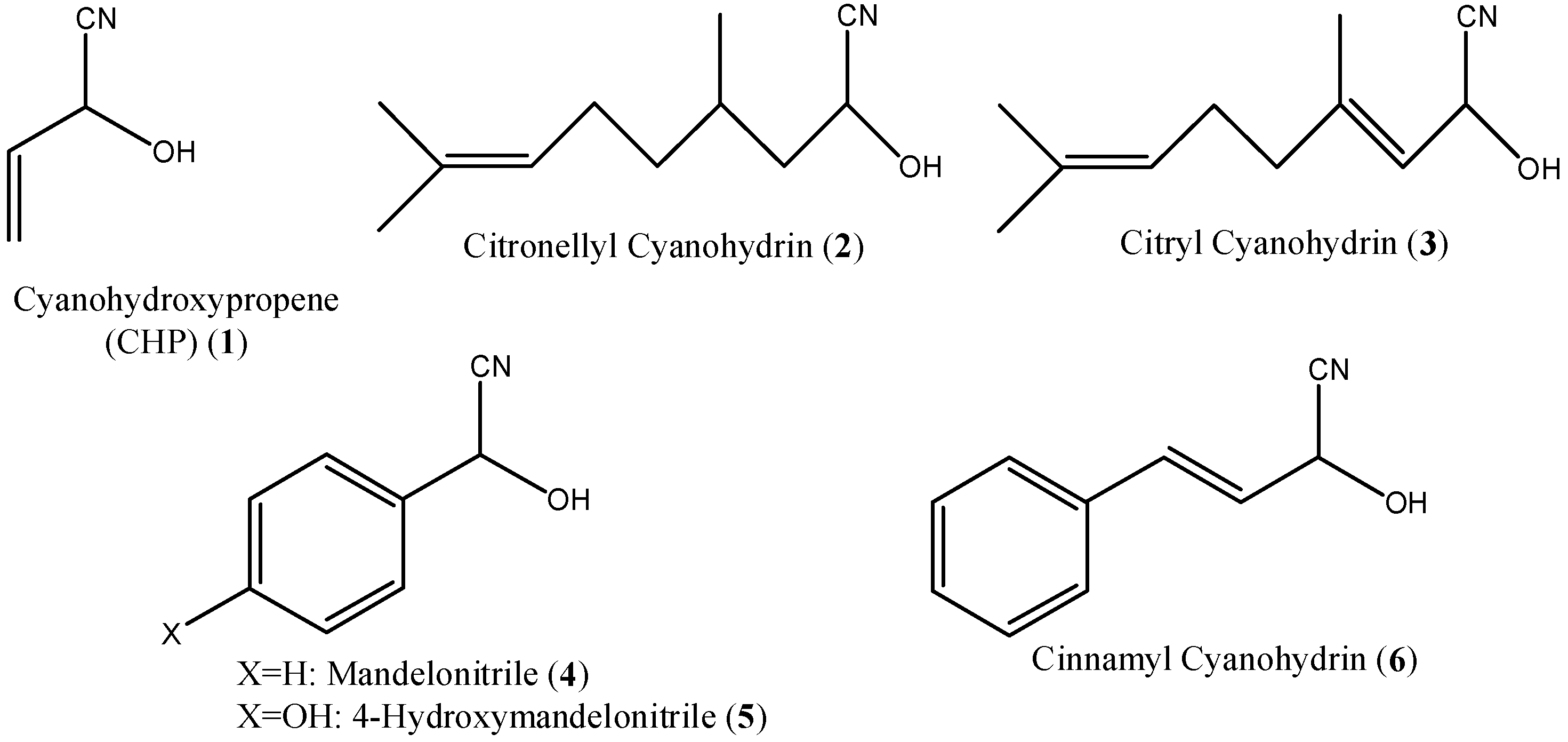
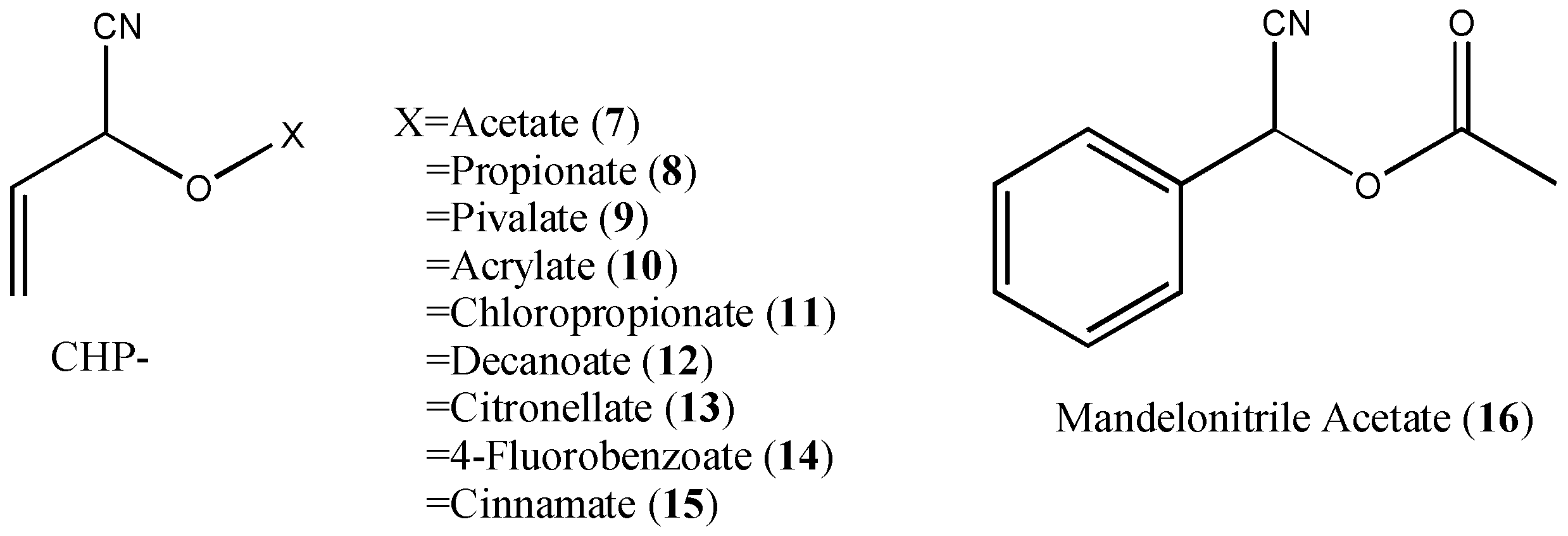
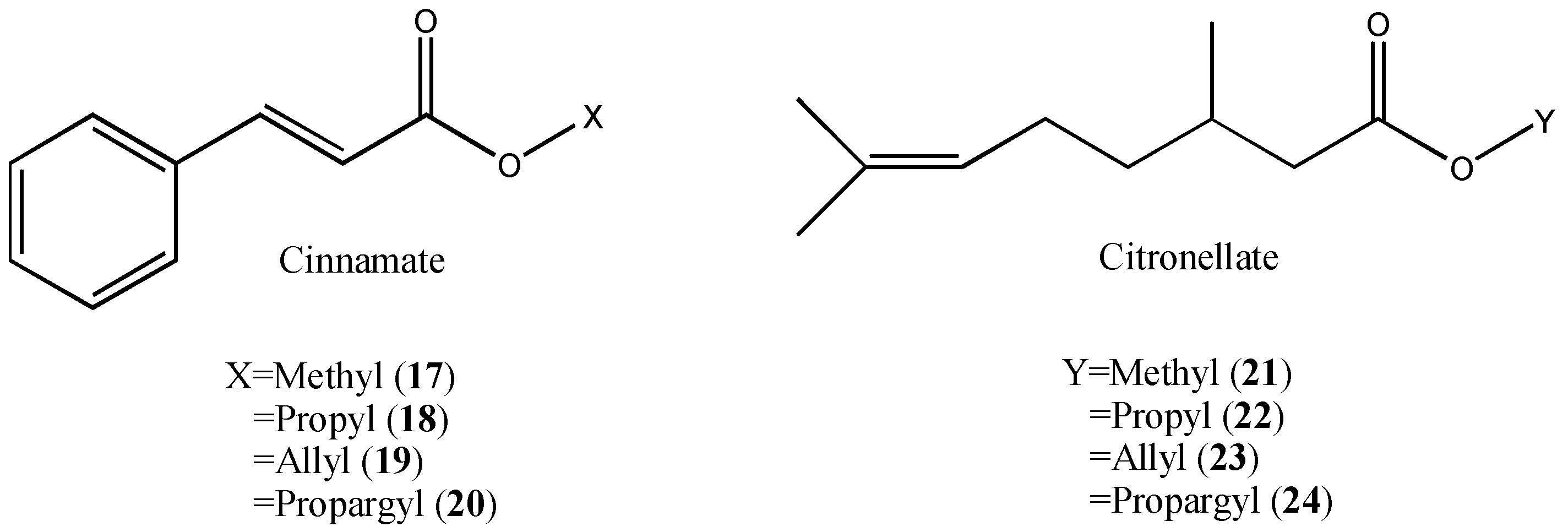
| N° | Compound | 100 μg/fly | 10 μg/fly | 1 μg/fly |
| 1 | CHP | 100 | 0 | 5 |
| 7 | CHP acetate | 100 | 0 | 0 |
| 8 | CHP propionate | 100 | 0 | 0 |
| 9 | CHP pivalate | 80 | 0 | 0 |
| 12 | CHP decanoate | 100 | 64 | 0 |
| 13 | CHP citronellate | 100 | 91 | 0 |
| 15 | CHP cinnamate | 87 | 50 | 0 |
| 17 | Methyl cinnamate | 50 | 0 | 10 |
| 18 | Propyl cinnamate | 38 | 0 | 0 |
| 19 | Allyl cinnamate | 100 | 40 | 4 |
| 20 | Propargyl cinnamate | 100 | 35 | 0 |
| 21 | Methyl citronellate | 57 | 5 | 4 |
| 22 | Propyl citronellate | 41 | 0 | 0 |
| 23 | Allyl citronellate | 100 | 17 | 5 |
| 24 | Propargyl citronellate | 100 | 15 | 0 |
| Control (acetone blank) | 0 |
| N° | Compound | LD50 | 95% FL |
| 2 | Citronellyl cyanohydrin | > 50 | |
| 3 | Citryl cyanohydrin | > 50 | |
| 4 | Mandelonitrile | 7.06 | 5.45, 8.54 |
| 5 | 4-Hydroxy mandelonitrile | 33.1 | 25.5, 47.5 |
| 6 | Cinnamyl cyanohydrin | 12.7 | 10.2, 15.7 |
| 16 | Mandelonitrile acetate | 14.5 | 11.8, 17.8 |
| N° | Compound | 100 ppm | 10 ppm | 1 ppm |
| 1 | CHP | 100 | 63 | 20 |
| 3 | Citryl cyanohydrin | 87 | 0 | 0 |
| 6 | Cinnamyl cyanohydrin | 100 | 15 | 25 |
| 7 | CHP acetate | 0 | 0 | 0 |
| 9 | CHP pivalate | 100 | 30 | 23 |
| 10 | CHP acrylate | 100 | 43 | 30 |
| 11 | CHP chloropropionate | 100 | 43 | 33 |
| 12 | CHP decanoate | 100 | 95 | 15 |
| 13 | CHP citronellate | 100 | 100 | 40 |
| 14 | CHP 4-fluorobenzoate | 20 | 27 | 13 |
| 15 | CHP cinnamate | 100 | 100 | 95 |
| 17 | Methyl cinnamate | 100 | 80 | 35 |
| 18 | Propyl cinnamate | 100 | 100 | 65 |
| 19 | Allyl cinnamate | 100 | 100 | 95 |
| 20 | Propargyl cinnamate | 100 | 100 | 90 |
| 21 | Methyl citronellate | 100 | 100 | 45 |
| 22 | Propyl citronellate | 100 | 100 | 100 |
| 23 | Allyl citronellate | 100 | 100 | 100 |
| 24 | Propargyl citronellate | 100 | 64 | 25 |
| Control | 4 |
| N° | Compound | LC50 | 95% FL |
| 1 | CHP | 2.75 | 1.94, 3.80 |
| 9 | CHP pivalate | 8.22 | 4.59, 17.8 |
| 10 | CHP acrylate | 5.19 | 3.55, 7.72 |
| 11 | CHP chloropropionate | 14.0 | 9.68, 23.9 |
| 14 | CHP 4-fluorobenzoate | 3.77 | 1.78, 7.78 |
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Peterson, C.J.; Tsao, R.; Eggler, A.L.; Coats, J.R. Insecticidal Activity of Cyanohydrin and Monoterpenoid Compounds. Molecules 2000, 5, 648-654. https://doi.org/10.3390/50400648
Peterson CJ, Tsao R, Eggler AL, Coats JR. Insecticidal Activity of Cyanohydrin and Monoterpenoid Compounds. Molecules. 2000; 5(4):648-654. https://doi.org/10.3390/50400648
Chicago/Turabian StylePeterson, Chris J., Rong Tsao, Aimee L. Eggler, and Joel R. Coats. 2000. "Insecticidal Activity of Cyanohydrin and Monoterpenoid Compounds" Molecules 5, no. 4: 648-654. https://doi.org/10.3390/50400648
APA StylePeterson, C. J., Tsao, R., Eggler, A. L., & Coats, J. R. (2000). Insecticidal Activity of Cyanohydrin and Monoterpenoid Compounds. Molecules, 5(4), 648-654. https://doi.org/10.3390/50400648





